INTRODUCTION
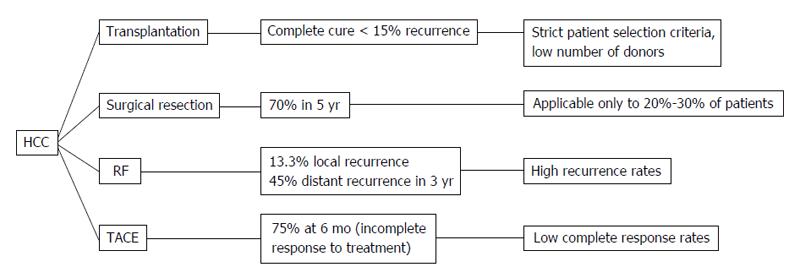
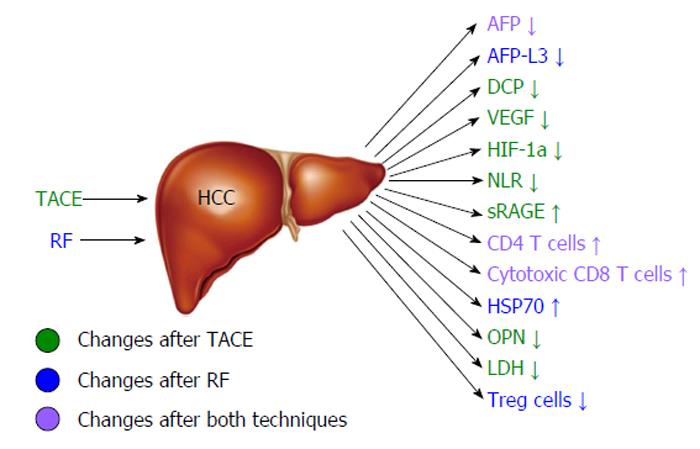
Hepatocellular cancer (HCC) is the 5th most common cancer in the world and the third cause of death by malignant disease[1]. Despite its high occurrence, the survival rates are not yet satisfying. The therapeutic tools that we possess are variable depending on the stage of HCC and the severity of the underlying liver disease. However, the only one that provides complete cure is liver transplantation. Unfortunately, due to the specific selection criteria and the low availability of liver transplants, this treatment is implemented in a limited number of patients[2]. Moreover, surgical resection is applicable to a confined number of patients, as over 70% of them have advanced hepatic disease often combined with portal hypertension or multifocal disease, conditions that preclude any possibility of hepatic surgery[3,4]. Therefore, locoregional therapies have been developed in order to treat patients that did not fulfill the criteria for surgical interventions. Transarterial chemoembolization (TACE) is a minimally invasive technique combining the impact of chemotherapy and obstruction of blood supply on the tumor area without systematic effects. The patients that benefit mostly are those with intermediate stage of HCC[5,6]. On the other hand, radiofrequency ablation (RFA) is a technique suitable for small tumors (< 3 cm) and early stage disease[7]. Its action is based on the direct destruction of the tumor by radiofrequency waves[7]. Despite their efficacy, both these techniques have high rates of local and distant recurrence[8,9] (Figure 1). The main diagnostic tools for the early detection of recurrence are the radiological techniques such as 4-phase computed tomography or dynamic contrast enhanced magnetic resonance imaging based on the mRECIST criteria[10]. The tumor response is evaluated according to target lesions’ diameters after locoregional treatment. Complete response indicates disappearance of targeted lesions, while partial response indicates that the the sum of the greatest one-dimensional diameters is decreased more than 30%. When the targeted lesions’ diameters are increased more than 20% according to baseline the tumor is characterized as progressive. Finally in cases that do not qualify for partial response or progressive disease the term “stable disease” is used[10]. However, these tools are not adequate for the exact estimation of treatment response, the immediate diagnosis of disease recurrence and the patients’ prognosis. For the above reasons, there is a current need for biomarkers that may provide the possibility to predict the course of the disease post-treatment and to recognize certain groups of patients with different prognosis. In other words, there is a need for biomarkers that could allow the personalization of therapy aiming to better treatment results and reduction of recurrence rates. To achieve this goal, a number of traditional as well as recently discovered serum biomarkers have been measured before and after locoregional therapies, in order to identify patterns that could be indicative of treatment efficacy and prognosis. For example, the prognostic value of diagnostic markers’ post-treatment changes such as alpha-fetoprotein (AFP)[11-13], lens culinaris agglutinin A-reactive fraction of AFP (AFP-L3)[14] and des-gamma-carboxy prothrombin (DCP)[15,16] has been put under investigation. At the same time, the response of vascular endothelial growth factor (VEGF) and other angiogenetic factors to hypoxic conditions caused by TACE or RF was evaluated according to tumor response[17-21]. Another intriguing observation was the immunomodulatory effect of loco-regional techniques, affecting CD4+, CD8+ T cells and T regulatory cells (Treg)[22-24] and its potential impact on patients’ survival. Finally, the post-treatment behavior of various new serum HCC markers such as nucleosomes, osteopontin (OPN)[25], soluble receptor of advanced glycation end products (sRAGE)[26] and heat shock proteins[27,28] has been examined along with its role in treatment outcome (Figure 2). The aim of this review is to present the results of these studies and analyze the emerging conclusions.
Figure 1 Hepatocellular cancer therapeutic methods.
HCC: Hepatocellular cancer; TACE: Transarterial chemoembolization; RF: Radiofrequency.
Figure 2 Changes of circulating markers and immune parameters associated with good hepatocellular cancer response after radiofrequency or transarterial chemoembolization.
HCC: Hepatocellular cancer; TACE: Transarterial chemoembolization; RF: Radiofrequency; AFP: Alpha-fetoprotein; DCP: Des-gamma-carboxy prothrombin; VEGF: Vascular endothelial growth factor; HIF-1a: Hypoxia inducible factor-1a; NLR: Neutrophil-to-lymphocyte ratio; sRAGE: Soluble receptor of advanced glycation end products; HSP70: Heat shock protein 70; OPN: Osteopontin; LDH: Lactate dehydrogenase.
AFP
AFP is one of the first markers used for the detection and prognosis of HCC. It has been used in clinical practice for many years despite its limitations in the diagnosis of small tumors and the fact that other diseases apart from HCC may cause a mild to moderate rise of its levels[12]. The diagnostic values of this marker vary according to the chosen cut-off values. In cirrhotic patients, when the cut-off value is 20 ng/mL, its sensitivity and specificity are 60% and 90% respectively[12]. Lately, the use of AFP as a screening test for HCC is not strongly recommended as curable tumors smaller than 3 cm may not cause a detectable rise and thus may not be immediately diagnosed. However, AFP is regarded as a reliable prognostic marker of recurrence[13].
It has been shown that high levels of AFP in the serum of patients 24 h after TACE are a strong independent prognostic factor for poor survival[29]. Additionally, patients with poor response of AFP levels after TACE (decrease less than 50% of baseline) had a hazard ratio for progression free survival up to 4.2 (95%CI: 2.4 to 7.2) in comparison to patients with a higher response[30]. According to another study, reduction of AFP less than 20% from the baseline level was correlated to the progression free survival as well as the overall survival of treatment naïve patients after TACE (P = 0.009)[31]. The decrease of AFP is also predictive one month post-treatment as the patients without strong AFP response at that point of time have lower overall survival (34.9 mo vs 13.2 mo; P = 0.002)[32] and are more likely to have extrahepatic metastasis six months of TACE initiation (P < 0.001)[33]. Consequently, the impact of TACE on AFP levels may reflect the efficacy of the method and the patients’ overall survival. Therefore, AFP could be used as a possible tool for further treatment choices.
As far as RFA is concerned, pre and post-treatment levels of AFP have been also associated with the response as well. It is believed that patients without an adequate decrease of AFP after RFA (AFP half-life less than 7 d) have not a complete response to treatment and thus have a lower disease free survival even if the radiological findings show successful outcome (P = 0.003)[34]. Moreover, high post treatment AFP levels (less than 20% reduction from baseline) one month after RFA are an independent risk factor for tumor recurrence (P < 0.001) as well as for low overall survival (P = 0.023) according to multivariative analysis[35].
AFP-L3
AFP-L3 is a biomarker detected in the serum of HCC patients even in cases of small tumors (35% of patients with tumors < 3 cm)[36]. Its sensitivity varies from 45% for tumors < 2 cm to 90% for tumors > 5 cm and its specificity reaches 95%. It is considered to be a marker of poor prognosis as it is often combined with tumor size, higher possibility of early metastasis and limited liver function[37]. It is believed that in combination with AFP or other markers may enhance the sensitivity and specificity of HCC diagnostic tools[12]. However, the utility of this biomarker is not yet adequately established and AFP-L3 is not currently integrated in clinical practice.
Positive pre-treatment values of AFP-L3 (> 24.4%) along with tumor size were found to be the two statistically significant predicting factors of treatment response[14]. Additionally, AFP-L3 positivity before TACE was significantly associated with 2-year survival rates (P = 0.01)[14]. Recently, it has been shown that An AFP-L3 decrease > 20% after 2 cycles of TACE is indicative of median overall survival (P < 0.0001)[38]. Therefore, the evaluation and the surveillance of its values in the course of therapy could be useful for the estimation of disease progression.
In contrast to TACE, the role of AFP-L3 changes in the serum of patients before and after RFA has been more thoroughly investigated. AFP-L3 fragment positivity (> 15%) before and 2 mo after ablation was found to be indicative of high risk of recurrence (P = 0.0096) and possibly a marker of residual HCC that cannot be depicted by radiological techniques[39]. On the other hand, the patients who had positive pre-treatment values of AFP-L3 and became negative post-treatment, did not show significantly higher rates of recurrence[39]. In another study, AFP-L3 was the only significant predictor of disease free and overall survival in comparison to AFP and DCP when measured before and after RFA[40].
DCP
DCP is an abnormal form of prothrombin produced by malignant hepatocytes. It has been used as a diagnostic marker (sensitivity 72%, specificity 90%) mainly in Japan and is associated with microvascular invasion of tumor cells[41-44]. Due to its correlation with HCC angiogenesis[41], it is thought to be indicative of high recurrence incidence. Like AFP, the elevation of its levels may be induced by chronic hepatitis C or advanced cirrhosis[41] and consequently DCP is not suitable for surveillance protocols. However, if combined with AFP, the sensitivity of the screening test may be increased. As a marker of tumor invasiveness, it is another potential biomarker for the effectiveness of locoregional treatments for HCC.
As suggested by a recent study, the response of DCP values to TACE may be useful for the estimation of treatment outcome. Decrease of DCP values greater than 50% was significantly associated with radiologic response to therapy (P < 0.001) as well as higher disease free and overall survival (P < 0.001)[45]. Moreover, DCP levels trend before and after TACE is considered to correlate both with treatment response (P = 0.009) (according to Response Evaluation Criteria in Solid Tumors) and overall survival[15].
On the other hand, it has not been proved so far that the change of DCP levels before and after RFA reflects the progress of the disease. Despite the fact that high DCP pre-treatment values are considered as a marker of high possibility of local recurrence, no study has yet proposed that the response of DCP levels after RFA has a statistically significant correlation with recurrence free survival or overall survival[46]. However, in a study examining the vascular invasion predictive factors after RFA in 1057 HCC patients, DCP was found to be the most significant predictor[16].
ANGIOGENETIC FACTORS
Angiogenesis is a key process for the expansion of hepatocellular carcinoma involving a number of factors stimulating, inhibiting or regulating various cellular pathways[47]. VEGF for example, is an angiogenetic factor often up regulated in the serum of HCC patients. The VEGF serum levels are proven to reflect the tumor mass size as well as the tumor potential to infiltrate nearby tissues. Moreover, VEGF elevated values are associated with portal vein invasion and extended disease[48]. Therefore, it is a possible tool to predict HCC prognosis and metastasis. Nevertheless, more extended investigation is needed to prove its value in HCC patients’ monitoring. Hypoxia inducible factor-1a (HIF-1a) plays also an important role in the angiogenetic process[49]. The survival of malignant cells under hypoxic conditions is mainly regulated by this factor, as it is responsible for the expression of a number of proteins including VEGF[49]. As a result, the evaluation of its behavior in HCC patients is really interesting.
Especially for TACE, a technique that induces hypoxic conditions in the tumor environment, the response of factors such as VEGF and HIF-1a has been put under investigation in a number of studies. Patients with complete response to TACE were found to have lower pretreatment levels of serum VEGF (P < 0.001) than patients with partial or no response[50]. Serum VEGF, when measured after TACE, seems to reach the highest value 1 d after treatment and then it is gradually reduced[17]. It has been shown that patients with higher levels of VEGF 7 d after TACE had a rapid progression of HCC in a 3-mo period (P < 0.05)[18]. Additionally, decreased levels of serum VEGF receptor-2 4 wk after TACE are predictive of higher survival rates (19.0 mo vs 9.8 mo, P < 0.001)[19]. Moreover, HIF-1a, a factor responsible for the regulation of the expression of VEGF[20], showed the same response as VEGF in the serum of patients 1 d, 1 wk and 1 mo after TACE[21]. The above support the correlation between the two angiogenetic factors. Additionally, patients with complete response to TACE had lower levels of both HIF-1a and VEGF 1 mo after TACE than those with partial response, stable or progressive disease (all P < 0.01)[21]. Recently, the changes of the two angiogenetic markers in the serum of 22 patients 30 to 40 d after TACE were correlated with tumor’s hepatic artery perfusion (HAP) and hepatic artery perfusion index (HPI). According to the study, VEGF and HIF-1a post-treatment levels were higher while HAP and HPI were lower in patients with recurrent disease comparing to the baseline values (P < 0.05)[51]. As a result, VEFG and HIF-1a should be further studied as potential markers of response to this therapeutic technique.
The levels of VEGF in the serum of patients treated with RFA are also considered to be a potential prognostic factor. High pre-treatment levels (> 240 pg/mL) are an independent prognostic factor of the recurrence free survival as well as the overall survival after RFA (P = 0.005 and 0.002, respectively)[52]. However, when serum VEGF levels were measured 2 and 5 d after RFA, there was no significant change between the pre- and post-treatment levels[53]. This is probably explained by the mechanism of action of RFA. In other words, this treatment method is based on the direct necrosis of the tumor tissue and has not as a clear antiangiogenetic effect as TACE. Therefore the impact of RFA on the levels of angiogenetic factors and tumor angiogenesis is not yet adequately delineated and should be investigated in future studies.
OSTEOPONTIN
OPN is an integrin-binding glycophosphoprotein with an important role in bone metabolism, immune responses and vascular remodeling. It is produced by various tissues including macrophages, activated lymphocytes and Kupffer cells and is considered to have a cytokine’s action[54,55]. Additionally, OPN due to its immunogenic function is involved in the pathogenesis of alcoholic and nonalcoholic liver disease and T cell mediated hepatitis. Its expression has been found to be up regulated in HCC tumors and especially in metastatic HCC tumors. As a result, OPN has been associated with advanced disease, portal vein and lymph node invasion and early metastasis[55]. However, its value as a prognostic HCC biomarker is not yet proven by a large study in broader HCC populations. The above OPN functions though, could explain the fact that low baseline OPN levels and their decrease (> 10%) 4 wk after TACE, have been correlated to better response to treatment and better cumulative survival[25]. Nevertheless, when evaluated in a multivariative analysis, this relationship was not statistically significant[25].
IMMUNE RESPONSE CIRCULATING PARAMETERS
Locoregional therapies for HCC cause tumoral necrosis resulting in an immunomodulatory effect. Necrotic cell death provides a source of antigens that stimulate a strong immune response firstly mediated by antigen presenting cells[56].
The outcome of TACE has been associated to the blood neutrophil-to-lymphocyte ratio (NLR), a marker of immune activity often up regulated in patients with HCC[25,57]. In a recent study 42 patients with an elevated pre-treatment NLR (> 1.85) had a median survival of 8 mo while 136 patients with normal NLR had a median survival of 17.5 mo (P < 0.001)[58]. In addition, the down regulation of NLR after TACE is indicative of higher overall survival and thus an independent prognostic factor with possible clinical value (P = 0.006)[59]. TACE may also influence the levels of sRAGE, a biomarker still under investigation, related to immunogenic cell death with a possible role in stimulation of immune response and angiogenesis[60]. Patients that exhibited higher levels of sRAGE before and 24 h after treatment had a better treatment response[26]. Moreover, TACE induces a specific CD4+ T cell response targeting the tumor tissue. It is possible that the acute inflammation caused by the necrosis of the tumor sensitizes the previously tolerant immune system and promotes the activation of AFP- specific CD4+ T Cells[22]. These cells through the production of interferon-gamma (IFN-γ) further promote the destruction of tumor cells by cytotoxic CD8+ T cells[22]. Finally, according to a recent paper, higher levels of Th17 cells 30 d post-TACE were found to be significantly associated with elevated overall survival (P = 0.007)[23]. Th17 cells through the production of interleukin-17 (IL-17) are responsible for the accumulation of neutrophils after acute tissue injury[24]. Consequently it is possible that TACE due to its hypoxic effect on HCC tissue promotes the activation of Th17 cells resulting it the recruitment of neutrophils in the damaged area.
RFA causes direct destruction of the targeted HCC lesion, resulting in the induction of acute inflammation and extended immune response. It has been shown in vitro that the ablated malignant tissue promotes intensely the maturation of antigen presenting cells in comparison to the non-ablated malignant tissue or normal liver tissue. This possibly happens due to the release of previously “hidden” intracellular antigens of malignant cells[61]. Additionally, the dendritic cells (DCs) that were activated in the presence of ablated HCC tissue produce higher amounts of IL-12, a cytokine promoting T helper 1 cells responses, while DCs activated by non-ablated HCC tissue produce mainly IL-10 resulting in the induction of T helper 2 cells responses[61]. The post-RFA activated DCs were also found to secrete high amounts of IL-1 and tumor necrosis factor-a[61]. In another study, RFA caused a significant 5-6 fold rise (P < 0.0001) of HCC specific CD4+ T cells, cytotoxic T cells and IFN-γ 8 wk after treatment[62]. Interestingly, this strong anti-tumor T cell immune response has been proven to be mediated mainly by CD4+ T cells. However, the pool of circulating lymphocytes when measured 1 mo after RFA, presented an elevation of CD56 differentiation antigens of T cells and natural killer cells. In other words, RFA caused the rise of circulating effector cytotoxic cells. Despite this fact, the tumor recurrence rate was not correlated to the extent of the immune response. In fact, the antigens extracted from the recurrent tumor tissue did not initiate an intense response of DCs and T cells that was produced by the ablated tumor tissue[62]. The effect of RFA on the levels of cytotoxic T cells was further confirmed recently, as 5 out of 9 patients presented with elevated glypican-3 specific cytotoxic T cells after treatment[63]. On the contrary, only 1 of 9 patients treated with surgical resection had increased levels of this specific type of cells[63]. Moreover, the number of tumor-associated antigen - specific T cells produced after RFA has been correlated to HCC recurrence rates[64].
Additionally, a recent study focusing on the role of CD4+CD25+Foxp3+ Treg cells in the prognosis of HCC after cryoablation has produced interesting results[65]. This type of cells is known to affect the immune response against malignant cells through the production of specific cytokines such as transforming growth factor-β or by direct cell contact. The above functions result in the suppression of APCs maturation and T cell differentiation and the apoptosis of effector cells[66]. According to the study, patients with higher levels of circulating CD4+CD25+FoxP3+ Treg had significantly higher rates of reccurence after cryoablation (P = 0.026). Moreover, among 31 patients subjected to cryoablation, those who presented with tumor progression during the follow-up period (12-48 wk after treatment), had elevated Treg frequency. In fact, the Treg cells isolated from 6 patients with recurrent HCC had increased immunosuppressive effect against PBMCs isolated from healthy controls. On the contrary, Treg cells extracted from 6 patients with good tumor response did not have such a strong immunosuppressive effect[65].
Finally, as suggested by studies on animal models and patients with HCC as well, RFA enhances the production of heat shock proteins such as heat shock protein 70 (HSP70)[67,68]. In HCC tissue extracted immediately before and 24 h after RFA the expression of HSP70 and HSP90 was found to be increased 8-fold and 1.2-fold respectively[27]. In a study with a limited number of patients with liver, kidney or lung malignancies, the levels of serum HSP70 were significantly higher 1 d after RFA (paired t test, P = 0.001)[28]. Moreover the patients with the higher increase tended to have lower recurrence rates than those without detectable increase post-RFA[28]. Heat shock proteins are thought to play an important role in the activation of dendritic cells and may be the local stimuli for the strong immune response taking part after RFA[69].
All the above mechanisms could be the basis of new treatment strategies combining immunotherapy with locoregional therapies in order to enhance the therapeutic effect of these techniques.
CELL DEATH PARAMETERS
As mentioned above, due to the induction of tumor necrosis by local therapies, a number of cell death products are released into the circulation directly after treatment[61]. Some of these products could be put under investigation in order to acquire useful markers for the evaluation of early treatment response.
Recently, the kinetics of serum cell death products, such as nucleosomes, cytokeratine-19 fragments (CYFRA 21-1) and lactate dehydrogenase (LDH) pre- and post-TACE, have been studied along with their correlation with treatment response[70]. The results showed that all three parameters were increased 24 h after TACE. However, the levels of nucleosomes were the only marker that was significantly different between the group of responders and the group of non-responders (P < 0.001)[70]. In other words, higher percentage changes between the baseline levels and the levels of serum nucleosomes 24 h post-treatment were correlated with disease progression. Interestingly, in the multivariative analysis the combination of nucleosomes (24 h), alkaline phosphatase (24 h) and number of TACE was the best prognostic model for treatment response[70]. LDH is considered to be another potential prognostic marker for patients treated with TACE. It has been shown that patients with increased post-treatment LDH values had lower disease free and overall survival in comparison to patients with decreased values within 1 mo after TACE[71]. This difference was found to be statistically significant both for disease free and overall survival (P < 0.0087 and P < 0.0001 respectively)[71]. Nevertheless, due to the limited patient number in this study, the exploitation of cell death markers needs to be extensively studied by further clinical trials.
DISCUSSION
In comparison to other types of cancer, HCC is characterized by the ability to produce a significant variety of potential biomarkers. This aspect is an important advantage for the amelioration of the existing diagnostic and prognostic tools as well as the development of new ones. As mentioned above, the immediate estimation of treatment response and disease prognosis plays a pivotal role for the clinical outcome of patients treated with locoregional therapies, due to the high rates of recurrence. Towards this direction, the evaluation of circulating biomarkers’ values after TACE or RFA may reflect the tumor behavior and the possibility of disease progression. According to the available studies, there is a correlation between the changes of classical or newer biomarkers such as AFP, AFP-L3 or DCP and the patients’ survival rates. Although each of the above biomarkers is not established as a separate, useful diagnostic or prognostic tool, their combination in a prognostic model could prove beneficial. Moreover, since angiogenesis plays a key role in the pathophysiology of HCC, the response of angiogenetic factors’ levels to therapy could be indicative of treatment efficacy and future outcome. This is of great importance especially for TACE, a therapeutic technique with anti-angiogenetic mechanism of action. Another interesting development is the measurement of cell death products caused by the destruction of the malignant tissue. The extent of tumor necrosis is indicative of treatment efficiency. Therefore, the quantification of tumor necrosis with the use of circulating cell death parameters provides a direct measure of treatment outcome even more specific than mRECIST criteria. Finally, the unique ability of locoregional therapies to induce a major immune response could be also exploited either by associating the intensity of specific HCC-targeting cell production with treatment outcome or by the implementation of a new combination of treatment strategies.
CONCLUSION
As locoregional therapies are currently the most common treatment choice for patients with early or intermediate stage of HCC, the discovery of prognostic models for patients stratification is undoubtedly of great importance. Apart from the above biomarkers, there is a need for new molecular parameters that could enhance the understanding of HCC behavior and susceptibility to different therapeutic techniques. In other words, the identification of more specific molecular markers for HCC may permit the generation of specific HCC molecular profiles resulting in more targeted treatment strategies or perhaps new combinations of them. In order to achieve this, a number of clinical trials should be conducted in the future.
P- Reviewer: Cao GW, Razek A, Tarazov PG S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ