Published online Jul 18, 2015. doi: 10.4254/wjh.v7.i14.1875
Peer-review started: April 24, 2015
First decision: May 13, 2015
Revised: May 29, 2015
Accepted: June 30, 2015
Article in press: July 2, 2015
Published online: July 18, 2015
Processing time: 101 Days and 18.4 Hours
AIM: To assess serum cartilage oligomeric matrix protein (COMP) as a marker of cirrhosis and risk of progression to hepatocellular carcinoma (HCC).
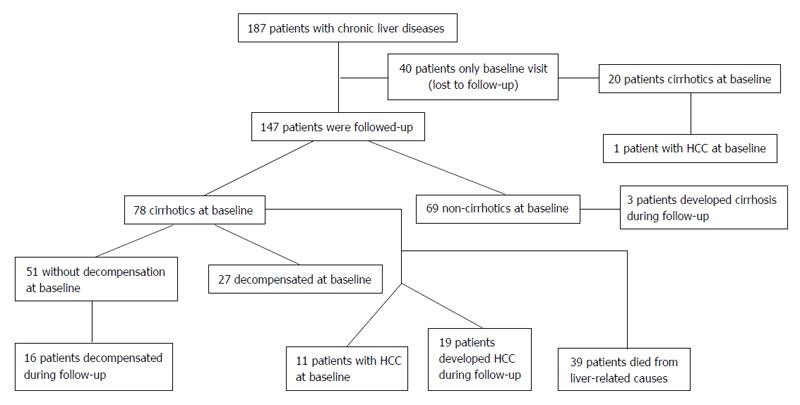
METHODS: A COMP enzyme-linked immunosorbent assay was used to test 187 patients with chronic liver diseases at the time point of first evaluation. The selected patients included 72 with chronic hepatitis B infection, 75 with chronic hepatitis C infection, 22 with primary biliary cirrhosis, 7 with autoimmune hepatitis type 1, and 11 with alcoholic liver disease. Demographic, biochemical, histological and clinical characteristics of the patients were recorded at the first evaluation. One hundred and forty-seven patients were followed for a median [interquartile range (IQR)] duration of 96.5 (102) mo. The clinical, biochemical and histological data, as well as the development of cirrhosis, HCC according to internationally accepted criteria and in case of death, a liver-related cause during the follow-up period, were recorded at the electronic database of our clinic. COMP determination was also performed in 43 healthy individuals who served as the control study group.
RESULTS: COMP positivity (> 15 U/L) was detected in 22%-36% among chronic liver disease groups. Strikingly, almost 83% of COMP-positive patients were cirrhotic at baseline, independently of cause of liver disease. Among the patients who developed HCC during follow-up, 73.7% (14/19) were COMP positive at baseline. COMP positivity was significantly associated with older age (P < 0.001), advanced fibrosis (P = 0.001) and necroinflammatory activity (P = 0.001), higher aspartate aminotransferase (P < 0.001), alanine aminotransferase (P < 0.02), γ-glutamyl transpeptidase (P = 0.003), alkaline phosphatase (P = 0.001), bilirubin (P < 0.05), international normalized ratio (P = 0.002) and alpha-fetoprotein levels (P < 0.02), and lower albumin (P < 0.001), and platelet count (P = 0.008). COMP levels [median (IQR)] were significantly higher in cirrhotics compared to non-cirrhotics [13.8 (7.9) U/L vs 9.8 (4.6) U/L, respectively; P < 0.001]. On multivariate logistic regression analysis, COMP-positivity was independently associated only with cirrhosis (OR = 4.40, 95%CI: 1.33-14.69, P = 0.015). Kaplan-Meier analysis showed that COMP positivity was significantly associated with HCC development (P = 0.007) and higher incidence of liver-related death (P < 0.001).
CONCLUSION: Elevated COMP levels are strongly associated with cirrhosis and HCC progression. Serum COMP is a new promising non-invasive biomarker for HCC risk assessment in surveillance programs.
Core tip: We report our first results regarding the utility of serum cartilage oligomeric matrix protein (COMP), an antigen over-expressed in developing liver, as a novel non-invasive marker of liver fibrosis and risk of progression to hepatocellular carcinoma (HCC). HCC is the third leading cause of cancer deaths worldwide, therefore non-invasive tests of fibrosis, as well as tests that can predict which patients are at high risk to develop HCC are needed. Our results suggest that COMP levels are associated with cirrhosis and a worse prognosis, thus serum COMP may assist clinicians as a non-invasive biomarker for risk assessment in surveillance programs.
- Citation: Norman GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos GN. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol 2015; 7(14): 1875-1883
- URL: https://www.wjgnet.com/1948-5182/full/v7/i14/1875.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i14.1875
Hepatocellular carcinoma (HCC) accounts for almost 90% of all primary liver cancer cases, being the third most common cause of tumor-related death among males and the sixth among females[1-4]. The major causes of HCC are hepatitis B virus (HBV)- or hepatitis C virus (HCV)-related cirrhosis and alcoholic cirrhosis[1-5]. In Greece, data from the HEPNET-GREECE Study Group has shown a cumulative 5-year incidence of HCC approaching 20% in decompensated and 10% in compensated patients with HBV-related cirrhosis. The incidence in non-cirrhotic HBV infected patients is less than 4%. This is in contrast to a lower incidence (1.4%) in HCV patients[4-7].
Prompt diagnosis of early or very early stage HCC is difficult due to the lack of specific symptoms and the relatively limited prognostic value of the serological and radiological approaches currently used for surveillance. The prognosis of HCC is generally poor, as a result of the aggressive nature of the disease, concurrent liver decompensation and the sometimes limited availability of potential treatment options[2,3,8-13]. Screening using determinations of serum alpha-fetoprotein (AFP) levels and ultrasonography every 6 mo appear to identify only a minority of cases with early stage HCC and therefore its use is not recommended by several international authorities[9-14].
Increasing evidence suggests that fibrosis progression is a key parameter in estimating the risk of HCC development[12,14]. Therefore, there is a need for non-invasive tests of fibrosis, as well as tests that can predict which patients are at high risk to develop HCC. Non-invasive markers currently reported are not sufficiently accurate, largely because they can only identify non-cirrhotic patients or those with advanced cirrhosis and are least useful in the early stages of HCC, when detection could be life-saving[14,15]. In this context, despite considerable controversy, AFP continues to be used extensively because it is inexpensive and there is a long clinical history supporting its use[14].
Progressive damage of the liver leading to fibrosis, cirrhosis, and eventually HCC, is associated with a remodeling of the liver as a consequence of both the degradation of the extracellular matrix and the accumulation of fibrotic scar tissue. This led us to consider that cartilage oligomeric matrix protein (COMP) could be a potential marker of liver fibrosis and early HCC. COMP, the fifth member of the thrombospondin family, is a pentameric extracellular, calcium-binding glycoprotein that modulates the cellular phenotype during tissue genesis and remodeling[16]. This glycoprotein is predominantly expressed in articular cartilage, but also in other tissues, including the developing liver[17-20]. Diseases that cause damage to the cartilage lead to the release of COMP into the blood[21] and thus it is reasonable that changes in serum COMP levels may reflect alterations in cartilage breakdown[22]. Hence, measurement of serum COMP levels has been used diagnostically in the non-invasive estimation of the degree of cartilage damage in patients with inflammatory joint diseases such as rheumatoid arthritis (RA) and osteoarthritis (OA)[23-26].
In the present study, we speculated that serum COMP could be an early marker of fibrosis, and that increased serum COMP levels could reflect the degree of cartilage breakdown during liver destruction and re-modeling. Our assumption is supported by data showing an over-expression of COMP in liver tissue specimens from patients with viral hepatitis-related HCCs[27]. These data have led researchers to speculate that COMP may have a central role early in the development of cirrhosis and liver carcinogenesis[27]. If this holds true, the presence of COMP could be a non-invasive tool to assist in selectively identifying individuals at significantly increased risk of progressing to cirrhosis and hepatocellular carcinogenesis.
To investigate this hypothesis, we measured COMP levels in serum samples from cirrhotic patients, including patients who developed HCC over time, as well as non-cirrhotic patients with chronic HBV and HCV infections, autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and alcoholic liver disease (ALD).
Serum samples from 187 Caucasian patients with chronic liver diseases followed at the outpatient clinic of the Department of Medicine, Medical School, University of Thessaly, Larissa, Greece during the period 2000-2013, were chosen at the time point of first evaluation from available specimens in the biobanking facility of the Research Laboratory of Internal Medicine and were stratified according to the presence (n = 98) or absence of cirrhosis (n = 89). The serum samples were randomly selected in order to avoid any potential bias selection and were stored at -80 °C (never-thawed) until the determination of COMP levels. The demographic, biochemical, histological and clinical baseline characteristics of patients are shown in Table 1. All patients had negative history of RA and OA or other autoimmune rheumatic diseases at the time of investigation and during follow-up. The selected patients included 72 with chronic HBV infection (45 males, mean age 55 ± 12 years); 75 with chronic HCV infection (34 males, mean age 50 ± 16 years); 22 with PBC (4 males, mean age 57 ± 17 years); 7 with AIH-type 1 (1 male, mean age 67 ± 21 years); and 11 with ALD (11 males, mean age 54 ± 12 years).
| Sex (male/female), n (%) | 95 (50.8%)/92 (49.2%) |
| Age (yr), mean ± SD | 53.7 ± 15.2 |
| HBV/HCV/PBC/AIH/ALD, n | 72/75/22/7/11 |
| Duration of follow-up (mo), median (IQR) | 96.5 (102) |
| INR, median (IQR), (normal range: 0.85-1.15) | 1.04 (0.24) |
| Platelets (× 103/μL), median (IQR), (normal range: 140-440) | 190 (116) |
| AST (U/L), median (IQR), (UNL: 40 U/L) | 38 (45) |
| ALT (U/L), median (IQR), (UNL: 40 U/L) | 40 (46) |
| γ-GT (U/L), median (IQR), (UNL: 37 U/L) | 33 (52) |
| ALP (U/L), median (IQR), (UNL: 104 U/L) | 90 (74) |
| Bilirubin (mg/dL), median (IQR), (UNL: 1.1 mg/dL) | 0.8 (0.8) |
| Albumin (g/dL), median (IQR), (normal range: 3.5-5.2 g/dL) | 4.3 (1.0) |
| IgG (mg/dL), mean ± SD, (UNL: 1650 mg/dL) | 1583 ± 508 |
| IgM (mg/dL), median (IQR), (UNL: 200 mg/dL) | 135 (147) |
| IgA (mg/dL), mean ± SD, (UNL: 300 mg/dL) | 312 ± 192 |
| AFP (ng/mL), median (IQR), (UNL: 10 ng/mL) | 4.6 (6.4) |
| Cirrhosis (yes/no), n (%) | 98 (52.4%)/89 (47.6%) |
| Decompensation of cirrhosis1 (yes/no), n (%) | 27 (27.6%)/71 (72.4%) |
| HCC (yes/no), n (%) | 12 (6.4%)/175 (93.6%) |
| Histological grade (none/minimal/mild vs moderate/severe), n (%) | 61 (55.5%)/49 (44.5%) |
| Histological stage (none/minimal/mild vs moderate/severe/cirrhosis), n (%) | 62 (56.4%)/48 (43.6%) |
Histological data were available for 110 patients. The histologic evaluation for inflammation and fibrosis was assessed using the Knodell histologic activity index[28]. According to previous publications of our group[29-31], for statistical reasons the patients were divided into two groups: (1) according to inflammation: minimal/mild (score 0-8) and moderate/severe (score 9-18); and (2) according to fibrosis: none/mild (score 0-1) and moderate/severe/cirrhosis (score 2-3).
Ninety-eight patients (52.4%) were classified as cirrhotic at initial presentation (Table 1) based on histological findings where available and/or ultrasonographic findings (nodules in the hepatic parenchyma, spleen > 12 cm, portal vein > 16 mm) and/or endoscopic findings of cirrhosis (varices, portal gastropathy) and/or clinical findings of decompensation (ascites, variceal bleeding, encephalopathy)[32,33]; 38/72 with HBV, 36/75 HCV, 6/22 PBC, 7/7 AIH-1 and 11/11 ALD. Among the 98 patients with cirrhosis, 12 had developed HCC at the time of serum collection. One hundred and forty-seven patients were followed for a median [interquartile range (IQR)] duration of 96.5 (102) mo. Ultrasonography and AFP measurements were performed every 6 mo in cirrhotic patients and every 12 mo approximately in the non-cirrhotics. The clinical, biochemical and histological data, as well as the development of cirrhosis, HCC according to internationally accepted criteria for its diagnosis[10,12,13] and in case of death, a liver-related cause during the follow-up period, was recorded in the electronic database of our clinic. COMP determination was also performed in 43 healthy individuals who served as the control group of the study.
COMP levels in serum were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (AnaMar Diagnostics, Sweden). The COMP ELISA is a solid-phase, two-site enzyme immunoassay. It is based on the direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the COMP molecule. During incubation, COMP in the sample reacts with peroxidase-conjugated anti-COMP antibodies and anti-COMP antibodies bound to the well of the microwell plate. A washing step removes unbound enzyme-labeled antibody and the bound conjugate is detected by a reaction with 3,3’,5,5’-tetramethylbenzidine. The reaction is stopped by adding acid to give a colorimetric endpoint that is read spectrophotometrically. A calibration curve is obtained using 5 calibrators corresponding to 0.4, 0.7, 1.2, 1.8 and 3.2 U/L. According to the manufacturer, patients with inflammatory joint disease and serum COMP levels lower than 12 U/L have lower risk of joint destruction in the future compared to those with 12-15 U/L, who have an increasing risk, and those with more than 15 U/L, who have a higher risk for aggressive joint destruction. Using these values for guidance, we evaluated the effectiveness of two different cut-offs (12 and 15 U/L) for assessing positivity in our cohort of patients with chronic liver diseases. Based on studies of normal as well as disease controls, it was determined that the more rigorous and specific cut-off of 15 U/L was more appropriate for patients with chronic liver diseases.
Levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), bilirubin, albumin, international normalized ratio (INR), serum immunoglobulin IgA, IgG and IgM, and AFP were determined using standard techniques. Serological markers of HBV infection (HBsAg, anti-HBs, anti-HBc, HBeAg and anti-HBe) and antibodies to HCV were determined by the AxSYM system using the respective MEIA kits (Abbott Laboratories, Diagnostics Division, 100 Abbott Park Road, Abbott Park, IL).
All subjects provided written informed consent to participate in the study. The ethical committee of Thessaly University Medical School approved the study protocol.
Kolmogorov-Smirnov test was used to assess the normality of the distribution of variables. Normally distributed values are expressed as mean ± SD, while non-normally distributed values as median (IQR). Data were analyzed by t-test, Mann-Whitney U test, χ2 test (two by two with Yate’s correction), Fischer’s exact test and Spearman’s rho correlation where applicable. The parameters that were significant in the univariate analysis entered a binary logistic regression model, in order to identify independent risk factors. Survival analysis was carried out using the Kaplan-Meier plot for COMP-positive or COMP-negative patients up to the time patients reached the following study end-points: development of cirrhosis, decompensation, HCC, or death due to liver disease. The comparisons were done by log-rank test. Two-sided P values less than 0.05 were considered statistically significant.
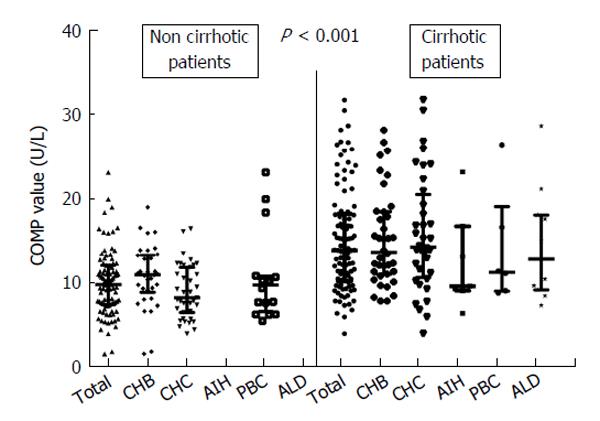
COMP antigen levels (> 15 U/L) were detected in 52 of the total cohort of 187 (27.8%) patients. The frequency of COMP positivity was comparable among patients with various chronic liver diseases (30.6% in HBV, 25.3% in HCV, 22.7% in PBC, 28.6% in AIH-1 and 36.4% in ALD; P = 0.880). Strikingly however, 82.6% (43/52) of the COMP positive sera originated from patients with cirrhosis (Figure 1 and Table 2). The increased frequency of COMP levels in patients with cirrhosis was similar when patients were stratified according to disease group (Figure 1). All 43 healthy controls had serum levels of COMP less than 15 U/L.
| COMP-positive (n = 52) | COMP-negative (n = 135) | P value | |
| Sex (male/female), n (%) | 26/26 (50%/50%) | 69/66 (51.1%/48.9%) | 1.000 |
| Age (yr), mean ± SD | 61.7 ± 11.9 | 50.9 ± 15.3 | < 0.001 |
| Duration of follow-up (mo) | 45 (91) | 107 (95) | < 0.01 |
| INR | 1.15 (0.22) | 1 (0.17) | 0.002 |
| Platelets (× 103/μL) | 166 (125) | 199 (104) | 0.008 |
| AST (U/L) | 59 (57) | 31 (33) | < 0.001 |
| ALT (U/L) | 46 (50) | 38 (39) | < 0.02 |
| γ-GT (U/L) | 50 (65) | 27 (48) | 0.003 |
| ALP (U/L) | 115 (102) | 83 (63) | 0.001 |
| Bilirubin (mg/dL) | 1.1 (1.5) | 0.7 (0.6) | < 0.05 |
| Albumin (g/dL) | 3.9 (1) | 4.4 (1) | < 0.001 |
| IgG (mg/dL), mean ± SD | 1724 ± 614 | 1533 ± 461 | 0.151 |
| IgM (mg/dL) | 134 (180) | 135 (120) | 0.265 |
| IgA (mg/dL), mean ± SD | 359 ± 196 | 295 ± 190 | 0.196 |
| AFP (ng/mL) | 6.9 (10.5) | 3.8 (5.5) | < 0.02 |
| Cirrhosis (yes/no), n (%) | 43/9 (82.7%/17.3%) | 55/80 (40.7%/59.3%) | < 0.001 |
| Decompensation of cirrhosis1 (yes/no), n (%) | 11/32 (25.6%/74.4%) | 16/39 (29.1%/70.9%) | 0.874 |
| HCC (yes/no), n (%) | 7/45 (13.5%/86.5%) | 5/130 (3.7%/96.3%) | < 0.05 |
| Histological grade (none/minimal/mild vs moderate/severe), n (%) | 5/17 (22.7%/77.3%) | 56/32 (63.6%/36.4%) | 0.001 |
| Histological stage (none/minimal/mild vs moderate/severe/cirrhosis), n (%) | 5/17 (22.7%/77.3%) | 57/31 (64.8%/35.2%) | 0.001 |
The association of the presence of COMP antigen with the demographic, clinical, laboratory, and histological parameters of patients at baseline are shown in Table 2. COMP positivity was significantly associated with older age (P < 0.001), higher levels of AST (P < 0.001) and ALT (P < 0.02), higher levels of γ-GT (P = 0.003), ALP (P = 0.001), bilirubin (P < 0.05) and INR (P = 0.002) and lower levels of albumin (P < 0.001) and platelet count (P = 0.008) (Table 2). Moreover, COMP positivity was significantly correlated with advanced fibrosis (P = 0.001), necroinflammatory activity (P = 0.001), higher levels of AFP (P < 0.02), the presence of cirrhosis (P < 0.001), and the presence of HCC (P < 0.05) (Table 2). Moreover, COMP levels were positively correlated with age (r = 0.417; P < 0.001), AST (r = 0.474, P < 0.001), ALT (r = 0.324, P < 0.001), γ-GT (r = 0.268; P < 0.001), ALP (r = 0.212; P = 0.005), bilirubin (r = 0.192; P = 0.02), INR (r = 0.275; P = 0.002) and AFP (r = 0.261; P = 0.003), while were negatively correlated with platelet count (r = -0.192, P < 0.02) and albumin (r = -0.343, P < 0.001).
Restricting the analysis in the subgroup of patients with available histological data (n = 110), showed that COMP positivity was similarly associated with older age (P < 0.001), higher levels of AST (P < 0.001) and ALT (P < 0.02), γ-GT (P = 0.005), ALP (P < 0.001), bilirubin (P = 0.005), INR (P < 0.001), lower levels of albumin (P < 0.001), the presence of cirrhosis (P < 0.001) and the presence of HCC (P = 0.001) (data not shown).
All parameters that were univariately associated with COMP positivity were entered in a multivariate logistic regression model. COMP antigen positivity was independently associated only with the presence of cirrhosis (OR = 4.40, 95%CI: 1.33-14.69, P = 0.015). Of note, COMP antigen titers [median (IQR)] in cirrhotic patients [13.8 (7.9) U/L] were significantly higher compared to non-cirrhotic patients [9.8 (4.6) U/L; P < 0.001, Figure 1].
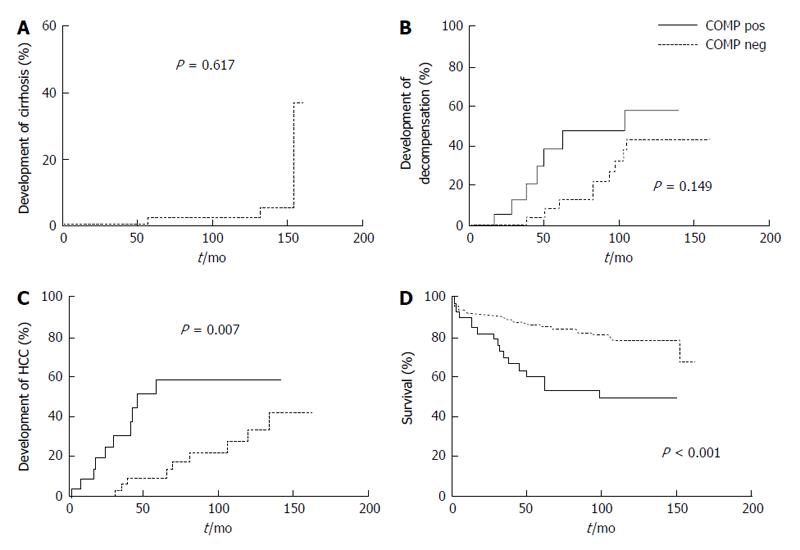
As shown in Figure 2, 147 patients had a long-term follow-up of 96.5 (102) mo. Seventy-eight of these 147 patients were cirrhotic at the baseline visit, while 3 out of the remaining 69 non-cirrhotic patients developed cirrhosis during the follow-up period (Figure 3A). Twenty-seven cirrhotic patients had decompensated cirrhosis at baseline visit, while 16 subjects out of the remaining 51 cirrhotic patients developed decompensation during follow-up. HCC was diagnosed in 12 patients at baseline visit, including 11 with long-term follow-up and one lost in follow-up (7/12, 58.3% were COMP positive), whereas HCC developed in other 19 patients during follow-up at least 6 mo after baseline visit. After excluding the 11 HCC cases diagnosed at baseline, the remaining 136 patients were evaluated for HCC development during long-term follow-up. Of interest, development of HCC was observed in 14/34 (41.2%) of the patients positive for COMP at baseline compared to only 5/102 (4.9%) of the patients negative for COMP at baseline (P = 0.008). In addition, 14 out of the 19 (73.7%) patients who developed HCC on follow-up had tested positive for COMP prior to the diagnosis of HCC. Similarly, in the subgroup of non-HCC patients with advanced fibrosis (moderate, severe fibrosis or cirrhosis) as it was determined by liver biopsy (n = 39), development of HCC was observed in 6/11 (54.5%) of COMP positive patients at baseline compared to 4/28 (14.3%) of COMP negative patients at baseline (P < 0.02).
While there was a trend for more rapid development of decompensation in COMP positive compared to COMP negative patients (Figure 3B), this difference did not reach statistical significance (P = 0.149). In contrast, the Kaplan-Meier analysis in cirrhotic non-HCC patients during long-term follow-up (n = 70) revealed a significant difference regarding the development of HCC between COMP-positive (14/25; 56%) and COMP-negative (5/45; 11.1%) cirrhotic patients (Figure 3C). Moreover, COMP-positive patients demonstrated a statistically higher incidence of liver-related deaths (17/39; 43.6%) compared to COMP-negative patients (22/108; 20.4%) (Figure 3D). Similarly, Kaplan-Meier analysis in the sub-group of patients with available histological data at baseline that were followed-up (n = 84), revealed that COMP-positive patients at baseline had a higher incidence of development of HCC (P = 0.001) and liver-related deaths (P < 0.001) during follow up (data not shown).
In the present study we have demonstrated for the first time that the presence of COMP in the sera of patients with chronic liver diseases is strongly associated with liver cirrhosis and that increased COMP levels appear to identify a subgroup of patients who are at an increased risk of progressing to HCC and liver-related mortality. In fact, after multivariate logistic regression analysis, COMP antigen positivity was independently associated only with the presence of cirrhosis. In addition, Kaplan-Meier analysis showed significantly higher rates of HCC development and liver-related mortality during follow-up in COMP-positive patients compared to those with a negative test.
The COMP assay measures peptides released during the breakdown of cartilage[17-19]. Clinically COMP has been primarily used to assess the destruction of cartilage in patients with RA and OA[21-26]. Our hypothesis that during liver remodeling, COMP fragments could be detected in patients’ sera has been proven valid, and the amount of COMP likely indicates the level of fibrogenic activity. As we postulated, the frequency of COMP positivity was clearly increased in patients with chronic liver diseases compared to healthy controls. Indeed, a dramatic increase of COMP was largely seen in patients with cirrhosis, regardless of the etiology of liver disease. Thus, 44% (43/98) of the cirrhotic patients were positive for COMP, compared to just 10% (9/89) of the non-cirrhotic patients. Notably, the great majority of patients with a positive COMP result (43/52, 82.7%) had well-documented cirrhosis. Furthermore, 73.7% (14/19) of patients who developed HCC during follow-up were COMP positive prior to the diagnosis of HCC. Although the presence of cirrhosis is clearly associated with an increased risk of disease progression, our findings suggest the detection of COMP in cirrhotic patients is a potentially useful marker to identify a subgroup of cirrhotic patients with a higher likelihood of development of HCC.
Cirrhosis represents a critical milestone in the decline of liver function and the progression of individuals towards decompensation and HCC[34-36]. The absence of fibrosis, as well as the presence of advanced fibrosis, can be established by physical examination and current non-invasive techniques. However, early fibrosis, as well as the identification of patients with a higher likelihood of progressing to cirrhosis, cannot be identified with certainty, leading to significant delay in implementing proper surveillance and rigorous management[34-36]. Currently, several markers have been considered diagnostically meaningful for assessing the development and extent of liver fibrosis[14]. These include costly profiles consisting of biochemical markers and physical measurement techniques such as ultrasonography, fibroscan, and magnetic resonance imaging. Despite the ever-increasing number of described fibrosis markers, most are used for research purposes only and have not been incorporated in routine clinical practice[37-41].
According to our data, COMP levels above 15 U/L are associated with an increased likelihood of cirrhosis, development of HCC, and liver-related death. Although transient expression of megakaryocyte-derived protein immunoreactive with antiserum to COMP in the developing rat liver has been described[42], it is generally accepted that COMP is not expressed in normal liver tissue[16-18]. Only one small study of 30 patients with cirrhosis or HCC investigated the presence of COMP in liver diseases[27]. Consistent with our results, these authors documented an increased COMP mRNA expression in HCC tissue samples, suggesting that COMP is upregulated and overexpressed in HCC tissues[27]. They also showed that COMP was only weakly expressed in cirrhotic liver tissues, indicating that this gene might have a function early in the course of liver carcinogenesis, and this was further supported by their findings that COMP expression was not associated with the stage of HCC[27]. Taking into account that tumors often express genes that are normally restricted to the development of an organ, the observation of overexpression in the liver of a COMP-like protein during embryogenesis, but not shortly after birth[42], may explain the findings of the former study[27].
In conclusion, our novel results support the notion that determination of serum COMP levels may assist clinicians in identifying patients with cirrhosis and those at an increased risk of liver-related death and the development of HCC. Single measurement of COMP shows utility on its own, but it will be certainly of greater diagnostic value with serial determinations obtained during follow-up visits or in combination with other tests, by casting the net wider. The present exploratory study has provided intriguing results and may assist enhanced management of hepatic fibrosis, in particular the assessment of regression or progression of fibrosis before and after specific therapeutic treatments. While larger studies of prospectively collected serum samples will be needed to better address these possibilities, COMP appears to be a promising, simple, non-invasive serological biomarker that may help guide the management of patients with chronic liver disease.
Hepatocellular carcinoma (HCC) is one of the most frequent cancers worldwide and accounts approximately one-third of all malignancies. Early diagnosis is a prerequisite for radical treatment, such as surgical resection or liver transplantation.
The study of novel biomarkers is an increasingly important field in the early detection of HCC, taking account that currently prompt diagnosis is difficult due to the lack of specific symptoms and the relatively limited prognostic value of the available serological and radiological methods used for surveillance.
In the present study, the authors demonstrate for the first time that the presence of cartilage oligomeric matrix protein (COMP) in the sera of patients with chronic liver diseases is strongly associated with liver cirrhosis and that increased COMP levels appear to identify a subgroup of patients at increased risk of progressing to HCC and liver-related mortality.
The study results suggest that COMP is a new promising, non-invasive biomarker for risk-assessment and surveillance of patients with chronic liver diseases at risk to develop HCC.
Extracellular matrix degradation is closely associated with fibrosis, cirrhosis and cancer development. COMP is an antigen expressed in articular cartilage, but also in other tissues, including the developing liver.
Starting part of this paper is excellent, specially the abstract. It is concise and organized. The study is a timely research. Objectives are consistent with literature review and analysis.
P- Reviewer: Kapoor S, Penkova-Radicheva MP, Zielinski J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4260] [Article Influence: 236.7] [Reference Citation Analysis (2)] |
| 2. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 3. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 4. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Mazioti A, Gatselis NK, Rountas C, Zachou K, Filippiadis DK, Tepetes K, Koukoulis GK, Fezoulidis I, Dalekos GN. Safety and efficacy of transcatheter arterial chemoemboliazation in the real-life management of unresectable hepatocellular carcinoma. Hepat Mon. 2013;13:e7070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Manesis EK, Papatheodoridis GV, Touloumi G, Karafoulidou A, Ketikoglou J, Kitis GE, Antoniou A, Kanatakis S, Koutsounas SJ, Vafiadis I. Natural course of treated and untreated chronic HCV infection: results of the nationwide Hepnet.Greece cohort study. Aliment Pharmacol Ther. 2009;29:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3280] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 10. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4512] [Article Influence: 347.1] [Reference Citation Analysis (2)] |
| 11. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 865] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6560] [Article Influence: 468.6] [Reference Citation Analysis (1)] |
| 13. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [PubMed] |
| 14. | Lencioni R. Surveillance and early diagnosis of hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S223-S227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Tremosini S, Reig M, de Lope CR, Forner A, Bruix J. Treatment of early hepatocellular carcinoma: Towards personalized therapy. Dig Liver Dis. 2010;42 Suppl 3:S242-S248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Oldberg A, Antonsson P, Lindblom K, Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267:22346-22350. [PubMed] |
| 17. | Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heinegård D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132-6136. [PubMed] |
| 18. | Halász K, Kassner A, Mörgelin M, Heinegård D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166-31173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279:25294-25298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354:237-240. [PubMed] |
| 21. | Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31:583-591. [PubMed] |
| 22. | Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Häuselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36:1151-1160. [PubMed] |
| 23. | Skoumal M, Haberhauer G, Feyertag J, Kittl EM, Bauer K, Dunky A. Serum levels of cartilage oligomeric matrix protein are elevated in rheumatoid arthritis, but not in inflammatory rheumatic diseases such as psoriatic arthritis, reactive arthritis, Raynaud’s syndrome, scleroderma, systemic lupus erythematosus, vasculitis and Sjögren’s syndrome. Arthritis Res Ther. 2004;6:73-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Skoumal M, Haberhauer G, Feyertag J, Kittl EM, Bauer K, Dunky A. Serum levels of cartilage oligomeric matrix protein (COMP): a rapid decrease in patients with active rheumatoid arthritis undergoing intravenous steroid treatment. Rheumatol Int. 2006;26:1001-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wisłowska M, Jabłońska B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin Rheumatol. 2005;24:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Tseng S, Reddi AH, Di Cesare PE. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights. 2009;4:33-44. [PubMed] |
| 27. | Xiao Y, Kleeff J, Guo J, Gazdhar A, Liao Q, Di Cesare PE, Büchler MW, Friess H. Cartilage oligomeric matrix protein expression in hepatocellular carcinoma and the cirrhotic liver. J Gastroenterol Hepatol. 2004;19:296-302. [PubMed] |
| 28. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [PubMed] |
| 29. | Gatselis NK, Georgiadou SP, Koukoulis GK, Tassopoulos N, Zachou K, Liaskos C, Hatzakis A, Dalekos GN. Clinical significance of organ- and non-organ-specific autoantibodies on the response to anti-viral treatment of patients with chronic hepatitis C. Aliment Pharmacol Ther. 2006;24:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Stefos A, Gatselis N, Zachou K, Rigopoulou E, Hadjichristodoulou C, Dalekos GN. Descriptive epidemiology of chronic hepatitis B by using data from a hepatitis registry in Central Greece. Eur J Intern Med. 2009;20:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Gatselis NK, Zachou K, Norman GL, Tzellas G, Speletas M, Gabeta S, Germenis A, Koukoulis GK, Dalekos GN. IgA antibodies against deamidated gliadin peptides in patients with chronic liver diseases. Clin Chim Acta. 2012;413:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P, Sypsa V, Manolakopoulos S, Mangia G, Gatselis N. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol. 2015;62:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Papatheodoridis GV, Dalekos GN, Sypsa V, Yurdaydin C, Buti M, Goulis J, Chi H, Manolakopoulos S, Mangia G, Gatselis N. Timing of hepatocellular carcinoma development and predictability of a modified PAGE-B risk score in caucasian chronic hepatitis B patients treated with entecavir or tenofovir. Hepatology. 2014;60 Suppl 1:S316A-317A. |
| 34. | Rahimi R, Yopp A, Singal A. Current issues and future trends in surveillance for hepatocellular carcinoma. Clin Liver Disease. 2012;1:186-189. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Yang JD, Kim WR. Surveillance for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Kim do Y, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 37. | Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol. 2014;20:16820-16830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 125] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 38. | Branchi F, Conti CB, Baccarin A, Lampertico P, Conte D, Fraquelli M. Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2014;20:14568-14580. [PubMed] |
| 39. | Schiavon Lde L, Narciso-Schiavon JL, de Carvalho-Filho RJ. Non-invasive diagnosis of liver fibrosis in chronic hepatitis C. World J Gastroenterol. 2014;20:2854-2866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Chrostek L, Panasiuk A. Liver fibrosis markers in alcoholic liver disease. World J Gastroenterol. 2014;20:8018-8023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Piao RL, Brigstock DR, Zhu J, Zhang ML, Gao RP. Clinical significance of connective tissue growth factor in hepatitis B virus-induced hepatic fibrosis. World J Gastroenterol. 2012;18:2280-2286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Onodera S, Tonozuka Y, Tashiro S. Transient expression of megakaryocyte-derived protein immunoreactive with an antiserum to cartilage oligomeric matrix protein in developing rat liver. Biochem Biophys Res Commun. 2000;271:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |