Published online Jul 28, 2015. doi: 10.4254/wjh.v7.i15.1936
Peer-review started: February 12, 2015
First decision: March 20, 2015
Revised: June 24, 2015
Accepted: July 21, 2015
Article in press: July 23, 2015
Published online: July 28, 2015
Processing time: 178 Days and 11.3 Hours
A substantial proportion of individuals with chronic hepatitis C virus (HCV) are co-infected with human immunodeficiency virus (HIV). Co-infected individuals are traditionally considered as one of the “special populations” amongst those with chronic HCV, mainly because of faster progression to end-stage liver disease and suboptimal responses to treatment with pegylated interferon alpha and ribavirin, the benefits of which are often outweighed by toxicity. The advent of the newer direct acting antivirals (DAAs) has given hope that the majority of co-infected individuals can clear HCV. However the “special population” designation may prove an obstacle for those with co-infection to gain access to the new agents, in terms of requirement for separate pre-licensing clinical trials and extensive drug-drug interaction studies. We review the global epidemiology, natural history and pathogenesis of chronic hepatitis C in HIV co-infection. The accelerated course of chronic hepatitis C in HIV co-infection is not adequately offset by successful combination antiretroviral therapy. We also review the treatment trials of chronic hepatitis C in HIV co-infected individuals with DAAs and compare them to trials in the HCV mono-infected. There is convincing evidence that HIV co-infection no longer diminishes the response to treatment against HCV in the new era of DAA-based therapy. The management of HCV co-infection should therefore become a priority in the care of HIV infected individuals, along with public health efforts to prevent new HCV infections, focusing particularly on specific patient groups at risk, such as men who have sex with men and injecting drug users.
Core tip: This manuscript focuses on hepatitis C virus/human immunodeficiency virus (HIV) co-infection, two intersecting epidemics with great global health interest. It reviews the epidemiology, pathogenesis and natural history of chronic hepatitis C in HIV infected individuals. It also reviews the impact of antiretroviral therapy on the natural history of chronic hepatitis C and the liver. Moreover, it shows that the outcomes of treatment with the newer direct acting antivirals against hepatitis C are similar in the mono-infected and co-infected patients, providing informative data extracted from relevant clinical trials. It argues that HIV infected individuals should no longer be designated as a “special population” among those with chronic hepatitis C, as this could delay their access to the new treatments.
- Citation: Karageorgopoulos DE, Allen J, Bhagani S. Hepatitis C in human immunodeficiency virus co-infected individuals: Is this still a “special population”? World J Hepatol 2015; 7(15): 1936-1952
- URL: https://www.wjgnet.com/1948-5182/full/v7/i15/1936.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i15.1936
Co-infection with the blood-borne hepatitis C Virus (HCV) and human immunodeficiency virus (HIV) is common due to their shared routes of transmission and the fact that individuals with HIV are at higher risk of contracting HCV. Estimates of HCV prevalence in the general population overall vary from 0.3% in Austria, England and Germany to 8.5% in Egypt[1]. However, in the HIV population, the prevalence of HCV/HIV co-infection has been reported between 9.2%-37.3%[2,3]. This population has long been considered a special risk population both in terms of disease progression and subsequent mortality, and in terms of their inferior responses to traditional HCV therapies. However, in the ever-evolving era of direct-acting antivirals (DAAs) we ask the question “Is this still a special population?”.
Following its discovery 25 years ago and until recently, HCV was considered a disease of parenteral transmission, affecting people who inject drugs (PWIDs) who share needles or drug-taking equipment and of individuals who received infected blood products prior to the introduction of reliable screening in the 1990s. Largely this is still the case in less developed regions such as sub-Saharan Africa where HCV/HIV co-infected individuals tend to be older than those with HIV mono-infection, likely reflecting improvements to healthcare sterility and blood screening[4]. In almost all countries in the world there is a male preponderance for HCV infection overall, in keeping with higher levels of intravenous drug use in men, except in France and Germany where more women are affected, with the risk factor for acquisition being blood transfusion after childbirth[1].
It is estimated that in 2010 around 10 million PWIDs (range 6.0-15.2 million) were HCV seropositive. This is over three and a half times higher than the 2.8 million people estimated to be infected with HIV[5]. A review of worldwide systematic reviews demonstrated that the midpoint prevalence of co-infection in PWIDs varies greatly between countries ranging from 9.8% in Paraguay to 97% in Mexico. Those countries with the highest estimated populations of PWIDs were China, Russia and the United States with HCV prevalence of 67%, 72.5% and 73.4%, respectively. However, these statistics under-represent the total burden of HCV from drug use as they do not include former PWIDs who have previously been infected with HCV.
Blood-borne viruses account for much of the morbidity and cost associated with intravenous drug use and many countries around the world have invested in programmes to both treat drug addiction and promote safe injection practices. This may partly account for the reduction in HCV incidence in HIV-infected PWIDs that is currently being seen. The Swiss Cohort reported a decrease in incidence from 13.89 (95%CI: 8.20-22.39) per 100 person-years in 1998 to 2.24 (95%CI: 0.55-10.66) in 2011[6]. However, European surveillance data shows that although the number of newly diagnosed HIV infections related to intravenous drug use is decreasing following its peak in 2001-2002, only half the twelve countries with available data show decreases in HCV prevalence during 2005-2010[7]. These data however report prevalence rather than incidence and do not take into account probable increases in awareness and testing over the past decade. The economic recession that has struck countries like Greece worsens the efforts for tackling the HIV and HCV epidemics amongst PWIDs[8].
Transmission of HCV outside of these populations with healthcare-associated risks and intravenous drug-use has always been considered to be negligible and restricted to low numbers of new cases in regular sexual partners of individuals with HCV infection. However, a few years ago clinicians began to notice a significant rise in new HCV among men who have sex with men (MSM) and denied intravenous drug use and had no healthcare-associated risks. One survey of United Kingdom urban centre-based HIV clinics revealed a doubling of new HCV in MSM from 6.86 cases/person-years in 2002 to 11.58 in 2006, without an apparent change in testing policy[9].
This observed shift of new HCV infections from PWIDs to MSM was confirmed in analysis of the Swiss Cohort showing an alarming 18-fold increase in the incidence of new cases in MSM from 0.23 (95%CI: 0.08-0.54) per 100 person-years in 1998 to 4.09 (95%CI: 2.57-6.18) in 2011. This resulted in an increase in the proportion of MSM among incident HCV from 20% prior to 2006 to 75% after (P < 0.001)[10]. Significant increases were also shown in the Amsterdam MSM cohort from 2000 to 2005 (incidence rate ratio 3.41, 95%CI: 1.58-7.34), though there was a levelling off of new HCV infections in MSM from 2005 onwards[11].
Virus sequencing and phylogenetic analysis of 226 HIV-infected MSM diagnosed with acute HCV from urban centres in England, Netherlands, Germany, France and Australia revealed that the independently reported European outbreaks were actually part of a large European MSM-specific transmission network[12]. A second MSM-specific transmission network was found in Australia, with very little overlap with the European network. In contrast to the European network, only 18% of transmissions in this cohort were thought to be from sexual exposure, with intravenous drug exposure still the predominate risk factor (73%)[13]. Eighty-six percent of sexual transmissions were in MSM and all of those were HIV-positive. In this cohort social networks exist in HIV-infected MSM that contain both PWIDs and non-PWIDs.
In the European transmission networks of MSM the predominant HCV genotype has been shown to be genotype 1a (59%), with an unexpectedly high proportion of genotype 4d (23%)[12]. Thus, the difficult-to-treat genotypes 1 and 4 accounted for 90% of infections compared to 67% of the Australian cohort. This has clear implications for treatment and healthcare planning.
The cause of this epidemic of HCV in MSM is likely to be multi-factorial. There is certainly some evidence that risk of HCV transmission in HIV-infected MSM is associated with non-intravenous recreational drug taking. Recreational drug use may increase the risk of unprotected sex and may be associated with group sex or with more traumatic sexual practices. In the Multicenter AIDS Cohort Study (MACS) non-intravenous recreational drug use was found to double risk, however, in the Swiss cohort no association was found[6,14].
Risk of HCV acquisition in MSM has, unsurprisingly been found to be related to multiple sexual partners, receptive anal sex and inconsistent condom use[6,14]. The spread of HCV may in part, be related to the practice of serosorting. Though men may select sexual partners on the basis of HIV status, they may well be at risk of HCV; almost a third of HIV-positive individuals are unknowingly infected with HCV[15]. However, if HCV transmission among MSM was solely due to behavioural factors, higher rates would be expected in the HIV-negative MSM population, even taking into account serosorting. Interestingly, there has been no increase in HCV in HIV-negative MSM observed, and risk has been shown to be comparable or lower than the risks in the heterosexual population[16]. More recently, however, there are emerging reports of HCV infection amongst HIV-negative MSM, so this may well represent an emerging epidemic[17].
In both the Swiss and the MACS cohorts, risk was associated with past or recent syphilis infection, confirming either a shared route of acquisition or suggesting that potentially ulcerative sexually transmitted infection could increase the risk of HCV transmission[6,14]. Individuals with HCV/HIV co-infection have been shown to have higher HCV viral loads than HCV mono-infected individuals, which may well increase the risk of transmission, particularly in the presence of ulcerative lesions, as recognised in HIV transmission[18]. A change in the virulence of circulating HCV does not appear to account for the HCV epidemic is not supported by phylogenetic analysis showing strains in the populations belong to several different genotypes and subtypes[12]. There has also been the suggestion that transmission risk may increase with decreasing CD4 count. In the MACS cohort every 100 cells/mm3 decrease was associated with a 7% increase in risk of transmission below 500, though no association was shown in other studies[6,14].
The reports of HCV outbreaks in MSM around 2000, shortly followed the introduction of combination antiretroviral therapy (cART) in 1996. It was thought that individuals on cART had increased their sexual risk taking as a result of having suppressed HIV viral loads and reduced risk perception from HIV and there is some data to support this[19]. However, no association with HCV seroconversion and use of cART has been found, and cohort analyses have shown incident infections since the 1980s, and increases in incidence since the 1990s, well before the introduction of cART[6,14,20]. Furthermore, in the phylogenetic studies described, for each cluster of new HCV infections, the date of the common ancestor was calculated using the molecular clock approach. In the Australian networks the earliest events were estimated to have occurred around 1989 and in the European clusters 15% of transmissions were estimated to have occurred prior 1996, though the majority of infections (63%) did occur after 2000[13,20].
Acute hepatitis C is usually asymptomatic or minimally symptomatic and rarely causes severe hepatitis. Depending on the characteristics of the population examined, around 80% of patients with newly acquired hepatitis C mono-infection will develop chronic hepatitis C[21]. Most of the chronically mono-infected hepatitis C are asymptomatic, but 20%-30% will progress to develop cirrhosis over about 30 years[22]. The risk of progression from advanced fibrosis to cirrhosis has been estimated at about 10% per year[23]. Individuals with compensated cirrhosis have approximately a 4% annual risk for hepatic decompensation and a 1%-2% annual risk for development of hepatocellular carcinoma[24,25]. Among those with a first episode of hepatic decompensation, almost half will die within the next 5 years[24,26].
Higher HCV plasma viral loads are seen in HIV co-infected individuals[27]. The level of HCV viraemia has been inversely correlated with CD4 counts[28]. Although HCV viraemia is not thought to play a role in the rate of progression of liver disease, it is important in the treatment response to pegylated interferon (PegIFN)/ribavirin (RBV) therapy and may also play a role in the length of therapy required for likelihood of response to PegIFN-free therapy[29,30].
The likelihood of spontaneous clearance of acute hepatitis C infection appears to be lower in HIV-co-infected individuals[28]. This could correlate to weaker HCV-specific T-cell responses in individuals with HIV infection[31]. Immunogenetic factors, particularly a favourable interleukin-28B (IL28B) genotype, play a role in this regard, as in mono-infected individuals. Some high-risk HIV-infected individuals present with one or more reinfections after spontaneous clearance or successful treatment of hepatitis C. It has been suggested that the likelihood of clearance of a new episode of acute hepatitis C increases with the prior number of spontaneous clearances[32].
Numerous clinical studies have shown that HIV infection is an accelerator of hepatitis C related outcomes[33]. Whether the sequence of acquisition of the viruses is important in this regard has not been fully elucidated. Some experts argue that hepatitis C may progress more rapidly if acquired in a patient with pre-existing immunosuppression, as seen in the setting of the recurrence of hepatitis C after orthotopic liver transplantation[34]. There are case reports of HIV infected individuals developing decompensated cirrhosis and death within as soon as 2-8 years after HCV acquisition. Other studies, however, have not found such a malignant course after acute hepatitis C in HIV infected individuals[35].
The rate of fibrosis progression has been found to be faster in HCV/HIV co-infected individuals compared with HCV mono-infected ones. Lower CD4 counts and higher HIV viral load have been associated with a greater likelihood of fibrosis progression. Other risk factors for faster fibrosis progression among co-infected individuals include advanced age, alcohol use, viral co-infection, obesity, insulin resistance, and hepatic steatosis, the latter being more common with genotype 3 HCV infection[36-40].
In HCV/HIV co-infected individuals, as in those with HCV mono-infection, the likelihood of hepatic decompensation is associated with the stage of liver disease[41]. However, the likelihood of decompensation is higher for co-infected vs mono-infected individuals with a similar stage of liver disease, even if HIV control is achieved with antiretroviral therapy[42]. The prognosis following hepatic decompensation in co-infected individuals is generally poor. A median survival of 13 mo was noted in a prospective cohort of 153 HCV/HIV co-infected individuals after the first episode of hepatic decompensation[43]. The definitive treatment in decompensated cirrhosis is liver transplantation, the outcomes of which are less favourable for co-infected individuals[44]. HCV/HIV co-infected individuals have also a greater incidence of hepatocellular carcinoma than those with HCV mono-infection, which is observed at a younger age, is typically more advanced and more likely to be symptomatic at diagnosis, and has a worse prognosis[45].
With the effective control of HIV infection with potent antiretroviral therapy, non-AIDS related causes of death have become more prominent[46]. Among these, liver-related death, associated with chronic hepatitis, is one of the most common causes of death in the HIV-infected population[47,48]. Although the upwards trend of liver-related deaths among HIV infected individuals appears to be reversing, the proportion remains considerably high[46,48]. Liver-related death may be more likely in patients with lower CD4 counts[49]. The presence of HCV infection among HIV infected individuals has been associated with a negative impact on overall mortality[50].
All-cause hospitalization is also more likely in HCV/HIV co-infected compared with HIV mono-infected individuals[51]. Certain comorbid conditions have been observed more frequently in HCV/HIV co-infected individuals than those with HIV mono-infection. These include cardiovascular disease, neurocognitive disorders, chronic kidney disease, osteoporosis and bone fractures, as well as diabetes mellitus[52].
The achievement of a sustained virological response with anti-HCV treatment in HIV co-infected individuals has been associated with the same benefits on liver disease as those seen in HCV mono-infection, including decreases in fibrosis progression and greater likelihood for regression of fibrosis, as well as decreases in the rate of hepatic decompensation and liver-related mortality[39,53-55]. Although co-infected individuals with advanced fibrosis who have failed prior PegIFN/RBV therapy may fare better in terms of fibrosis progression than untreated individuals, maintenance PegIFN/RBV therapy in the setting of treatment failure has not proven beneficial[55,56]. Hepatocellular carcinoma can develop in individuals with cirrhosis despite effective treatment of chronic hepatitis C.
The effect of HCV infection on the natural history of HIV infection has not been well characterised[57]. Most studies suggest that chronic hepatitis C does not alter the course of HIV infection, however, in a multi-national HIV seroconvertor cohort, HCV appeared to increase risk of progression to AIDS and death[58].
Several pathogenetic mechanisms could explain the faster liver disease progression rate in HCV/HIV co-infected individuals. Although HIV does not directly infect hepatocytes, it has been shown that HIV enhances the replication of HCV in hepatocytes in vitro. This effect could be mediated by the interaction between HIV and the co-receptors CCR5 and CXCR4 on hepatocytes, via a transforming growth factor (TGF)-β1-mediated pathway[59]. TGF-β1 is a key mediator in the process of liver fibrosis, as it is one of the most pro-fibrinogenic cytokines. HIV can also promote fibrogenesis via the induction of production of reactive oxygen species by hepatocytes and hepatic stellate cells, via an nuclear factor kappa-B-dependent pathway; this effect is enhanced in the presence of HCV. HIV can also induce hepatocyte apoptosis through increased sensitivity to tumor necrosis factor-related apoptosis-inducing ligand[60]. The systemic immune activation in HIV-infection has been associated with activation of hepatic stellate cells, which have a central role in the development of fibrosis[61]. Direct infection of hepatic stellate cells by HIV has been documented, although the exact mechanism is unclear[62]. The activation of hepatic stellate cells may also relate to the diminished natural killer-cell cytotoxic responses against these cells that is seen in HIV infection, owing to loss and impaired function of CD4+ cells[63].
HIV infection has been associated with higher hepcidin blood levels than HCV mono-infection or HCV/HIV co-infection[64,65]. Hepcidin is a peptide hormone that regulates iron homeostasis. Whether increased hepcidin results in increased liver iron stores in co-infected individuals than HCV mono-infected ones, remains to be proven. Of note, liver iron has been shown to stimulate hepatic stellate cells and negatively affects fibrosis progression in HCV mono-infected individuals[66].
The immune responses against HCV are compromised in HIV co-infected individuals. Lower CD4 counts lead to attenuated CD8+ T cell HCV-specific immune responses[31,67]. The HCV infecting viral population appears to be genetically more diverse in co-infection[31,68]. This reflects weaker selection pressure from the immune system. Higher quasispecies heterogeneity might negatively affect the response to interferon-based treatment[69].
HIV leads to a state of immune activation and dysregulation. Decrease in CD4 cells in gut-associated lymphoid tissue, which occurs early in the course of HIV infection, leads to increase in microbial translocation through the intestinal mucosa[70]. This is evident by increase in the circulating lipopolysaccharide (LPS) and other relevant markers. Higher levels of circulating LPS have been associated with a higher likelihood of development of cirrhosis in HCV/HIV co-infected individuals[71].
Since the introduction of cART, life expectancy for individuals living with HIV has become comparable to that associated with other long term conditions, though it is still lower than the general population[3,72]. Less people with HIV are dying from HIV/AIDS-related causes and with the increasing length of survival, the relative importance of comorbidities such as viral hepatitis has increased[73]. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study found that since the introduction of cART, there has been a proportional increase in liver-related deaths (LRD) and that this was the most common cause of non-AIDS related death[49]. There was initial concern that this was a consequence of ART-related hepatotoxicity, however, in subsequent analyses it became clear that this excess liver-related mortality is largely a product of viral hepatitis, and that half of those that died in the D:A:D study had active HCV[73,74].
Individuals with HCV/HIV continue to do significantly worse in terms of mortality when compared with their HIV mono-infected peers. In a Spanish cohort, all cause mortality reduced by almost 50% in HIV mono-infected individuals, but no significant change was found in HCV/HIV co-infection[75]. A meta-analysis of cohort studies showed no increased risk of mortality associated with HCV/HIV co-infection in the pre-cART era, but since the introduction of cART the risk ratio was 1.12 (95%CI: 0.82-1.51) for AIDS-defining events and 1.35 (95%CI: 1.11-1.63) for overall mortality among co-infected patients, compared with that among patients with HIV mono-infection[50].
Though there is a risk of hepatitis flare when first initiating cART, there is no evidence that cumulative exposure to cART in itself is related to increased mortality[76,77]. The increased number of deaths in co-infected individuals after the introduction of cART is likely to reflect those individuals who would not have previously survived from HIV/AIDS related events rather indicating a hepatotoxic effect of cART. Moreover, any potential risk is outweighed by the benefits of treatment.
Though the benefits of cART to HIV disease are clear, its impact on liver disease progression in co-infected individuals has been debated. A meta-analysis in 2008 failed to show that cART had any significant effect on fibrosis progression or risk of cirrhosis. However, it did show that the risk of cirrhosis in the post-cART era was slightly lower than pre-cART[78]. Other studies since then have shown an association between the use of cART and a slower rate of liver fibrosis[79,80]. One study of 638 co-infected individuals, 69% of whom were on cART, showed that both current cART and HIV viral suppression were independently associated with decreased incidence of all-cause events and a 66% reduction in liver-related events such as end-stage liver disease (ESLD), hepatocellular carcinoma (HCC) and LRD[41]. Another study found evidence that cART reduces the risk of hepatic decompensation in those with advanced liver disease[81].
As a result of these benefits, national and international guidelines have changed to recommend initiation of cART in HCV/HIV co-infection at earlier stages of HIV disease[82,83]. One mathematical model has been used in South Africa to estimate the benefit of expanding cART eligibility from those with CD4 < 350 cells/mm3 to those with CD4 < 500 cells/mm3. Factoring in the assumptions that co-infection accelerates liver disease 2.5-fold and that cART reduces progression by one third, this model simulated disease progression in both HIV mono-infection and individuals co-infected with viral hepatitis. Significant benefits were shown in hepatitis B virus (HBV)/HIV co-infection, and HIV mono-infection in terms of deaths averted and disability-adjusted life-years (DALY). However, in HCV/HIV co-infection, expanding eligibility of cART actually increased the share of LRD by 34% as individuals survive for longer. Expanding eligibility was estimated to avert only 3.9 DALYs compared to 4.8 for HIV mono-infection and 5.1 for HBV/HIV co-infection. Authors estimated cART would need to reduce liver disease progression by 70% to show any significant benefit[84].
The obvious reason for this discrepancy in the benefit of cART between HBV/HIV and HCV/HIV co-infections is that while HBV can be easily controlled with well tolerated anti-hepatitis B containing cART, HCV has, until now, required long courses of poorly-tolerated subcutaneous PegIFN with RBV. The EuroSIDA study demonstrated that from 1998 to 2007, 22% of patients with at least F2 fibrosis remained untreated and that there were significant variations of treatment uptake across Europe and across transmission groups[85]. There has been a rise observed in HCV treatment uptake in co-infected individuals from 22% in 1991 one study to 88% in 2012[10]. However, a decline in 2013 was noted as individuals with less advanced disease inevitably await the availability of newer treatments[1].
Several studies have confirmed that PWIDs are less likely to receive treatment for their HCV than MSM[10,85]. Intravenous drug use is independently associated with poorer outcome in terms of all-cause mortality, LRD, ESLD and HCC[3,41,49]. Barriers to treatment in PWIDs include persistent drug or alcohol addiction, difficulties accessing care, concerns over drug side effects, poverty, discrimination and general poor health. Co-infected PWIDs are less likely to complete HCV treatment compared to mono-infected PWIDs. Adherence however, can be improved with addiction treatments, though coverage of opioid substitution and needle/syringe programmes is very variable across Europe with particularly low coverage in central and south-east Europe[7,86]. Addiction treatment can also have benefits for HIV treatment in terms of compliance and improved virological success[87]. After the roll-out of shorter, oral treatments for HCV with fewer side effects, requiring less intensive monitoring, it is hoped that uptake of HCV treatments will improve, but particular focus must still given to the hard-to-reach PWID population.
The response to PegIFN alpha plus RBV for the treatment of chronic hepatitis C in individuals with HIV co-infection is lower than in those with HCV mono-infection[88,89]. The basis of the viral clearance of HCV with PegIFN/RBV therapy is immunologic. PegIFN acts primarily by enhancing the innate antiviral immune response and can also potentiate adaptive immune responses[90]. Ribavirin is thought to exert various, not well characterised antiviral effects and to potentiate the effect of PegIFN[91]. An important predictor of the treatment success with PegIFN/RBV against chronic hepatitis C genotype 1 or 4 infection is a favourable interleukin 28B genotype[29,92].
The integrity of the immune system appears to be less important when direct acting antivirals are used for the treatment of HCV infection. The introduction in 2011 of the direct acting antivirals boceprevir and telaprevir, which are first generation, first wave NS3/4A protease inhibitors, has allowed for a substantial increase in the likelihood for sustained virological response (SVR) in genotype 1 HCV/HIV co-infected patients (Table 1). The absolute treatment benefit achieved with these agents is similar to that observed in HCV mono-infected individuals[93,94]. In a recently published study, telaprevir in combination with PegIFN/RBV had high effectiveness (SVR24 80%) in PegIFN/RBV treatment-experienced genotype 1 HCV/HIV co-infected individuals[95]. This population would have been considered to be a “difficult-to-treat” one in the era before DAAs. The likelihood of treatment success did not differ by the fibrosis stage, IL28B genotype, HCV 1a or 1b genotype, CD4 cell count, type of previous response to HCV treatment, baseline HCV-RNA level or the rapidity of HCV-RNA response.
| Study | GT | Tx regimen | Tx duration (wk) | Hepatitis C characteristics | SVR12 | ||
| GT | Fibrosis stage | Treatment status | |||||
| P05411[100] | 1 | PegIFNα-2b + RBV | BOC: 44 | Tx naïve | BOC | ||
| wb (600-1400 mg) + | PegIFN/RBV: 48 | Metavir F0-4 | GT1: 42/64 (66%) | ||||
| BOC vs placebo | Cirrhosis: 3% | GT1a: 32/51 (63%) | |||||
| GT1b: 7/12 (58%) | |||||||
| Placebo GT1: 9/34 (26%) | |||||||
| VX08-950-110[101] | 1 | PegIFNα-2a + RBV | TPV: 12 | Tx naïve | TPV | ||
| 800 mg or wb | PegIFN/RBV: 48 | Metavir F0-4 | GT1: 28/38 (74%) | ||||
| (1000-1200 mg) + | GT1a: 20/27 (74%) | ||||||
| TVR vs placebo | GT1b: 7/11 (64%) | ||||||
| Placebo | |||||||
| GT1: 10/22 (45%) | |||||||
| GT1a: 4/14 (29%) | |||||||
| GT1b: 4/6 (67%) | |||||||
| ANRS HC26 | 1 | PegIFNα-2a + RBV | TPV: 12 | Tx exp | GT1: 55/69 (80%) | F1-2 | Relapse |
| TelapreVIH[95] | wb (1000-1200 mg) | PegIFN/RBV (RGT): 44 or 72 | Metavir F0-4 | GT1a: 36/48 (75%) | 34/42 (81%) | 20/27 (74%) | |
| + TVR | Both cirrhosis and prior | GT1b: 19/21 (90%) | F3-4 | Breakthrough | |||
| null-response excluded | 21/27 (78%) | 5/6 (83%) | |||||
| Partial response | |||||||
| 15/15 (100%) | |||||||
| Null response | |||||||
| 15/21 (71%) | |||||||
| P7977-1910[102] | 1-4 | SOF + PegIFNα-2a | 12 | Tx naïve | All GTs: 21/23 (91%) | ||
| + RBV wb | No cirrhosis | GT1: 17/19 (89%) | |||||
| (1000-1200 mg) | [GT1a: 13/15 (87%) | ||||||
| GT1b: 4/4] | |||||||
| GT2: 1/1 | |||||||
| GT3: 2/2 | |||||||
| GT4: 1/1 | |||||||
| STARTVerso4[103,104] | 1 | FDV 240 mg (EFV) or 120 mg | FDV: 24 or (12 vs 24), | PegIFN/RBV Tx naïve | GT1: 221/308 (72%) | No Cirrhosis | Tx naïve |
| (ATV/r or DRV/r) or 120 vs 240 mg | PegIFN/RBV (RGT): 48 or | or prior relapse | GT1a: 171/242 (71%) | 187/261 (72%) | 164/239 (69%) | ||
| (RAL or MVC or no ART) + | 24 wk vs 48 wk | Compensated cirrhosis: 15% | GT1b: 50/66 (76%) | Cirrhosis | Relapse | ||
| PegIFNα-2a + RBV wb (1000-1200 mg) | 33/45 (73%) | 57/69 (83%) | |||||
| C212[105] | 1 | SMV + PegIFNα-2a + RBV wb | SMV: 12 | PegIFN/RBV Tx naïve | GT1: 78/106 (74%) | F0-2 | Tx naïve |
| (1000-1200 mg) | PegIFN/RBV RGT: (24 vs 48) | or Tx exp | GT1a: 62/88 (70%) | 36/45 (80%) | 42/53 (79%) | ||
| for Tx naïve or prior relapse | Metavir F0-4; Cirrhosis: | GT1b: 16/18 (89%) | F3-4 | Relapse | |||
| or 48 for partial- | 6%-30% by Tx arm | 14/22 (64%) | 13/15 (87%) | ||||
| or null-response or cirrhosis | Partial response | ||||||
| 7/10 (70%) | |||||||
| Null response | |||||||
| 16/28 (57%) | |||||||
| PHOTON-1[106] | 1-3 | SOF + RBV wb (1000-1200 mg) | GT 1: 24 | Tx naïve (GT1-3) or exp (GT2-3) | GT1: 87/114 (76%) | No Cirrhosis | Tx naïve |
| GT 2-3 Tx naïve: 12 | Cirrhosis ≤ 20% | [GT1a: 74/90 (82%) | GT1: 84/109 (77%) | GT1: 87/114 (76%) | |||
| GT 2-3 Tx exp: 24 | GT1b: 13/24 (54%)] | GT2: 22/25 (88%) | GT2: 23/26 (88%) | ||||
| GT2: 44/50 (88%) | GT3: 24/36 (67%) | GT3: 28/42 (67%) | |||||
| GT3: 44/59 (75%) | Cirrhosis | Tx exp | |||||
| GT1: 3/5 (60%) | GT2: 22/24 (92%) | ||||||
| GT2: 1/1 | GT3: 16/17 (94%) | ||||||
| GT3: 4/6 (67%) | |||||||
| PHOTON-2[107] | 1-4 | SOF + RBV wb (1000-1200 mg) | GT 1, 3, 4: 24 | Tx naïve (GT1-4) or exp (GT 2-3) | GT1: 95/112 (85%) | No Cirrhosis | Tx naïve |
| GT 2 Tx naïve: 12 | Compensated cirrhosis: 20% of patients | [GT1a: 84/100 (84%) | GT1: 84/95 (88%) | GT 1: 95/112 (85%) | |||
| GT 2 Tx exp: 24 | GT1b: 10/11 (91%)] | GT2: 19/22 (86%) | GT2: 17/19 (89%) | ||||
| GT2: 22/25 (88%) | GT3: 73/80 (91%) | GT3: 52/57 (91%) | |||||
| GT3: 94/106 (89%) | GT4: 19/23 (83%) | GT4: 26/31 (84%) | |||||
| GT4: 26/31 (84%) | Cirrhosis | Tx exp: | |||||
| GT1: 11/17 (65%) | GT2: 5/6 (83%) | ||||||
| GT2: 3/3 | GT3: 41/49 (84%) | ||||||
| GT3: 21/26 (81%) | |||||||
| GT4: 7/8 (88%) | |||||||
| TURQUOISE-I[108] | 1 | Paritaprevir/r/Ombitasvir + | 12 or 24 | Tx naïve or PegIFN/RBV exp | Tx for 12 wk | ||
| Dasabuvir + RBV wb (1000-1200 mg) | Cirrhosis ≤ 30% | GT1: 29/31 (94%) | |||||
| Tx for 24 wk | |||||||
| 19/20 (95%) | |||||||
| ERADICATE[109] | 1 | SOF/LDV | 12 | Tx naïve | Untreated HIV Infection | ||
| Knodell F0-3 | GT1: 10/10 | ||||||
| C-WORTHY[110] | 1 | Grazoprevir + MK-8742 +/- RBV | 12 | Tx naïve | With RBV | ||
| No cirrhosis | 28/29 (97%) | ||||||
| Metavir F0-3 | Without RBV | ||||||
| 26/29 (90%) | |||||||
Despite their antiviral activity, boceprevir and telaprevir have many limitations including the requirement for a long course of therapy in combination with PegIFN/RBV, a high rate of adverse effects, the need for multiple daily dosing including the requirement for co-administration with food, high pill burden, low barrier to resistance, and a high potential for drug-drug interactions[96]. Some of these issues are particularly important in the context of HIV co-infection and concomitant antiretroviral therapy.
Newer DAAs have now been marketed and numerous others are in the later stages of clinical development[97]. The drug targets include the NS3/4A serine protease, the NS5A replication complex, and the NS5B RNA polymerase; the latter enzyme can be targeted by nucleos(t)ide or non-nucleoside inhibitors. Following the paradigm of combination antiretroviral therapy, the combination of two or more antiviral agents (depending on potency, genetic barrier to resistance and activity against the different HCV genotypes), has made interferon-free therapy possible[98,99]. As of this writing, sofosbuvir (NS5B RNA polymerase nucleotide inhibitor), simeprevir (NS3/4A protease inhibitor), daclatasvir and ledipasvir (NS5A inhibitors) have been approved by the European Medicines Agency.
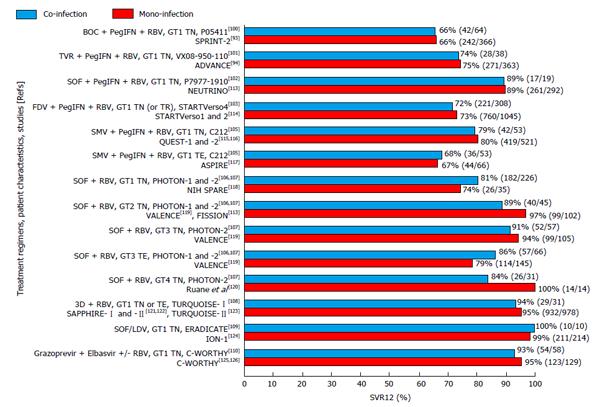
Table 1 presents the characteristics and findings of the main trials of direct acting antivirals, with or without PegIFN, in HCV/HIV co-infection[95,100-110]. The newer interferon-free regimens are expected to allow for a high likelihood (> 90%) of achieving SVR, with shorter treatment duration, a favourable side-effect profile (similar to that observed in mono-infected individuals), and convenient dosing schedules. The genotype 3 now appears as the most challenging HCV genotype to treat, as few of the newer DAAs have potent specific antiviral activity. This is important as genotype 3 is more common amongst PWIDs, who also have a substantial rate of HIV co-infection[111,112].
The effectiveness of DAA-based therapy does not appear to differ between HCV mono-infected and HCV/HIV co-infected individuals according to data from different trials, as shown in Figure 1[93,94,100-103,105-110,113-126]. For that reason, experts recommend that the same DAA-based regimens should be used in HCV/HIV co-infected individuals as those used in HCV mono-infected individuals[127,128]. The same position is held by the European Association for the Study of Liver, with a note for attention to potential drug-drug interactions[129]. In the United States, the pertinent guidelines consider HIV co-infected patients as one of four “unique patient populations”[130]. The United States Food and Drug Administration regards the HCV/HIV co-infected population as “a population with unmet medical needs”[131]. It warrants that industry sponsors for the development of DAAs to present specific clinical trial data on drug-drug interactions with the antiretroviral agents and the safety for HIV infection. In our opinion, drug developers must make sure that such recommendations do not lead to unnecessary delay in the licensure and availability of the newer agents for HCV/HIV co-infected individuals.
Routine screening for hepatitis C infection in HIV-infected individuals allows for many cases of acute hepatitis C infection to be recognised. This is important given the rising incidence of HCV acquisition among HIV-infected MSM in many countries seen over the past 10 years and the high incidence of HCV acquisition among PWIDs. Treatment of acute HCV infection with PegIFN results in a high likelihood of treatment success in mono-infected individuals, although in HIV co-infected individuals the addition of RBV is recommended[132]. Trials are under way for testing new DAA-based regimens for this indication, which might allow for a shorter duration of treatment, fewer side effects and great treatment effectiveness[133].
Reinfection with hepatitis C is not infrequent in certain HIV infected populations, particularly those with ongoing sexual risk-taking behaviour and illicit drug use[134]. Reinfection with a new HCV strain can in some cases complicate the assessment of the effectiveness of treatment against hepatitis C if it occurs during or shortly after the completion of treatment[135]. Data from phylogenetic analyses of paired samples from the same individuals are reassuring that true late relapses are generally rare in co-infected individuals, as is the case for mono-infected individuals[136].
Despite the effectiveness of DAAs in achieving SVR in HCV/HIV co-infected individuals, there are several issues that should be considered in the management of this population. As discussed, the treatment regimens must be selected carefully in view of the potential for drug-drug interactions between several DAAs and antiretroviral agents. This might require changes in dosage, as is the case for daclatasvir when used with efavirenz or boosted HIV protease inhibitors, or even avoidance of certain combinations. Moreover, the pharmacokinetics of different agents might change with different degrees of hepatic insufficiency and there is clearly a need for more data in this field[137]. Polypharmacy is common in HIV-infected individuals and a careful review of all drugs is needed before the addition of DAAs.
The sequence of treatment against each infection in newly diagnosed HCV/HIV co-infection could have been important with regard to treatment with PegIFN/RBV. Although chronic hepatitis C treatment can be treated prior to the commencement of antiretroviral therapy in patients with a high (> 500 per mm3) CD4 cell count, achieving HIV-RNA suppression first might increase the likelihood of SVR[138]. These considerations might not be important in the era of new DAAs.
Interferon alpha has been shown to reduce HIV-RNA plasma viral load by about 1 log10 after one week of therapy and to have sustained activity against HIV over a treatment period of 24-28 wk[139,140]. This effect might be protective in terms of control of HIV in the case that drug-drug interactions result in lower exposure to antiretroviral drugs. With the newer DAAs, interferon-free treatment courses as short as 12 wk have been used and even shorter courses are under investigation. Treatment for such a short duration can mitigate the effect of any potential drug-drug interactions of DAAs with ARVs on the control of HIV infection.
Adverse drug reactions in patients receiving treatment for hepatitis C might be more common in those co-infected with HIV. This has been a problem with boceprevir and telaprevir, but newer DAAs appear to have a similar safety profile in mono-infected compared to co-infected individuals.
Many of the HIV-infected individuals who are engaged into care have built over time strong relationships with their treating physicians and are well educated about several health issues[141-143]. Their care generally includes screening and immunisation against hepatitis A and B (if at risk) and monitoring for drug adherence and substance abuse disorders. The health-care structures for these individuals often provide social and psychological support for those with social/financial problems, substance abuse issues or psychiatric comorbidity. Thus, many HIV infected individuals could be well-prepared for receiving treatment for concomitant hepatitis C infection. In the case of MSM, preventing transmission of HCV to their sexual partners might be an additional incentive for a successful treatment outcome. Moreover, HIV-infected individuals receiving long-term antiretroviral therapy are familiar with the need of taking a long-term drug regimen[144]. It may be easier for them to incorporate DAAs in their daily schedule than individuals who have never taken long-term therapy.
The substantial drug costs of DAAs raise the issue of the access to the new treatments and of treatment prioritisation. Clearly those with advanced liver disease, whether mono-infected or HCV/HIV co-infected are in the greatest need for the new treatments.
In general, HCV/HIV co-infected individuals should be considered as a population in need for treatment of hepatitis C with the new DAAs. The uptake of PegIFN/RBV treatment has been low in this population, due to the presence of comorbidity or other conditions that render many patients ineligible, low treatment effectiveness, difficulties in staging of liver disease, or relative inexperience of some infectious diseases/HIV-medicine providers[145-147]. As mentioned above, the progression of HCV infection is accelerated in the presence of HIV co-infection and a not insignificant minority of individuals can progress rapidly after acute infection. The fact that the HIV population is ageing is another factor that makes treatment of hepatitis C important, as liver-related complications increase in the elderly[148]. HIV co-infected individuals may not have good access to liver transplantation in case that decompensated cirrhosis or HCC develops, while the management of these patients post-transplant is still challenging[149]. Thus, a decision to defer treatment for hepatitis C must be weighed against the above considerations.
The access to the new DAAs for the co-infected population is also important from a public health perspective in order to decrease the incidence of new infections, which is particularly high for certain subgroups[150]. In contrast, many mono-infected individuals have acquired HCV iatrogenically in the distant past, and are of relatively low risk of transmitting the virus to others. The ultimate goal would be the eradication of HCV[151]. Although this necessitates the allocation of substantial healthcare resources, the containment of the epidemic in certain high-risk subgroups through active screening and administration of effective and highly tolerable treatment can be a more feasible goal[150]. Treatment cannot however constitute the only form of prevention; public health efforts including reaching and educating high-risk populations about prevention and treatment, screening for HCV infection and providing good linkage to care are also important in this regard.
Although HIV co-infected individuals represent a substantial minority, they have traditionally been considered to be one of the “special populations” amongst the HCV infected ones. This was mainly attributed to the lower likelihood of cure from PegIFN/RBV therapy. Moreover, the uptake of this type of therapy has generally been low due to various complicating factors. The advent of newer DAA-based therapy offers the opportunity of a very high rate of treatment success with short treatment courses and a favourable side effect profile. Yet, the HCV/HIV co-infected population remains one with unmet medical needs, given the faster progression of liver disease compared with mono-infected individuals. Although successful cART ameliorates the course of chronic hepatitis C in HIV co-infected individuals, they retain increased liver-related risk when compared with the HCV mono-infected individuals. Specific issues relating to the treatment of hepatitis C in HIV co-infected individuals, particularly drug-drug interactions, should be addressed in a timely manner in the process of DAA drug development so that the newer treatment options become readily available to this population. Significant and sustained improvements in mortality and morbidity and control of the current HCV epidemic in HIV-infected subgroups could then become a feasible goal.
P- Reviewer: Ratnasari N, Silva LD, Wu PF S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Bruggmann P, Berg T, Øvrehus AL, Moreno C, Brandão Mello CE, Roudot-Thoraval F, Marinho RT, Sherman M, Ryder SD, Sperl J. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21 Suppl 1:5-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Hua L, Andersen JW, Daar ES, Glesby MJ, Hollabaugh K, Tierney C. Hepatitis C virus/HIV coinfection and responses to initial antiretroviral treatment. AIDS. 2013;27:2725-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Hernando V, Alejos B, Monge S, Berenguer J, Anta L, Vinuesa D, Palacios R, Muga R, Moreno S, Jarrin I. All-cause mortality in the cohorts of the Spanish AIDS Research Network (RIS) compared with the general population: 1997-2010. BMC Infect Dis. 2013;13:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol. 2014;61:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 973] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 6. | Wandeler G, Gsponer T, Bregenzer A, Günthard HF, Clerc O, Calmy A, Stöckle M, Bernasconi E, Furrer H, Rauch A. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Wiessing L, Likatavicius G, Hedrich D, Guarita B, van de Laar MJ, Vicente J. Trends in HIV and hepatitis C virus infections among injecting drug users in Europe, 2005 to 2010. Euro Surveill. 2011;16. [PubMed] |
| 8. | Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, Lazanas M, Gargalianos P, Psichogiou M, Skoutelis A. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Giraudon I, Ruf M, Maguire H, Charlett A, Ncube F, Turner J, Gilson R, Fisher M, Bhagani S, Johnson M. Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002-2006: is this an outbreak? Sex Transm Infect. 2008;84:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Wandeler G, Rohrbach J, Metzner K, Fehr J, Stöckle M, Cavassini M, Ambrosioni J, Keiser O, Furrer H, Rauch A. Incident HCV Infections in the Swiss HIV Cohort Study: Natural History and Treatment Outcomes. Abstract 643. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA 2014; Available from: http://www.croiconference.org/sessions/incident-hcv-infections-swiss-hiv-cohort-study-natural-history-and-treatment-outcomes. |
| 11. | Vanhommerig JW, Stolte IG, Lambers FAE, Geskus RB, van de Laar TJW, Bruisten SM, Schinkel J, Prins M. Hepatitis C Virus Incidence in the Amsterdam Cohort Study Among Men Who Have Sex With Men: 1984-2011. Abstract 673. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA 2014; Available from: http://www.croiconference.org/sessions/hepatitis-c-virus-incidence-amsterdam-cohort-study-among-men-who-have-sex-men-1984-2011. |
| 12. | van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, Vogel M, Baumgarten A, Chaix ML, Fisher M. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609-1617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Matthews GV, Pham ST, Hellard M, Grebely J, Zhang L, Oon A, Marks P, van Beek I, Rawlinson W, Kaldor JM. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Witt MD, Seaberg EC, Darilay A, Young S, Badri S, Rinaldo CR, Jacobson LP, Detels R, Thio CL. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984-2011. Clin Infect Dis. 2013;57:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Branch AD, Van Natta ML, Vachon ML, Dieterich DT, Meinert CL, Jabs DA. Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in the era of combination antiretroviral therapy. Clin Infect Dis. 2012;55:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Yaphe S, Bozinoff N, Kyle R, Shivkumar S, Pai NP, Klein M. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | McFaul K, Maghlaoui A, Nzuruba M, Farnworth S, Foxton M, Anderson M, Nelson M, Devitt E. Acute hepatitis C infection in HIV-negative men who have sex with men. J Viral Hepat. 2015;22:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Sherman KE, Shire NJ, Rouster SD, Peters MG, James Koziel M, Chung RT, Horn PS. Viral kinetics in hepatitis C or hepatitis C/human immunodeficiency virus-infected patients. Gastroenterology. 2005;128:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Bavinton BR, Jin F, Zablotska I, Prestage G, Grulich A. The Opposites Attract Study Team. Undetectable viral load is associated with increased unprotected anal intercourse in gay serodiscordant couples. Abstract MOLBPE30. In: 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Kuala Lumpur, Malaysia 2013; . |
| 20. | van der Helm JJ, Prins M, del Amo J, Bucher HC, Chêne G, Dorrucci M, Gill J, Hamouda O, Sannes M, Porter K. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450-456. [PubMed] |
| 22. | Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 23. | Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, Szabo G, Wright EC, Sterling RK. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [PubMed] |
| 26. | Planas R, Ballesté B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, Santos J, Coll S, Morillas RM, Solà R. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020-1023. [PubMed] |
| 28. | Daar ES, Lynn H, Donfield S, Gomperts E, Hilgartner MW, Hoots WK, Chernoff D, Arkin S, Wong WY, Winkler CA. Relation between HIV-1 and hepatitis C viral load in patients with hemophilia. J Acquir Immune Defic Syndr. 2001;26:466-472. [PubMed] |
| 29. | Corchado S, López-Cortés LF, Rivero-Juárez A, Torres-Cornejo A, Rivero A, Márquez-Coello M, Girón-González JA. Liver fibrosis, host genetic and hepatitis C virus related parameters as predictive factors of response to therapy against hepatitis C virus in HIV/HCV coinfected patients. PLoS One. 2014;9:e101760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Danta M, Semmo N, Fabris P, Brown D, Pybus OG, Sabin CA, Bhagani S, Emery VC, Dusheiko GM, Klenerman P. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J Infect Dis. 2008;197:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Ingiliz P, Boesecke C, Martin TC, Mauss S, Rodger A, Mandorfer M, Baumgarten A, Stellbrink HJ, Bhagani S, Rockstroh JK. NEAT Study Group. P781 Spontaneous clearance rates increase with HCV reinfection episode in HIV-positive men who have sex with men (MSM) independent of HCV subtype. J Hepatol. 2014;60:S331. [DOI] [Full Text] |
| 33. | Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 34. | Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clin Infect Dis. 2012;55 Suppl 1:S33-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Boesecke C, Ingiliz P, Mandorfer M, Schwarze-Zander C, Berger F, Valantin MA, Wasmuth JC, Reiberger T, Mauss S, Rockstroh JK, the NEAT study group. Is There Long-Term Evidence of Advanced Liver Fibrosis After Acute Hepatitis C in HIV Coinfection? Abstract 644. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA 2014; Available from: http://www.croiconference.org/sessions/there-long-term-evidence-advanced-liver-fibrosis-after-acute-hepatitis-c-hiv-coinfection. |
| 36. | Merchante N, Rivero A, de Los Santos-Gil I, Merino D, Márquez M, López-Ruz MA, Rodríguez-Baño J, Del Valle J, Camacho A, Sanz-Sanz J. Insulin resistance is associated with liver stiffness in HIV/HCV co-infected patients. Gut. 2009;58:1654-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Bhagani S. HIV/hepatitis C co-infection and hepatic fibrosis: looking beyond HIV-associated immune suppression; the contribution of hepatic steatosis and insulin resistance. Gut. 2009;58:1579-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Bailony MR, Scherzer R, Huhn G, Plankey MW, Peters MG, Tien PC. Association of HIV infection, hepatitis C virus infection, and metabolic factors with liver stiffness measured by transient elastography. J Infect Dis. 2013;208:1776-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Fernández-Montero JV, Vispo E, Barreiro P, Sierra-Enguita R, de Mendoza C, Labarga P, Soriano V. Hepatitis delta is a major determinant of liver decompensation events and death in HIV-infected patients. Clin Infect Dis. 2014;58:1549-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Marcellin F, Roux P, Loko MA, Lions C, Caumont-Prim A, Dabis F, Salmon-Ceron D, Spire B, Carrieri MP. High levels of alcohol consumption increase the risk of advanced hepatic fibrosis in HIV/hepatitis C virus-coinfected patients: a sex-based analysis using transient elastography at enrollment in the HEPAVIH ANRS CO13 cohort. Clin Infect Dis. 2014;59:1190-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, Moore RD, Thomas DL, Sulkowski MS. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 42. | Lo Re V, Kallan MJ, Tate JP, Localio AR, Lim JK, Goetz MB, Klein MB, Rimland D, Rodriguez-Barradas MC, Butt AA. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 43. | Merchante N, Girón-González JA, González-Serrano M, Torre-Cisneros J, García-García JA, Arizcorreta A, Ruiz-Morales J, Cano-Lliteras P, Lozano F, Martínez-Sierra C. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20:49-57. [PubMed] |
| 44. | Duclos-Vallée JC, Féray C, Sebagh M, Teicher E, Roque-Afonso AM, Roche B, Azoulay D, Adam R, Bismuth H, Castaing D. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Bräu N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, Trikha A, Sherman M, Sulkowski MS, Dieterich DT. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Morlat P, Roussillon C, Henard S, Salmon D, Bonnet F, Cacoub P, Georget A, Aouba A, Rosenthal E, May T. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS. 2014;28:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 47. | Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 48. | Smith C, Sabin CA, Lundgren JD, Thiebaut R, Weber R, Law M, Monforte Ad, Kirk O, Friis-Moller N, Phillips A. Factors associated with specific causes of death amongst HIV-positive individuals in the D: A: D Study. AIDS. 2010;24:1537-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 49. | Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 834] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 50. | Chen TY, Ding EL, Seage Iii GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009;49:1605-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 51. | Crowell TA, Gebo KA, Balagopal A, Fleishman JA, Agwu AL, Berry SA. Impact of hepatitis coinfection on hospitalization rates and causes in a multicenter cohort of persons living with HIV. J Acquir Immune Defic Syndr. 2014;65:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. 2011;8:12-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 53. | Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, Moreno A, González-Serrano M, Iribarren JA, Ortega E. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 54. | Casado JL, Quereda C, Moreno A, Pérez-Elías MJ, Martí-Belda P, Moreno S. Regression of liver fibrosis is progressive after sustained virological response to HCV therapy in patients with hepatitis C and HIV coinfection. J Viral Hepat. 2013;20:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Labarga P, Fernández-Montero JV, de Mendoza C, Barreiro P, Soriano V. Long-term survival and liver-related events after pegylated interferon/ribavirin therapy in HIV-infected patients with chronic hepatitis C. Antivir Ther. 2015;20:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Chapplain JM, Bellissant E, Guyader D, Molina JM, Poizot-Martin I, Perré P, Pialoux G, Turlin B, Mouchel C, Renault A. The effects of a maintenance therapy with peg-interferon alpha-2a on liver fibrosis in HIV/HCV co-infected patients: a randomized controlled trial. J Infect. 2013;67:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Mathews G, Bhagani S. The epidemiology and natural history of HIV/HBV and HIV/HCV co-infections. J HIV Ther. 2003;8:77-84. [PubMed] |
| 58. | van der Helm J, Geskus R, Sabin C, Meyer L, Del Amo J, Chêne G, Dorrucci M, Muga R, Porter K, Prins M. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology. 2013;144:751-760.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, Kim SS, Borges CB, Shao RX, Chung RT. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV induces TRAIL sensitivity in hepatocytes. PLoS One. 2009;4:e4623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Allison RD, Katsounas A, Koziol DE, Kleiner DE, Alter HJ, Lempicki RA, Wood B, Yang J, Fullmer B, Cortez KJ. Association of interleukin-15-induced peripheral immune activation with hepatic stellate cell activation in persons coinfected with hepatitis C virus and HIV. J Infect Dis. 2009;200:619-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, Chen P, Chen BK, Klotman ME, Bansal MB. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Glässner A, Eisenhardt M, Kokordelis P, Krämer B, Wolter F, Nischalke HD, Boesecke C, Sauerbruch T, Rockstroh JK, Spengler U. Impaired CD4+ T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J Hepatol. 2013;59:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, Smith NM, Huang X, Xu X, Pasricha SR. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci USA. 2014;111:12187-12192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Liu Y, Lv Q, Gao J, Long L, Duan Z, Liang H, Shen T, Lu F. Coinfection with HIV-1 alleviates iron accumulation in patients with chronic hepatitis C virus infection. PLoS One. 2014;9:e98039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Martinelli AL, Ramalho LN, Zucoloto S. Hepatic stellate cells in hepatitis C patients: relationship with liver iron deposits and severity of liver disease. J Gastroenterol Hepatol. 2004;19:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Kim AY, Lauer GM, Ouchi K, Addo MM, Lucas M, Schulze Zur Wiesch J, Timm J, Boczanowski M, Duncan JE, Wurcel AG. The magnitude and breadth of hepatitis C virus-specific CD8+ T cells depend on absolute CD4+ T-cell count in individuals coinfected with HIV-1. Blood. 2005;105:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Tanaka Y, Hanada K, Hanabusa H, Kurbanov F, Gojobori T, Mizokami M. Increasing genetic diversity of hepatitis C virus in haemophiliacs with human immunodeficiency virus coinfection. J Gen Virol. 2007;88:2513-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | González-Peralta RP, Qian K, She JY, Davis GL, Ohno T, Mizokami M, Lau JY. Clinical implications of viral quasispecies heterogeneity in chronic hepatitis C. J Med Virol. 1996;49:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2823] [Cited by in RCA: 2725] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 71. | Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 72. | Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, Delpech V, Phillips AN. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 73. | Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Cavassini M, Calmy A, Bernasconi E, Schmid P. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14:195-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 74. | Kovari H, Sabin CA, Ledergerber B, Ryom L, Worm SW, Smith C, Phillips A, Reiss P, Fontas E, Petoumenos K. Antiretroviral drug-related liver mortality among HIV-positive persons in the absence of hepatitis B or C virus coinfection: the data collection on adverse events of anti-HIV drugs study. Clin Infect Dis. 2013;56:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Berenguer J, Alejos B, Hernando V, Viciana P, Salavert M, Santos I, Gómez-Sirvent JL, Vidal F, Portilla J, Del Amo J. Trends in mortality according to hepatitis C virus serostatus in the era of combination antiretroviral therapy. AIDS. 2012;26:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Andrade BB, Hullsiek KH, Boulware DR, Rupert A, French MA, Ruxrungtham K, Montes ML, Price H, Barreiro P, Audsley J. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. J Infect Dis. 2013;207:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Kowalska JD, Reekie J, Mocroft A, Reiss P, Ledergerber B, Gatell J, d’Arminio Monforte A, Phillips A, Lundgren JD, Kirk O. Long-term exposure to combination antiretroviral therapy and risk of death from specific causes: no evidence for any previously unidentified increased risk due to antiretroviral therapy. AIDS. 2012;26:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 316] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 79. | Macías J, Viloria MM, Rivero A, de los Santos I, Márquez M, Portilla J, Di Lello F, Camacho A, Sanz-Sanz J, Ojeda G. Lack of short-term increase in serum mediators of fibrogenesis and in non-invasive markers of liver fibrosis in HIV/hepatitis C virus-coinfected patients starting maraviroc-based antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2012;31:2083-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Loko MA, Bani-Sadr F, Valantin MA, Lascoux-Combe C, Fontaine H, Bonnard P, Gervais A, Bouchaud O, Garipuy D, Quertainmont Y. Antiretroviral therapy and sustained virological response to HCV therapy are associated with slower liver fibrosis progression in HIV-HCV-coinfected patients: study from the ANRS CO 13 HEPAVIH cohort. Antivir Ther. 2012;17:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Anderson JP, Tchetgen Tchetgen EJ, Lo Re V, Tate JP, Williams PL, Seage GR, Horsburgh CR, Lim JK, Goetz MB, Rimland D. Antiretroviral therapy reduces the rate of hepatic decompensation among HIV- and hepatitis C virus-coinfected veterans. Clin Infect Dis. 2014;58:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Wilkins E, Nelson M, Agarwal K, Awoyemi D, Barnes E, Bhagani S, Brook G, Brown A, Castelino S, Cooke G. British HIV Association guidelines for the management of hepatitis viruses in adults infected with HIV 2013. HIV Med. 2013;14 Suppl 4:1-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | EACS European AIDS Clinical Society. EACS Guidelines Version 7.0 2. [Updated 2014 Jun]. Available from: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. |
| 84. | Martin NK, Devine A, Eaton JW, Miners A, Hallett TB, Foster GR, Dore GJ, Easterbrook PJ, Legood R, Vickerman P. Modeling the impact of early antiretroviral therapy for adults coinfected with HIV and hepatitis B or C in South Africa. AIDS. 2014;28 Suppl 1:S35-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Grint D, Peters L, Schwarze-Zander C, Beniowski M, Pradier C, Battegay M, Jevtovic D, Soriano V, Lundgren JD, Rockstroh JK. Temporal changes and regional differences in treatment uptake of hepatitis C therapy in EuroSIDA. HIV Med. 2013;14:614-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56:806-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 87. | Roux P, Carrieri MP, Villes V, Dellamonica P, Poizot-Martin I, Ravaux I, Spire B. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103:1828-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Bhagani S. Current treatment for chronic hepatitis C virus/HIV-infected individuals: the role of pegylated interferon-alpha and ribavirin. Curr Opin HIV AIDS. 2011;6:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 955] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 90. | Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 735] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 91. | Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011;53:32-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 92. | Chen Y, Xu HX, Wang LJ, Liu XX, Mahato RI, Zhao YR. Meta-analysis: IL28B polymorphisms predict sustained viral response in HCV patients treated with pegylated interferon-α and ribavirin. Aliment Pharmacol Ther. 2012;36:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 94. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] |
| 95. | Cotte L, Braun J, Lascoux-Combe C, Vincent C, Valantin MA, Sogni P, Lacombe K, Neau D, Aumaitre H, Batisse D. Telaprevir for HIV/hepatitis C virus-coinfected patients failing treatment with pegylated interferon/ribavirin (ANRS HC26 TelapreVIH): an open-label, single-arm, phase 2 trial. Clin Infect Dis. 2014;59:1768-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Karageorgopoulos DE, El-Sherif O, Bhagani S, Khoo SH. Drug interactions between antiretrovirals and new or emerging direct-acting antivirals in HIV/hepatitis C virus coinfection. Curr Opin Infect Dis. 2014;27:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Manns MP, von Hahn T. Novel therapies for hepatitis C - one pill fits all? Nat Rev Drug Discov. 2013;12:595-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 98. | Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. N Engl J Med. 2014;370:1576-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 99. | Rockstroh JK, Bhagani S. Managing HIV/hepatitis C co-infection in the era of direct acting antivirals. BMC Med. 2013;11:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, Rivero A, Mak C, Thompson S, Howe AY. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 101. | Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, Gharakhanian S, McCallister S, Henshaw J, Girard PM. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 102. | Rodriguez-Torres M, Rodriguez-Orengo J, Gaggar A, Shen G, Symonds B, McHutchison J, Gonzalez M. Sofosbuvir and Peginterferon alfa-2a/RibavirinTreatment of naïve Genotype 1-4 HCV infected patients that are co-infected with HIV. Abstract 714. 2013; Available from: http://www.natap.org/2013/IDSA/IDSA_24.htm. |
| 103. | Dieterich D, Tural C, Nelson M, Arasteh K, Soriano V, Guardiola J, Bhagani S, Rockstroh JK, Stern JO, Quinson A-M, on behalf of the STARTVerso4 Study Group. Faldaprevir plus pegylated interferon alfa-2a/ribavirin in HIV/HCV coinfection: STARTVerso4. Abstract #23. In: 21st Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA 2014; Available from: http://www.croiconference.org/sessions/faldaprevir-plus-pegylated-interferon-alfa-2aribavirin-hivhcv-co-infection-startverso4. |
| 104. | Dieterich D, Tural C, Nelson M, Arasteh K, Soriano V, Guardiola J, Bhagani S, Rockstroh JK, Stern JO, Quinson A-M, on behalf of the STARTVerso4 Study Group. Faldaprevir Plus Pegylated Interferon α-2a/Ribavirin in HIV/HCV Co infection: Abstract #681. STARTVerso4. In: (APASL) 23rd Conference of the Asian Pacific Association for the Study of the Liver. Brisbane, Australia 2014; Available from: http://apasl.info/. |
| 105. | Dieterich D, Rockstroh J, Orkin C, Gutierrez F, Klein MB, Reynes J, Jessner W, Lenz O, Peeters M, Beumont-Mauviel M. Simeprevir with pegylated interferon/ribavirin in patients co-infected with chronic hepatitis C and HIV-1: week-24 interim analysis of the TMC435-C212 study. Abstract 154LB. In: 20th Conference on Retroviruses and Opportunistic Infections (CROI). Atlanta, GA 2013; Available from: http://www.retroconference.org/. |
| 106. | Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, Luetkemeyer AF, Asmuth D, Gaggar A, Ni L. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 107. | Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, Massetto B, Gaggar A, Ni L, Svarovskaia E. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotypes 1, 2, 3 and 4 infection in patients co-infected with HIV (PHOTON-2). Abstract MOAB0105LB. In: 20th International AIDS Conference. Melbourne, Australia 2014; Available from: http://pag.aids2014.org/. |
| 108. | Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Slim J, Gathe J, Ruane PJ, Wang C, Elion R. Safety and efficacy of ABT-450/r/ombitasvir, dasabuvir, and ribavirin in patients co-infected with hepatitis C and HIV-1. Abstract MOAB0104LB. In: 20th International AIDS Conferenc. Melbourne, Australia 2014; Available from: http://pag.aids2014.org/. |
| 109. | Osinusi A, Townsend K, Nelson A, Kohli A, Gross C, Polis MA, Pang PS, Symonds WT, Talwani R, Sajadi MM, Hogan J, Benator D, Subramanian M, Mchutchison J, Masur H, Kottilil S, for the NIAID ERADICATE Study. O14 Use of sofosbuvir/ledipasvir fixed dose combination for treatment of HCV genotype-1 in patients coinfected with HIV. J Hepatol. 2014;60:S7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Sulkowski M, Mallolas J, Pol S, Bourliere M, Gerstoft J, Shibolet O, Nahass R, DeJesus E, Shaughnessy M, Hwang P. O63 Efficacy and safety of the all-oral regimen, MK-5172/MK-8742 ± RBV for 12 weeks in GT1 HCV/HIV co-infected patients: the C-WORTHY study. J Hepatol. 2014;60:S26. [DOI] [Full Text] |
| 111. | Ampuero J, Romero-Gómez M, Reddy KR. Review article: HCV genotype 3 - the new treatment challenge. Aliment Pharmacol Ther. 2014;39:686-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 112. | Morice Y, Cantaloube JF, Beaucourt S, Barbotte L, De Gendt S, Goncales FL, Butterworth L, Cooksley G, Gish RG, Beaugrand M. Molecular epidemiology of hepatitis C virus subtype 3a in injecting drug users. J Med Virol. 2006;78:1296-1303. [PubMed] |
| 113. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 114. | Jensen DM, Asselah T, Dieterich D, Foster GR, Sulkowski MS, Zeuzem S, P Mantry, C Moreno, D Ouzan, M Wright, LE Morano, R Buynak, M Bourlière, T Hassanein, S Nishiguchi, J-H Kao, M Omata, SW Paik, DK Wong, E Tam, K Kaita, SV Feinman, JO Stern, M Garcia, AM Quinson, F Voss, J-P Gallivan, WO Böcher, P Ferenci, on behalf of the STARTVerso1 and STARTVerso2 Study Groups. A pooled analysis of two randomized, double-blind placebo-controlled Phase III trials (STARTVerso1&2) of faldaprevir plus pegylated interferon alfa-2a and ribavirin in treatment- naïve patients with chronic hepatitis C genotype-1 infection. Abstract 1088. In: The Liver Meeting®, 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 1 - 5 November. Washington, DC 2013; Available from: http://www.aasld.org/. |
| 115. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 116. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 117. | Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, Hezode C, Hirschfield GM, Jacobson I, Nikitin I. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430-431.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 118. | Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 119. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 120. | Ruane PJ, Ain D, Meshrekey R, Riad J, Soliman M, Mikhail S, Wolfe PR, Kersey K, Doehle B, Jiang D. P1243 Sofosbuvir plus ribavirin, an interferon-free regimen, in the treatment of treatment-naive and treatment-experienced patients with chronic genotype 4 HCV infection. J Hepatol. 2014;60:S503-504. [DOI] [Full Text] |
| 121. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 656] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 122. | Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, Sulkowski MS, Wedemeyer H, Tam E, Desmond P. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 445] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 123. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 124. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1365] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 125. | Hezode C, Serfaty L, Vierling JM, Kugelmas M, Pearlman B, Sievert W, Ghesquiere W, Zuckerman E, Sund F, Howe A. Safety and efficacy of the all-oral regimen of MK-5172/MK-8742 ± ribavirin in treatment-naive, non-cirrhotic, patients with hepatitis C virus genotype 1 infection: the C-WORTHY study. Abstract 10. UK: London 2014; Available from: http://www.journal-of-hepatology.eu/. |
| 126. | Lawitz E, Hezode C, Gane E, Tam E, Lagging M, Balart L, Rossaro L, Ghalib R, Shaughnessy M, Hwang . O61 Efficacy and safety of MK-5172 and MK-8742 ± ribavirin in hepatitis C genotype 1 infected patients with cirrhosis or previous null-response: the C-WORTHY study. J Hepatol. 2014;60:S25-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 128. | Rockstroh JK. Does HIV remain a risk factor for achieving sustained virologic response under direct acting antiviral-based modern hepatitis C virus therapy? Clin Infect Dis. 2014;59:1777-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 129. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 130. | AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C [Internet]. [Cited 2014 Dec 28]. Available from: http://www.hcvguidelines.org. |
| 131. | United States. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry Chronic Hepatitis C Virus Infection: Developing Direct- Acting Antiviral Drugs for Treatment. Draft Guidance. Revision 1 2013; Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm225333.pdf. |
| 132. | European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS. 2011;25:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 133. | Fierer DS, Dieterich DT, Mullen MP, Branch AD, Uriel AJ, Carriero DC, van Seggelen WO, Hijdra RM, Cassagnol DG. Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men. Clin Infect Dis. 2014;58:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 134. | Martin TC, Martin NK, Hickman M, Vickerman P, Page EE, Everett R, Gazzard BG, Nelson M. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27:2551-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 135. | Abdelrahman T, Hughes J, Main J, McLauchlan J, Thursz M, Thomson E. Next-generation sequencing sheds light on the natural history of hepatitis C infection in patients who fail treatment. Hepatology. 2015;61:88-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 136. | Macartney M, Ricciardi A, Rodger A, Webster D, Karageorgopoulos D, Fernandez T, Ferro F, Sasadeusz J, Bhagani S. P745 Sequencing of initial and recurrent HCV infections in HIV-infected men who have sex with men (MSM). J Hepatol. 2014;60:S318-319. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 137. | Curry MP. HIV and hepatitis C virus: special concerns for patients with cirrhosis. J Infect Dis. 2013;207 Suppl 1:S40-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 138. | Bruno R, Cariti G, Nasta P, Capetti A, Ravasio V, Galli M, Raise E, Palmieri G, Iannacone C, Puoti M. OPERA: responses to peginterferon and ribavirin therapy in a subgroup of interferon-naïve patients with HIV/HCV genotype 2/3 co-infection in Italy. Liver Int. 2015;35:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 139. | Neumann A, Polis M, Rozenberg L, Jackson J, Reitano K, McLaughlin M, Koratich C, Dewar R, Masur H, Haagmans B. Differential antiviral effect of PEG-interferon-alpha-2b on HIV and HCV in the treatment of HIV/HCV co-infected patients. AIDS. 2007;21:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 141. | Blair JM, Fagan JL, Frazier EL, Do A, Bradley H, Valverde EE, McNaghten A, Beer L, Zhang S, Huang P. Behavioral and clinical characteristics of persons receiving medical care for HIV infection - Medical Monitoring Project, United States, 2009. MMWR Surveill Summ. 2014;63 Suppl 5:1-22. [PubMed] |
| 142. | Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 143. | Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;52 Suppl 2:S238-S246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 144. | Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, Sprangers MA, Nieuwkerk PT. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 145. | Oramasionwu CU, Moore HN, Toliver JC. Barriers to hepatitis C antiviral therapy in HIV/HCV co-infected patients in the United States: a review. AIDS Patient Care STDS. 2014;28:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 146. | Beisel C, Heuer M, Otto B, Jochum J, Schmiedel S, Hertling S, Degen O, Lüth S, van Lunzen J, Schulze Zur Wiesch J. German cohort of HCV mono-infected and HCV/HIV co-infected patients reveals relative under-treatment of co-infected patients. AIDS Res Ther. 2014;11:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 147. | Klein MB, Rollet KC, Hull M, Cooper C, Walmsley S, Conway B, Pick N. Who needs direct-acting antivirals for HCV? Challenges faced in advancing HCV therapy for HIV-HCV-coinfected individuals. Antivir Ther. 2013;18:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 148. | Qurishi N, Kreuzberg C, Lüchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh JK, Spengler U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 384] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 149. | Takatsuki M, Soyama A, Eguchi S. Liver transplantation for HIV/hepatitis C virus co-infected patients. Hepatol Res. 2014;44:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 150. | Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, Foster GR, Dillon JF, Goldberg DJ, Dore GJ. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 151. | Wei L, Lok AS. Impact of new hepatitis C treatments in different regions of the world. Gastroenterology. 2014;146:1145-50.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |