Published online Jul 18, 2015. doi: 10.4254/wjh.v7.i14.1866
Peer-review started: December 4, 2014
First decision: February 7, 2015
Revised: June 15, 2015
Accepted: July 11, 2015
Article in press: July 14, 2015
Published online: July 18, 2015
Processing time: 234 Days and 1.5 Hours
In the last years, the development in the oncology field has been huge and rapid. In particular, the evaluation of response to anti-tumour treatments has been being object of intense research, producing significant changes. Response assessment after therapy in solid neoplasias has always used radiological imaging techniques, with tumour size reduction representing a presumed therapeutic efficacy. However, with the introduction of anti-angiogenetic drugs the evaluation of tumour size has become unsuitable because some tumours, under treatment, show only tumour perfusion changes rather than lesion shrinkage. Between different imaging techniques with contrast-enhancement, contrast-enhanced ultrasound (CEUS) and, in particular, dynamic CEUS have arisen as a promising and non-invasive device for monitoring cancer treatments. Moreover, the introduction of perfusion software has even more refined the technique since it is able to provide quantitative parameters related to blood flow and blood volume that can be associated with tumour response and clinical outcome such as the progression free survival and the overall survival. Here, we give an overview of the current status of CEUS in monitoring hepatocellular carcinoma response to different kind of treatments.
Core tip: Hereby we present a literature revision about the current status of contrast enhanced ultrasound in monitoring hepatocellular carcinoma response to different kind of treatments. This is a very important topic because of the rapid development in the oncology field due to the introduction of novel anti-cancer therapies. Among different contrast enhanced imaging techniques, dynamic contrast-enhanced ultrasound has emerged as a versatile tool as standard radiological imaging has become unsatisfactory.
- Citation: Roccarina D, Garcovich M, Ainora ME, Riccardi L, Pompili M, Gasbarrini A, Zocco MA. Usefulness of contrast enhanced ultrasound in monitoring therapeutic response after hepatocellular carcinoma treatment. World J Hepatol 2015; 7(14): 1866-1874
- URL: https://www.wjgnet.com/1948-5182/full/v7/i14/1866.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i14.1866
The potential applications of ultrasound (US) imaging in the oncology field are vast, ranging from early cancer detection and tumour characterisation to treatment response monitoring[1]. In the last years, the evaluation of response to anti-tumour treatments has been being object of intense investigations and changes, since a number of new anti-cancer agents are progressively becoming available[2-4]. In this setting a proper evaluation of tumour response is very important in the achievement of therapeutic decisions.
Until now the classical response assessment criteria in solid cancers were based on tumour size measurement by radiological imaging techniques and a reduction in tumour size during treatment was associated with therapeutic and clinical benefit. However, with the recent development of molecularly targeted therapies it has become necessary to introduce different methods to evaluate treatment efficacy. To achieve this goal, the traditional response criteria based on tumour size [Response Evaluation Criteria In Solid Tumours (RECIST)] were lately modified introducing new criteria that evaluate changes in tumour vascularisation[5].
Among different contrast-enhanced imaging techniques, contrast-enhanced US (CEUS) and dynamic CEUS (D-CEUS) have arisen as a promising, non-invasive and cost-effective device for monitoring cancer treatments. Moreover, the introduction of perfusion software has refined the technique even more since it is able to provide quantitative parameters related to blood flow and blood volume[5-8].
The present review focused on the current standards and perspectives of application of both CEUS and D-CEUS in the evaluation of treatment response in patients affected from hepatocellular carcinoma (HCC).
Liver cancer is the sixth most common cancer, the third cause of cancer related death, and accounts for 7% of all cancers. HCC represents more than 90% of primary liver cancer, is a major global health problem and its worldwide incidence is growing up[9].
Diagnosis of HCC can be done using histopathology or by identifying the typical vascular hallmark (hyper-vascular in the arterial phase with washout in the portal venous or delayed phases) using contrast-enhanced imaging techniques.
The treatment depends on the tumour stage at the moment of the diagnosis. Liver resection, liver transplantation and ablative procedures such as radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI) are curative. Trans-catheter arterial chemo-embolisation (TACE) and systemic therapies such as anti-angiogenetic drugs and chemotherapies represent palliative treatments[10].
The advent of microbubble US contrast agents (UCA) has allowed the display of parenchyma microvasculature, impossible with B-mode and color-Doppler method[11]. The enhancement patterns of the tumours can be studied during arterial, portal venous, late and post-vascular phases, in real time and with a higher temporal resolution compared to other imaging modalities, allowing a deeper study of the lesion enhancement behavior. Moreover, the good safety profiles of UCA make possible to administer repeated boluses during the same exam, if necessary.
Recent European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines highlighted the role of CEUS, as a cost-effective technique with a good safety profile, not only in the characterisation and detection of focal liver lesion but also in monitoring tumour response after curative, loco-regional and systemic HCC treatments[12,13].
An accurate evaluation of treatment efficacy is fundamental both for phases II and III clinical trials and for clinician as a guide for therapeutic decisions.
When we evaluate the role of CEUS in monitoring tumour response it is important to distinguish between morphological and functional response.
In the first case vascular changes produced by the treatment are evaluated according modified-RECIST (mRECIST) by a qualitative or semi-quantitative CEUS. On the contrary, functional response can be assessed by D-CEUS that combines morphological and functional data leading to a more accurate measurement of tumour characteristics. The kinetics of microbubble flow through the tumour is evaluated by mathematical models applied to signal intensity vs time able to provide quantitative parameters associated to blood flow and blood volume. This application has encouraging clinical potential for delineating changes in tumour vascularisation secondary to anti-angiogenetic treatment[5-8].
All ablative procedures cause the destruction of both the tumour itself and its vasculature by alterations in the target lesions such as coagulative necrosis, apoptosis and tissue granulation. Contrast-enhanced computed tomography (CE-CT) performed 4-6 wk after the treatment is currently considered the “gold standard” for the evaluation of tumour response.
Tumour necrosis is identified according to the absence of hyper-enhancement areas in the context of the treated lesion[10].
Due to the ability in representing HCC micro-vessels, CEUS has been utilised to evaluate intra-tumoral vascularisation after ablative treatments. Response is complete when the tumour treatment has determined a coagulative and vascular necrosis of the entire lesion and in this case no contrast enhancement is detected during all contrastographic phases of the dynamic study. On the contrary, when the therapy has failed zones of well-perfused residual tumour remain in the target lesion and focal contrast enhancement is detected in these areas. The residual unablated tumour appears like an irregular, eccentric or nodular peripheral enhancement[14-17]. Sometimes, especially in HCC treated with PEI, septa enhancement as well as a vessel passing through the nodule may be detected.
In large tumours, incomplete ablations might look as zones with contrast up-taking, which usually are localised nearby the periphery of the lesions. In these cases, an accurate comparison between the pre- and post-ablation images is necessary to achieve a correct evaluation of treatment efficacy[17].
No unanimous strategy exists concerning the most appropriate timing schedule for the performance of CEUS. In fact, in the currently available studies on this topic CEUS has been performed at very heterogeneous time-points after the ablative treatments. In particular, tumour response can be evaluated in the immediate post-treatment, after 1 d, 1 mo or later during the follow-up[18].
The possibility to detect the residual enhancing tumour immediately after ablation by means of CEUS could be a tempting approach in the interventional setting as it may lead to a prompt retreatment in the same session[19]. In fact, when CEUS is carried out within 60 min after PEI or RFA, there is a fair agreement with standard radiological imaging performed 2 or 4 wk later. However, despite its high specificity (94%), CEUS is characterised by only 40% of sensitivity in the detection of viable remnant tumour, due to false negative results. This high number of false negative cases could be related to the difficult interpretation of the images obtained immediately after the procedure and, especially, to the presence of a thin marginal area of hyper-vascularity in the arterial phase not followed by a proper washout in the portal/venous phase[15]. More specifically, the differentiation between the hyper-vascularity produced by a localised tissue response (hyperemia) or arteriovenous shunting and the residual hypervascular tumour in the periphery of the ablated area may be challenging. Reactive hyperemia usually shows a diffuse and homogeneous peripheral enhancement, with uniform and ring-like thickness, no more than 4-5 mm thick, followed by iso-enhancement in the portal and late phase. In contrast, residual tumour shows a local, heterogeneous or irregular peripheral enhancement, a thickness greater than 7-8 mm and the pattern of enhancement is characterised by hyper-enhancement in the arterial phase, followed by hypo-enhancement in the portal and late phase. However, in some cases viable tumour could be associated with arterial enhancement without complete washout in the portal and late phase, usually likewise to the enhancement pattern of the tumour before treatment[20,21].
Other reasons justifying the high number of false negative results could be a scan plane not including the residual tumour, uncooperative patients under conscious sedation or general anesthesia and, finally, an incorrect scanning time. In fact, intra-lesional gas developing during RFA or PEI may hinder a proper evaluation in the immediate post-procedural follow-up. These artefacts may persist for 15-180 min[22], but a delay of at least 20 to 40 min after the procedure would help to adjust visibility minimizing gas development.
Based on the results of different studies the positive predictive value (PPV) and negative predictive value of immediate post-procedural CEUS in detecting viable tumour tissue are around 82% and 50%, respectively[15].
Accordingly, the only significant role of CEUS performed within 60 min after treatment is to detect viable tumour during the same ablative session and allow an immediate retreatment, thereby lowering the rate of unsuccessful treatment, improving the cost-effectiveness ratio and optimising patient care[23].
Some authors suggest that CEUS should be performed at least 24 h after RFA or PEI. However, this strategy seems to be less attractive than the immediate post-treatment assessment, not permitting an ablation refinement in the same session if required. Moreover, as immediate post-procedural CEUS may not be available in all clinical settings, a delayed CEUS should overcome some of the aforementioned technical issues of intra-operative CEUS.
Good concordance between CEUS and CE-CT performed at the same time point has been reported[24,25]. However, a recent study shows that 1 d after the procedure gas bubbles could be displayed within half of the tumour, with a reported sensitivity in detecting residual viable tumour only equal to 27%. In addition, one patient with suspected residual disease at this time point was finally classified as a false positive result. Thus, CEUS performed at 24 h after ablative treatment may show both false negative and false positive results hampering its routine application in clinical practice[24].
These results were confirmed by Meloni et al[26], who found that the sensitivity and specificity of CEUS performed at 24 h were 33% and 98%, respectively.
Overall, these data indicate that CE-CT and CEUS at 24 h are not always helpful in the evaluation of percutaneous ablation response, having only poor sensitivity and a specificity not equal to 100%.
These unsatisfactory results might be related to the gas persistence in the context of the tumour, as well as to the frequent post-treatment peritumoral inflammation. Both these conditions may be still detectable several days after treatment and, in some cases, may persist up to 1 or 2 mo[24].
Several studies evaluated the usefulness of CEUS performed 1 mo after ablative therapies compared to the CE-CT at the same time-point. These studies demonstrated almost the same diagnostic accuracy between CEUS and CE-CT. In particular, Vilana et al[24] found a sensitivity and specificity of 91% and 97%, respectively. Similarly, Pompili et al[27] reported a sensitivity of 87% and a specificity of 98.4%, with a good diagnostic agreement with CE-CT (94.6%).
Based on these results CEUS performed 1 mo after the procedure can be considered an appropriate, reliable, comparatively inexpensive and safe alternative technique to CE-CT in the assessment of therapeutic response after RFA or PEI[24,27,28].
A 2 years follow-up with an imaging technique is mandatory to detect HCC recurrence, satellites or seeding[29,30]. The ability of CEUS in detecting local tumour progression or new intrahepatic recurrence during follow-up has been evaluated in different studies. In all cases the sensitivity and the PPV of CEUS compared to CE-CT were unsatisfactory[31]. These results could be related to the short duration of the arterial phase that makes difficult to scan the whole liver or to the intrinsic shortcomings of US technique (small lesion, unfavorable location, etc.).
Thus, in the long time follow-up, CE-CT or contrast-enhanced magnetic resonance (CE-MRI) are the mainstay for the imaging of treated patients and the detection of local or remote intra-hepatic and extra-hepatic relapse[23].
However, even though several studies has been published regarding the role of CEUS vs CE-CT after ablative treatments, the results remain still controversial and an ideal imaging follow-up scheme is not yet available. Where CEUS is available both techniques should be recommended in order to combine the virtues and to reduce the limits of both modalities. Anyway, further studies are still needed to evaluate the efficacy of this approach[29].
Since TACE has been introduced as a palliative treatment in patients with unresectable HCC it has become one of the most common form of interventional therapies, although in many cases it is difficult to achieve complete necrosis of the tumour. Intratumoral vascularity after TACE has been shown to correlate with tumour viability and is used as the major criterion to assess treatment efficacy and to plan additional treatment.
Similarly as previously described for RFA and PEI, CEUS has been proved to be efficient in differentiating residual from necrotic tumour after TACE.
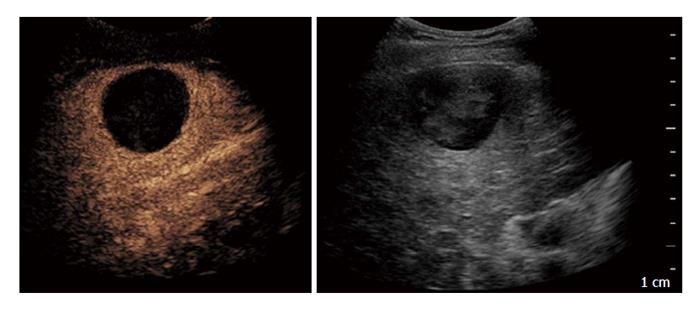
Moschouris et al[32] reported that the early assessment of treatment response by CEUS performed 48 h after drug-eluting bed TACE could underestimate the degree of necrosis in comparison with delayed evaluation (35-40 d after the procedure) with a percentage of tumour necrosis of 43.5% and 52.3%, respectively. The same authors found a good agreement between delayed post-TACE CEUS and CE-CT[32].
In another study CEUS resulted even more sensitive than CE-CT in the detection of residual vascular enhancement after TACE using angiography as reference standard. In fact, CE-CT performed 1 mo after treatment detected 20 of 23 incomplete responses whereas CEUS performed at the same time point detected all cases of incomplete response. Results of CEUS and CE-CT agreed with those of the reference standard (angiography) in 38/38 (100%) and in 35/38 (92.1%) nodules, respectively[33]. Another recent study from a Chinese group suggests a leading role of CEUS compared to CE-CT for detecting residual tumour after lipiodol-based TACE. Liu et al[34] evaluated treatment response in 130 HCC patients who underwent CEUS 15 to 90 d after procedure. The sensitivity and accuracy of detecting residual tumour by CEUS vs CE-CT were 95.9% vs 76.2% and 96.2% vs 77.7% respectively, thus recommending CEUS as an optional procedure for assessing the tumour response after TACE[34].
Based on these results, CEUS performed at 1-mo with second generation contrast agents can be regarded as a valid alternative technique to CE-CT in the assessment of therapeutic response after TACE for HCC (Figure 1).
Angiogenesis plays a critical role in the growth and spread of tumour. Cancer cells are able to produce some biochemical signals stimulating angiogenesis and to enhance the production of angiogenesis signaling molecules by the surrounding normal cells. Fed by new blood vessels cancer cells proliferate and progressively lose their differentiation, invading the around tissues, going in the blood and lymphatic vessels and forming new colonies of cancer cells far from the primitive cancer, called metastases[35].
The “gold-standard” to assess the angiogenesis is the histological evaluation of the average number of micro-vessels [microvascular density (MVD)][36]. However, biopsy is invasive and sampling bias may happen due to tumours heterogeneity, producing a possibly under- or overestimation of the angiogenesis grade[37].
Furthermore, MVD is not able to give information about changes of blood flow or vascular bed hyper-permeability. On the contrary, functional imaging is able to quantify these changes above all as an early consequence of the anti-angiogenesis therapy[38,39].
One of the most important recent steps in the oncology field is the development of anti-angiogenetic drugs. These agents act interfering with various steps in the angiogenesis process. Usually, they bind to receptors on the surface of endothelial cells or to other proteins in the downstream signaling pathways, inhibiting factors needed for new blood vessels arrangement.
The United States Food and Drug Administration approved different drugs showing anti-angiogenetic activity including sorafenib, sunitinib and bevacizumab. To date sorafenib is the only anti-angiogenetic approved for HCC treatment but other drugs with similar activity are under investigation in many phase II and III trials.
Angiogenesis inhibitors interfere with various steps in this process. In particular, sorafenib acts by inhibiting the serine-threonine kinases and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptor β, which have been implicated in the molecular pathogenesis of HCC[40-45].
The different mechanism of action of these new agents from classical cytostatic drugs requires a shift from standard efficacy evaluation criteria to new imaging modalities that assesses changes in tumour vascularisation. Although progression free survival (PFS) and overall survival (OS) represent the most significant efficacy end-points in the medium and long term, early assessment of tumour angiogenesis remains a crucial aim in this area as it allows optimisation of individualised treatment[46]. Especially, early evaluation of failed response allows a tailored therapy, avoiding needless toxicity, psychological burden and costs.
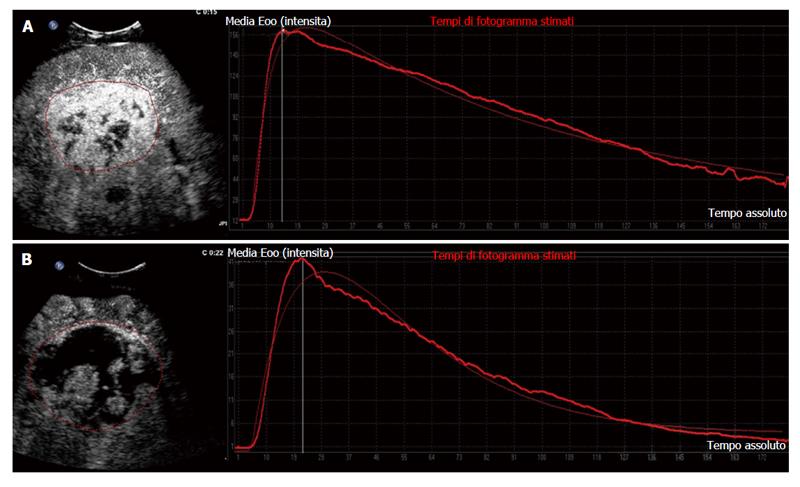
D-CEUS is a new functional technique enabling a quantitative assessment of solid tumour perfusion. This is achieved by a quantitative analysis performed on contrast uptake curves which are built up from raw linear data after automatic modelisation. The robustness of this approach relies on the fact that signal intensity is proportional to the microbubble concentration in the region of interest. Raw linear data are used to quantify parameters such as peak intensity (PI), time to PI, mean transit time, slope coefficient of wash-in (Tp), total area under the curve (AUC), AUC of wash-in and AUC of wash-out. All these parameters provide information about blood flow and volume, but an optimal parameter has not been clearly identified yet[47].
D-CEUS is supported by the French National Cancer Institute (INCa), which is currently evaluating such technique in different malignancy as well as in primary HCC to establish the reliable perfusion parameters and timing for quantitative anticancer efficacy assessments[48].
Reduction in tumour vascularisation can easily be detected in responders after 1 or 2 wk and is correlated with mRECIST response, PFS and OS in renal cell carcinoma and HCC[6,47].
As already said, VEGF plays a critical role in mediating angiogenesis in HCC, and the tumour expression of VEGF correlates with vascular density, tumour invasiveness and prognosis[49-51]. Several studies demonstrated the utility of D-CEUS for the quantification of tumour perfusion as a prognostic tool in patients with advanced HCC treated with anti-angiogenetics and identified quantitative parameters correlated with standard efficacy endpoints such as tumour response, PFS and OS.
In a recent experimental study D-CEUS was able to detect a reduction in tumour vascularisation as early as 3 d after bevacizumab therapy for HCC, with a good agreement with CE-CT performed at 2 mo in the identification of responders and non-responders patients[52].
Another study from our group corroborated that D-CEUS is a reliable method to identify early reduction in tumour vascularisation in patients undergoing treatment with sorafenib. Changes in selected quantitative parameters, detected after 14 d of therapy, agreed with tumour response evaluated by means of standard criteria at 2 mo. Between the parameters analysed PI, AUC and Tp showed a significant reduction soon after the beginning of therapy with sorafenib in most of the patients reaching long-term stable disease (Figure 2).
Moreover, a relationship was found between D-CEUS variables and improved clinical outcome such as prolonged OS and PFS[53].
Some researchers evaluated the usefulness of D-CEUS in the quantification of liver parenchymal perfusion for the early detection of major adverse events in patients with advanced HCC treated with sorafenib. The decrease in functional parameters related to blood volume (AUC and PI) between baseline and day 7 after the initiation of treatment was strongly associated with changes in laboratory data related to liver function and was able to predict the occurrence of major adverse events such as liver failure[54].
The dynamic enhancement parameters of D-CEUS can provide important references for clinical pathological factors in prognosis prediction such as VEGF expression and MVD. In fact, a recent study reported a good correlation between VEGF and CD34 expression, (evaluated by immune-histo-chemistry), MVD and some D-CEUS parameters (enhanced time, washout time and AUC)[55].
Over the past decades, different locoregional and systemic therapies have emerged as a suitable alternative to surgery in patients with HCC. An accurate assessment of therapeutic response is mandatory, as complete tumour necrosis significantly increases patient survival, whereas residual viable tumour requires additional treatment. CEUS suggests an effective procedure when a previously enhancing, hyper-vascularised HCC tumour shows lack of contrast enhancement after treatment, whereas still viable tumoral tissue is usually visualised as an arterial-enhancing area with subsequent washout[6].
Several studies demonstrated the usefulness of CEUS and D-CEUS in monitoring tumour response after HCC treatment. In fact, it is able to provide both morphological and functional data associated with low cost and good safety profile.
CEUS performed within 60 min after RFA or PEI with a correct timing scan seems to be reliable for the immediate post-treatment assessing, allowing an immediate retreatment during the same session, if necessary[23]. As concerning recently introduced devices for ablative treatment such as cryoablation and irreversible electroporation, the usefulness of CEUS was investigated only in few studies showing preliminary and inconclusive results[56]. One important information stemming from these studies is that CEUS pattern after cryoablation appears different compared to that after RFA because the margins of the lesions are less well defined and shrink significantly faster than RFA lesions, explaining why it is often difficult to identify them on B-mode or even CEUS more than 1 year after the procedure[57]. Overall, CEUS can be considered a reliable and safe alternative technique to CE-CT in the assessment of therapeutic response to ablative treatment and TACE after 1 mo[28].
Finally, CEUS associated with perfusion software and time intensity curves can be used as a new functional technique enabling a quantitative assessment of solid tumour perfusion by means of a quantitative analysis. This is very important in the early assessment of tumour vascularisation in HCC treated with vascular targeting agents since it would enable an optimisation of individualised treatment. Especially, early evaluation of failed response allows a tailored therapy, avoiding unnecessary toxicity, psychological burden and costs.
The effective application of CEUS and D-CEUS in clinical practice has been recently highlighted by EFSUMB guidelines. For example this panel of experts recognised the important role of CEUS in the very early evaluation of ablative treatment as a guidance for immediate re-treatment of residual unablated tumour[12].
Novel CEUS-based techniques may even exploit the advantages of this imaging modality in evaluating tumour response after HCC treatment. For instance, a technical development based on real-time fusion of CEUS with CE-CT or CE-MRI enables a precise mapping of tumour lesions in CEUS. This new technique allows a multi-plane display of tumour lesions and also shows small lesions which are normally hard to display in standard US. In a pilot study by Ross et al[58] the fusion of pre-interventional CE-CT or CE-MRI with post-interventional CEUS performed immediately after treatment showed an improved visualisation of microcirculation and residual tumour perfusion after TACE. A high correlation between early fusion study (CEUS with CE-CT or CE-MRI) and CE-CT performed 6 wk after TACE granted an early assessment of therapeutic success[58]. More recently, three-dimensional CEUS technique (3D CEUS) has been reported to improve the study of tumour vascularity, thus allowing the response evaluation of HCC treatments in the three orthogonal planes. Nevertheless, it has been suggested that the spatial resolution of the current 3D probes may be limited as 3D CEUS provided similar diagnostic performance compared to conventional CEUS in the assessment of therapeutic response of HCC treated with ablative treatments[59].
In conclusion, the perspectives about a large diffusion of CEUS and D-CEUS in clinical practice are very positive and promising, although further studies are warranted to determine the still unclear aspects such as the best timing and the best quantitative dynamic parameter for the assessment of response to HCC treatment.
P- Reviewer: Ferraioli G, Sugimoto K, Xu CS S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg. 2012;2:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 2. | Gwyther SJ. Current standards for response evaluation by imaging techniques. Eur J Nucl Med Mol Imaging. 2006;33 Suppl 1:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Provenzale JM. Imaging of angiogenesis: clinical techniques and novel imaging methods. AJR Am J Roentgenol. 2007;188:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Fournier LS, Cuénod CA, Clément O, Siauve N, Frija G. [Imaging of response to treatment in oncology]. J Radiol. 2007;88:829-843. [PubMed] |
| 5. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3277] [Article Influence: 218.5] [Reference Citation Analysis (36)] |
| 6. | Marcus CD, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72:217-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 8. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21477] [Article Influence: 1342.3] [Reference Citation Analysis (1)] |
| 9. | International Agency for Research on Cancer. Cancer Surveillance. 2011; Available from: http://www-dep.iarc.fr/. |
| 10. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4512] [Article Influence: 347.1] [Reference Citation Analysis (2)] |
| 11. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 12. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 13. | Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, Gloy V, Raatz H, Misso K, Severens J. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17:1-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Sparchez Z, Radu P, Anton O, Socaciu M, Badea R. Contrast enhanced ultrasound in assessing therapeutic response in ablative treatments of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2009;18:243-248. [PubMed] |
| 15. | Dill-Macky MJ, Asch M, Burns P, Wilson S. Radiofrequency ablation of hepatocellular carcinoma: predicting success using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:S287-S295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Solbiati L, Tonolini M, Cova L. Monitoring RF ablation. Eur Radiol. 2004;14 Suppl 8:P34-P42. [PubMed] |
| 17. | Dhamija E, Paul SB. Role of contrast enhanced ultrasound in hepatic imaging. Trop Gastroenterol. 2014;35:141-151. [PubMed] |
| 18. | Bartolotta TV, Taibbi A, Midiri M, De Maria M. Hepatocellular cancer response to radiofrequency tumor ablation: contrast-enhanced ultrasound. Abdom Imaging. 2008;33:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Goldberg SN, Walovitch RC, Straub JA, Shore MT, Gazelle GS. Radio-frequency-induced coagulation necrosis in rabbits: immediate detection at US with a synthetic microsphere contrast agent. Radiology. 1999;213:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, Lim JH, Kim SA. Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics. 2003;23:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Limanond P, Zimmerman P, Raman SS, Kadell BM, Lu DS. Interpretation of CT and MRI after radiofrequency ablation of hepatic malignancies. AJR Am J Roentgenol. 2003;181:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Goldberg SN, Gazelle GS, Solbiati L, Livraghi T, Tanabe KK, Hahn PF, Mueller PR. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51 Suppl:S19-S23. [PubMed] |
| 24. | Vilana R, Bianchi L, Varela M, Nicolau C, Sánchez M, Ayuso C, García M, Sala M, Llovet JM, Bruix J. Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma? Eur Radiol. 2006;16:2454-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Imai Y, Okamoto N, Tateiwa N, Hasebe O, Nagata A, Imai S, Makita H. Assessment of treatment efficacy in radiofrequency ablation for hepatocellular carcinoma: Comparison between multiplanar reconstruction by multi-detector row CT and contrast-enhanced ultrasonography by Truagent detection mode. Hepatol Res. 2006;35:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Meloni MF, Andreano A, Zimbaro F, Lava M, Lazzaroni S, Sironi S. Contrast enhanced ultrasound: Roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J Ultrasound. 2012;15:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, Gasbarrini G, Rapaccini GL. Contrast-enhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Bartolotta TV, Midiri M, Galia M, Runza G, Bellia M, Lagalla R. Usefulness of sonovue-enhanced pulse-inversion ultrasonography to assess hepatocellular carcinoma response after percutaneous radiofrequency thermal ablation therapy [Abstract]. In: RSNA Assembly and annual meeting program 2005; 73. |
| 29. | Liu LN, Xu HX, Zhang YF, Xu JM. Hepatocellular carcinoma after ablation: the imaging follow-up scheme. World J Gastroenterol. 2013;19:797-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, Lee JH, Lim JH, Choo IW. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, Liu LN. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol. 2013;19:855-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Moschouris H, Malagari K, Papadaki MG, Kornezos I, Matsaidonis D. Contrast-enhanced ultrasonography of hepatocellular carcinoma after chemoembolisation using drug-eluting beads: a pilot study focused on sustained tumor necrosis. Cardiovasc Intervent Radiol. 2010;33:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010;35:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Liu M, Lin MX, Lu MD, Xu ZF, Zheng KG, Wang W, Kuang M, Zhuang WQ, Xie XY. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol. 2015;25:2502-2511. [PubMed] |
| 35. | Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15-18. [PubMed] |
| 36. | Lu JP, Wang J, Wang T, Wang Y, Wu WQ, Gao L. Microvessel density of malignant and benign hepatic lesions and MRI evaluation. World J Gastroenterol. 2004;10:1730-1734. [PubMed] |
| 37. | Barrett T, Brechbiel M, Bernardo M, Choyke PL. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Knopp MV, von Tengg-Kobligk H, Choyke PL. Functional magnetic resonance imaging in oncology for diagnosis and therapy monitoring. Mol Cancer Ther. 2003;2:419-426. [PubMed] |
| 39. | Drevs J, Müller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015-4022. [PubMed] |
| 40. | Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 41. | Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 42. | Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 43. | Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 414] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 44. | Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 45. | Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev. 2011;37:63-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 450] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 46. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1323] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 47. | Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, Taieb S, Aziza R, Sarran A, Labbe-Devilliers C. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Tseng PL, Tai MH, Huang CC, Wang CC, Lin JW, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Brodsky SV, Mendelev N, Melamed M, Ramaswamy G. Vascular density and VEGF expression in hepatic lesions. J Gastrointestin Liver Dis. 2007;16:373-377. [PubMed] |
| 51. | Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, Wu W, Qiu LW, Meng XY. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220-226. [PubMed] |
| 52. | Lassau N, Koscielny S, Chami L, Chebil M, Benatsou B, Roche A, Ducreux M, Malka D, Boige V. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification--preliminary results. Radiology. 2011;258:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 53. | Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, Imai Y. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Yang YL, Yang RJ, Liu X, Liu J, Chao LJ, Duan YY. Correlations between the time-intensity parameters of contrast-enhanced ultrasound and clinical prognosis of hepatocellular carcinoma. Clin Imaging. 2013;37:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Wiggermann P, Zeman F, Niessen C, Agha A, Trabold B, Stroszczynski C, Jung EM. Percutaneous irreversible electroporation (IRE) of hepatic malignant tumours: contrast-enhanced ultrasound (CEUS) findings. Clin Hemorheol Microcirc. 2012;52:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Guibal A, Bertin C, Egels S, Savier E, Grenier PA, Lucidarme O. Contrast-enhanced ultrasound (CEUS) follow-up after radiofrequency ablation or cryoablation of focal liver lesions: treated-area patterns and their changes over time. Eur Radiol. 2013;23:1392-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Ross CJ, Rennert J, Schacherer D, Girlich C, Hoffstetter P, Heiss P, Jung W, Feuerbach S, Zorger N, Jung EM. Image fusion with volume navigation of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) for post-interventional follow-up after transcatheter arterial chemoembolization (TACE) of hepatocellular carcinomas (HCC): Preliminary results. Clin Hemorheol Microcirc. 2010;46:101-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Bartolotta TV, Taibbi A, Matranga D, Midiri M, Lagalla R. 3D versus 2D contrast-enhanced sonography in the evaluation of therapeutic response of hepatocellular carcinoma after locoregional therapies: preliminary findings. Radiol Med. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |