Published online Jul 8, 2015. doi: 10.4254/wjh.v7.i13.1797
Peer-review started: March 17, 2015
First decision: April 10, 2015
Revised: May 3, 2015
Accepted: June 9, 2015
Article in press: June 11, 2015
Published online: July 8, 2015
Processing time: 115 Days and 15.8 Hours
AIM: To assess the effectiveness of transjugular intrahepatic portosystemic stent shunt (TIPSS) in refractory hepatic hydrothorax (RHH) in a systematic review and cumulative meta-analysis.
METHODS: A comprehensive literature search was conducted on MEDLINE, EMBASE, and PubMed covering the period from January 1970 to August 2014. Two authors independently selected and abstracted data from eligible studies. Data were summarized using a random-effects model. Heterogeneity was assessed using the I2 test.
RESULTS: Six studies involving a total of 198 patients were included in the analysis. The mean (SD) age of patients was 56 (1.8) years. Most patients (56.9%) had Child-Turcott-Pugh class C disease. The mean duration of follow-up was 10 mo (range, 5.7-16 mo). Response to TIPSS was complete in 55.8% (95%CI: 44.7%-66.9%), partial in 17.6% (95%CI: 10.9%-24.2%), and absent in 21.2% (95%CI: 14.2%-28.3%). The mean change in hepatic venous pressure gradient post-TIPSS was 12.7 mmHg. The incidence of TIPSS-related encephalopathy was 11.7% (95%CI: 6.3%-17.2%), and the 45-d mortality was 17.7% (95%CI: 11.34%-24.13%).
CONCLUSION: TIPSS is associated with a clinically relevant response in RHH. TIPSS should be considered early in these patients, given its poor prognosis.
Core tip: Evidence on the effectiveness of transjugular intrahepatic portosystemic stent shunt (TIPSS) in patients with refractory hepatic hydrothorax (RHH) is scarce and variable. This paper summarizes available data on the effectiveness of TIPSS in RHH in a cumulative meta-analysis. The sum total of the evidence shows that TIPSS is associated with a clinically relevant response in three-quarters of patients with medically RHH. We suggest that TIPSS be considered early in patients with RHH, given its impact on quality of life and prognosis. However, caution should be exercised in older patients and those with severe underlying liver or renal dysfunction.
- Citation: Ditah IC, Al Bawardy BF, Saberi B, Ditah C, Kamath PS. Transjugular intrahepatic portosystemic stent shunt for medically refractory hepatic hydrothorax: A systematic review and cumulative meta-analysis. World J Hepatol 2015; 7(13): 1797-1806
- URL: https://www.wjgnet.com/1948-5182/full/v7/i13/1797.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i13.1797
Hepatic hydrothorax (HH) is the accumulation of significant pleural effusion, usually in excess of 500 mL, in a patient with cirrhosis without coexisting primary cardiopulmonary disease[1-3]. It is a relatively uncommon complication of end-stage liver disease, with an estimated prevalence among cirrhotic patients of 5% to 10%[1,3-5]. Although the exact mechanisms involved in the development of HH have not been completely elucidated, the most widely accepted mechanism is the passage of fluid from the peritoneal to the pleural cavity through diaphragmatic defects, usually less than 1 cm in diameter[6-9]. The one way flow of the ascitic fluid into the pleural cavity is also thought to be influenced by the negative intrathoracic pressure. The effusion, typically a transudate, most commonly occurs in the right hemithorax (85%)[3,10]. Ascites can be absent in up to 20% of patients with HH[11-13]. A diagnostic thoracentesis often confirms diagnosis and excludes infection.
The initial management of HH is similar to that for ascites. Maximal sodium restriction (< 70-90 mmol/d) and optimal tolerated diuretics are the first-line therapy. Therapeutic thoracentesis is a safe and effective way to rapidly relieve symptoms of dyspnea in patients with large effusions (1.5-2.0 L)[5]. However, when thoracentesis is required more than once every 2 to 3 wk in patients on maximal sodium restriction and optimal diuretics, it is considered refractory, and alternative treatments should be considered. Pleurodesis and peritoneovenous shunts are surgical options that are usually associated with rapid fluid reaccumulation and procedure-related complications, and they are not generally recommended as treatments for HH[14,15]. In the absence of a large pneumothorax, hemothorax, or frank empyema, a chest tube should not be inserted in patients with HH[16,17].
Up to 25% of patients with HH will become refractory to treatment[18], compared to only 10%[17] of patients with cirrhotic ascites. Refractory HH (RHH) has traditionally been associated with poor prognosis. Patients with RHH should therefore be considered for liver transplantation. The treatment strategies for RHH are similar but not identical to those for refractory ascites. In patients with prerenal azotemia, therapeutic thoracentesis as a long-term regular treatment is not recommended because of the risk for bleeding and pneumothorax[6]. Transjugular intrahepatic portosystemic stent shunt (TIPSS) is a nonsurgical, angiographic technique of reducing hepatic sinusoidal pressure, which then results in a reduction in the accumulation of fluid in the peritoneal and pleural space. The procedure is often used as a bridge to liver transplantation in patients with end-stage liver disease. Since RHH is an uncommon complication of cirrhosis, most of the studies on the effectiveness of TIPSS have been limited to small numbers of patients, primarily in the form of case reports[19-22] or case series[3,14,23-28]. Findings from these studies have varied substantially. The purpose of this study was to evaluate the effectiveness of TIPSS in patients with RHH by pooling all available evidence in a systematic review with cumulative meta-analysis.
A comprehensive literature search was conducted using Ovid on MEDLINE and EMBASE, PubMed Cochrane Library, and the Web of Science for the period from January 1970 to August 2014. The search terms included, in different combinations: “portosystemic shunt”, “transjugular intrahepatic stent shunt”, “liver cirrhosis or end-stage liver disease”, “hydrothorax”, “pleural effusion” and “ascites”. The search was limited to studies in humans published in English. References of articles meeting inclusion criteria and review articles on the subject were manually searched for other relevant studies that might have been missed.
The selection criteria were studies in: (1) patients with cirrhosis irrespective of etiology; (2) patients with medically RHH with or without ascites; and (3) series that included at least 10 patients. Case reports or series with fewer than 10 patients were excluded. Two reviewers (ICD and BFAB) independently screened article titles and abstracts for selection. Once unrelated articles were excluded, each eligible article was then reviewed in full.
Data were abstracted by the same 2 investigators onto standardized paper forms and entered into an Excel spreadsheet (Microsoft Corp, Redmond, Washington). The following information were abstracted from each study: author, time period of study, study methods and participants, outcome of interest [mortality/survival, response to TIPSS, TIPSS-related complications, incidence of hepatic encephalopathy (HE), mean change in hepatic venous pressure gradient (HVPG), and country of study]. Differences between the 2 abstracting investigators were settled by reviewing the article together and seeking an independent input from a third investigator (BS).
Medically RHH: Patients with underlying liver cirrhosis who underwent TIPSS because of symptomatic HH that had failed to respond to sodium (< 2 g/d) restriction, who had optimal diuretics dosing (maximal tolerated doses without electrolyte abnormalities or clinically significant side effects), and who required frequent (more than once every 2-3 wk) thoracentesis were classified as having medically RHH.
Response to TIPSS was based on clinical or radiographic evidence of hydrothorax post-TIPSS. Response was categorized as complete, partial, or absent. Response was classified as complete if the patients’ symptoms of shortness of breath resolved or returned to baseline, with no evidence of pleural effusion requiring thoracentesis. Partial response was defined as improvement of shortness of breath but without complete symptomatic resolution; thoracentesis was required less frequently than pre-TIPSS. Absent response was defined as persistent or worsening symptoms of shortness of breath and/or persistent need for thoracentesis. Radiologically, complete response was defined as undetectable pleural effusion on chest radiographs, computed tomogram, or ultrasonogram; partial response if pleural effusion decreased compared to pre-TIPSS; and absent response if pleural effusion was unchanged or increased. The studies used either radiologic and/or clinical criteria to assess response to TIPSS.
TIPSS-related complications: (1) HE. TIPSS related HE was defined as new onset (i.e., never existed prior to TIPSS) or worsening (increased in frequency or severity of encephalopathy, compared to pre-TIPSS status). One study considered HE as TIPSS related if it occurred within 30 d of the procedure[24]; and (2) Mortality After TIPSS. Death was evaluated as early (i.e., occurred within 45 d of the procedure) and overall (death irrespective of when the event occurred throughout the follow-up period). The follow-up period varied across the studies, with the longest duration being 5 years.
Data from eligible studies were pooled using a random-effects model with Stata version 11 (Stata Corp LP, College Station, Texas). Outcomes are expressed as proportions (percentages) with 95%CIs. The pooled analyses are presented as forest plots. Since there were only 6 eligible studies, we determined a priori that subgroup analyses would not be performed. Statistical heterogeneity between studies was assessed using the Cochran Q test and the I2 statistic. An I2 value of greater than 50% or a P value of less than 0.05 for the Q statistic was taken to indicate significant heterogeneity. All analyses were performed in accordance with the Meta-analysis of Observational Studies in Epidemiology guidelines (Table 1)[29]. Since this was a cumulative meta-analysis, publication bias was not assessed.
| MOOSE criteriaa | Met (yes/no) |
| Reporting background should include | |
| Problem definition | Yes |
| Hypothesis statement | No |
| Description of study outcome(s) | Yes |
| Type of exposure or intervention used | Yes |
| Type of study designs used | Yes |
| Study population | Yes |
| Reporting of search strategy should include | |
| Qualifications of searchers (e.g., librarians and investigators) | Yes |
| Search strategy, including time period included in the synthesis and keywords | Yes |
| Effort to include all available studies, including contact with authors | Yes |
| Databases and registries searched | Yes |
| Search software used, name and version, including special features used (e.g., explosion) | Yes |
| Use of hand searching (e.g., reference lists of obtained articles) | Yes |
| List of citations located and those excluded, including justification | Yes |
| Method of addressing articles published in languages other than English | Yes |
| Method of handling abstracts and unpublished studies | No |
| Description of any contact with authors | No |
| Reporting methods should include | |
| Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested | Yes |
| Rationale for the selection and coding of data (e.g., sound clinical principles or convenience) | Yes |
| Documentation of how data were classified and coded (e.g., multiple raters, blinding, and interrater reliability) | Yes |
| Assessment of confounding (e.g., comparability of cases and controls in studies where appropriate) | No |
| Assessment of study quality, including blinding of quality assessors; stratification or regression on possible predictors of study results | Yes |
| Assessment of heterogeneity | Yes |
| Description of statistical methods (e.g., complete description of fixed or random effects models, justification of whether the chosen models account for predictors of study results, dose-response models, or cumulative meta-analysis) in sufficient detail to be replicated | Yes |
| Provision of appropriate tables and graphics | Yes |
| Reporting of results should include | |
| Graphic summarizing individual study estimates and overall estimate | Yes |
| Table giving descriptive information for each study included | Yes |
| Results of sensitivity testing (e.g., subgroup analysis) | No |
| Indication of statistical uncertainty of findings | Yes |
| Reporting of discussion should include | |
| Quantitative assessment of bias (e.g., publication bias) | NA |
| Justification for exclusion (e.g., exclusion of non-English-language citations) | Yes |
| Assessment of quality of included studies | Yes |
| Reporting of conclusions should include | |
| Consideration of alternative explanations for observed results | Yes |
| Generalization of the conclusions (e.g., appropriate for the data presented and within the domain of the literature review) | Yes |
| Guidelines for future research | Yes |
| Disclosure of funding source | Yes |
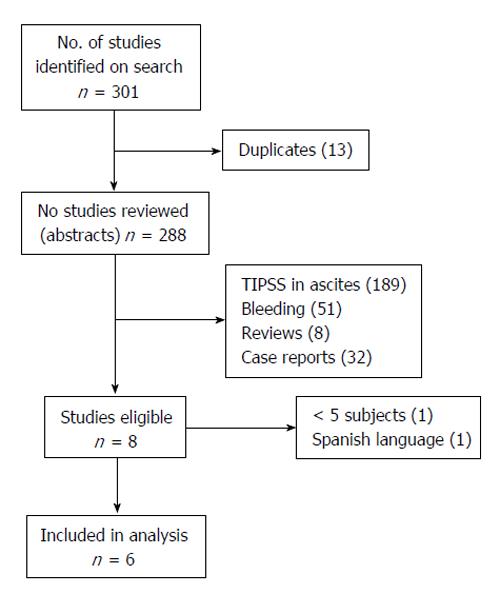
Six studies involving a total of 198 patients were included in the analyses. Two studies were excluded because each had a small number of study subjects and were judged by 2 of the reviewing authors to be of poor quality[3,25]. Figure 1 summarizes the results of the literature search, including the reasons for the exclusion of studies, and Table 2 summarizes the characteristics of the 6 studies that were included in the analysis.
| Ref. | Methods and patients | Outcomes/complications | Remarks |
| Gordon et al[14] | Retrospective chart review of 24 consecutive patients with medically RHH | Post-TIPSS response was categorized as complete, partial, or absent | 11 patients had variceal bleeding > 4 wk before TIPSS |
| Post-TIPSS patients underwent Doppler US studies every 3 to 6 mo | Mean change in HVPG | Stent revision if decreased flow noted | |
| Mean follow-up was 7.2 mo (range, 0.25-49.0 mo) | TIPSS patency was assessed by change in CTP score, survival, and new or worsened HE | 5 failures were CTP C | |
| Patients with infection were excluded | 12 patients had medically RHH; the rest of the 9 patients had TIPSS and RHH as a secondary indication with the primary indication being intractable ascites (n = 7) and gastric varices (n = 2) | ||
| Jeffries et al[24] | Retrospective chart review of 12 consecutive patients with medically RHH | Post-TIPSS response at ≤ 1 or > 1 mowas categorized as complete, partial, or absent | Immediate pre- and post-TIPSS prophylactic antibiotics given |
| Post-TIPSS, patients had Doppler US studies every 3 mo | TIPSS-related complications: ≤ 30 and > 30 d | Shunt thrombosis or decreased velocities requiredangioplastic revision | |
| Mean follow-up was 173 d (range, 7-926 d) | New-onset or worsened HE survival | 4 patients had shunt revisions | |
| Patients with heart failure, HCC, alcoholic hepatitis, or intrinsic renal disease were excluded | Mean change in HVPG | Patients who died or underwent transplant ≤ 30 d after TIPSS were classified as nonresponders to TIPSS | |
| Siegerstetter et al[26] | Retrospective chart review of 40 consecutive patients with medically RHH | Post-TIPSS response was categorized as complete, partial, or absent | 8 patients had no ascites; RHH was diagnosed by intraperitoneal methylene blue injection or technetium-Tc-99 |
| Post-TIPSS, patients had Doppler US studies at 4 wk, then every 3 mo | Predictors of survival: | 2 stent size reductions due to chronic HE | |
| Mean (SD) follow-up was 14 mo | Mean change in HVPG | ||
| [14 (range, 1-54 mo)] | New-onset or worsened HE | ||
| Patients with infection were excluded | CTP score improvement | ||
| Survival at 1 yr | |||
| Spencer et al[27] | Retrospective chart review of 21 consecutive patients with medically RHH | 30-d mortality | Prophylactic antibiotics administered |
| Post-TIPSS, patients had Doppler US studies at 1, 3, and 6 mo, then every 6 mo | Post-TIPSS complications: Early ( ≤ 30 d) or late(> 30 d) | Radiographic and clinical response | |
| Mean follow-up was 223 d | New-onset or worsened HE | TIPSS placement 100% successful | |
| Patients with severe right-sided heart failure and patients with PVT with cavernous transformation were excluded | Post-TIPSS response was categorized as complete, partial, or absent | 1 patient with a partial response was weaned off oxygen due to decreased pleural fluid | |
| Mean change in HVPG | |||
| Cumulative survival | |||
| Wilputte et al[28] | Retrospective chart review of 28 consecutive patients with medically RHH | Mean change in HVPG | Stent revised for stenosis, obstruction, or relapsing RHH |
| Post-TIPSS, patients had Doppler US at 24 h and at 1, 2, 3, 6, 9, and 12 mo, then every 6 mo | 30-d mortality post-TIPSS | Patients who underwent transplant were censored at surgery date | |
| Mean (SD) follow-up was 358 d (121 d); 3 patients were excluded due to grade 3 HE, HCC, cardiopulmonary disease, and infection | Response to TIPSS was categorized as complete, partial, and absent | 6 patients required TIPSS revision | |
| 2 patients had TIPSS reduction due to intractable HE | |||
| Both covered and uncovered stents were used | |||
| Dhanasekaran et al[23] | Retrospective chart review of 73 consecutive patients with medically RHH | Post-TIPSS response at 1 mo and 6 mo was categorized as complete, partial, or absent | TIPSS catheterization used if stenosis suspected or RHH reaccumulated |
| Patients had Doppler US every 3 mo for 12 mo, then annually | Evaluated predictors of response to TIPSS | Angioplasty performed, if needed | |
| Patients with heart failure, pulmonary disease, infection, severe HE, portal vein thrombosis, and multiple hepatic cysts were excluded | Assessed for new or worsening HE | Uncovered and covered stents used | |
| Mean change in HVPG | |||
| Overall and 30-d mortality |
The mean (SD) age of the 198 patients was 56 years (1.8 years) and 52% were male. The majority of patients had Child class C disease (56.9%), while 40.7% and 0.8% were Child class B and A, respectively. The mean pre- and post-TIPSS HVPG values were 20.14 mmHg (range, 17.4-26.0 mmHg) and 7.37 mmHg (range, 5.7-10.0 mmHg), respectively. The mean duration of follow-up was 10 mo (5.7-16.0 mo). Table 3 shows the results of the various outcomes of the individual studies.
| Ref. | No. of patients | Complete response (%) | Partial response (%) | 45-d mortality (%) | 1-yr survival (%) | Predictors of mortality |
| Gordon et al[14] | 24 | 58 | 21 | 21 | NA | TIPSS nonresponse |
| CTP class C | ||||||
| Jeffries et al[24] | 12 | 42 | 17 | 25 | NA | Age > 65 yr |
| Siegerstetter et al[26] | 40 | 53 | 28 | 13 | 64 | Age > 60 yr |
| Spencer et al[27] | 21 | 57 | 10 | 29 | NA | Medical comorbidities |
| Wilputte et al[28] | 28 | 57 | 11 | 14 | 41 | CTP score > 10 |
| Mayo score > 1.5 | ||||||
| Dhanasekaran | 73 | 59 | 21 | 19 | 48 | MELD > 15 |
| et al[23] | Nonresponse | |||||
| Elevated creatinine |
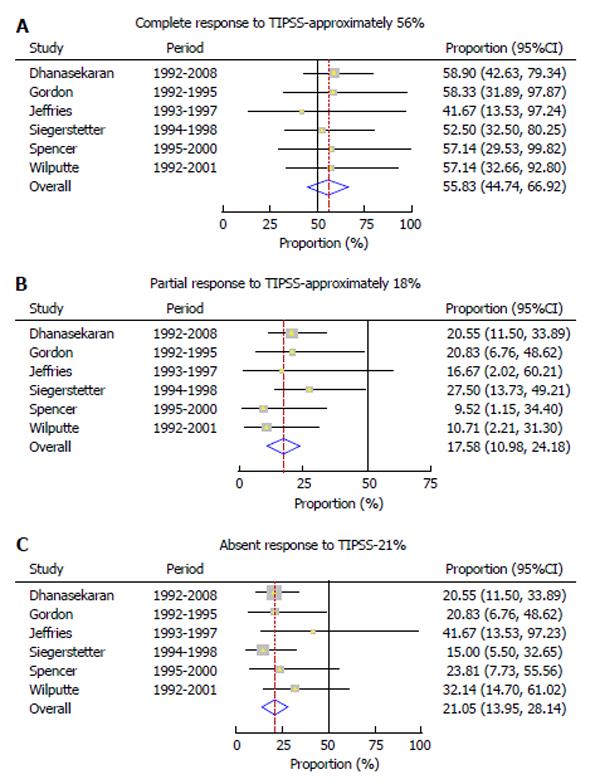
Response to TIPSS was complete in 55.8% (95%CI: 44.7%-66.9%) (Figure 2A) and partial in 17.6% (95%CI: 10.9%-24.2%) of patients (Figure 2B). There was absent response in 21.2% (95%CI: 14.2%-28.3%) of the patients (Figure 2C). There was no evidence of heterogeneity among the 6 studies (P = 0.99, P = 0.65, and P = 0.76) respectively.
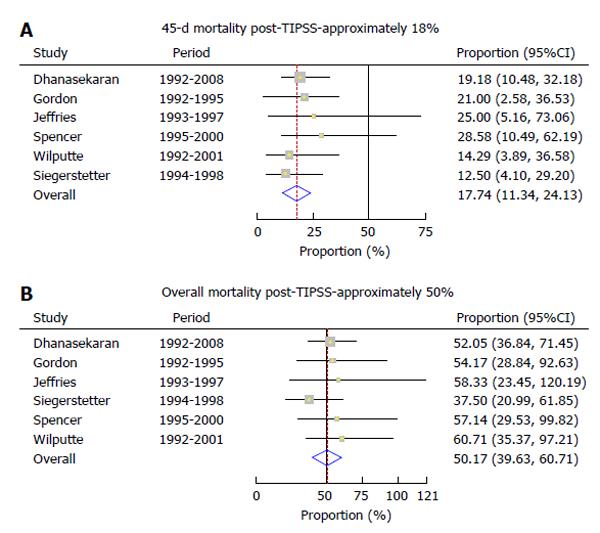
Mortality within 45 d (early mortality) of TIPSS placement was 17.74 (95%CI: 11.34%-24.13%) (Figure 3A), while the overall mortality post-TIPSS was 50.17% (95%CI: 39.63%-60.71%) (Figure 3B). Predictors of mortality included older age, severity of liver disease, elevated creatinine and nonresponse to TIPSS. There was no evidence of heterogeneity among the studies (P = 0.86 and P = 0.81, respectively).
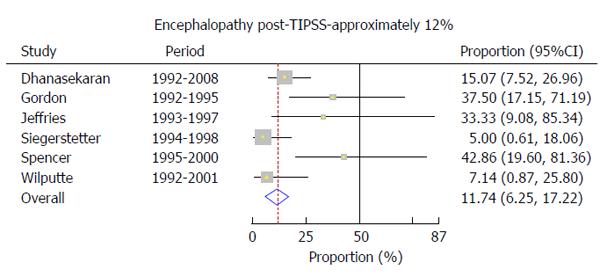
The incidence of post-TIPSS encephalopathy was 11.7% (95%CI: 6.3%-17.2%) (Figure 4). On this outcome, however, there was evidence of significant heterogeneity among the studies (P = 0.04).
This study shows that TIPSS relieves symptoms in close to three-fourths (73%) of patients with RHH. The 45-d mortality and the 1-year survival in patients with RHH are comparable to those seen in patients with refractory ascites and variceal hemorrhage. The most important predictors of poor outcomes after TIPSS for RHH include older age and severe underlying liver disease and/or associated renal dysfunction.
HH remains a rare complication of liver cirrhosis, with limited therapeutic options. When symptomatic HH fails to respond to medical treatment, repeat thoracentesis is often undertaken. Although thoracentesis is less invasive than TIPSS and is effective in quickly relieving symptoms of dyspnea, it can be associated with complications such as re-expansion pulmonary edema, pneumothorax, and empyema[5,30]. Repeated thoracentesis is also associated with deteriorating clinical status and poor quality of life[1,6]. TIPSS is a nonsurgical approach that decompresses the portal system, thereby addressing the mechanism of fluid collection in the abdomen and/or chest[31]. TIPSS is superior to other treatment modalities in the prevention of rebleeding from varices, and its control of refractory ascites has been well studied in controlled trials[32-36]. In contrast, controlled studies on its use in patients with RHH are lacking, and comparative studies with other treatment options may not be feasible[37,38]. Consequently, evidence on the effectiveness of TIPSS in RHH has been limited to case series with often small numbers of study participants. Results from the 6 studies included in this pooled analysis found a wide range of responses and complication rates, perhaps due to the lack of statistical power. In this study, we combined data from all the small studies, which allowed us to provide the best evidence on TIPSS effectiveness in RHH.
One-fifth of the patients died in the first 45 d after TIPSS placement. This number is well within the range for mortality following TIPSS use in patients with refractory ascites and variceal bleeding[39-44]. Early mortality was observed in patients who developed progressive liver failure, sepsis, renal failure, bleeding, cardiac complications, and pulmonary complications. Pre-TIPSS factors associated with post-TIPSS mortality included older age, severe liver disease as measured by the Child-Turcott-Pugh score, and renal dysfunction. Ideally, patients with a high likelihood of decompensation after TIPSS should also initiate evaluation for liver transplantation, with TIPSS serving only as a bridge. In a meta-analysis of individual patient data, Salerno et al[45] also found that a model composed of age (< 60 years), bilirubin (< 3 mg/dL), and sodium level reliably predicted successful outcomes after TIPSS placement in patients with refractory ascites.
Another important outcome of this study was estimating the incidence of TIPSS-related HE. HE has been shown to predict mortality after TIPSS placement, with survival decreasing from 8 years to about 2 years[46]. The overall incidence of TIPSS-related HE was noted to be 12%. This rate falls within the rate of HE observed with TIPSS for established indications[34,35,47]. The heterogeneity noted between the studies on HE incidence highlights the fact that its diagnosis is subjective.
These results should be interpreted bearing in mind the following: First, this summative analysis was based purely on the published medical literature. We did not have access to individual patient data, which could have allowed us to perform more detailed analysis, especially on factors associated with response to TIPSS and survival (e.g., acute liver failure and procedure related complications). Second, contrary to the extensive literature on refractory ascites, there is a complete lack of controlled trials comparing TIPSS to other therapeutic options for RHH. Conducting a randomized controlled trial on RHH is not feasible because of its relative rarity, and a step-up approach in management is often preferred by clinicians. Most of the 6 studies did not have information on what type of stents were used. Dhanasekaran et al[23] compared patients with covered and uncovered stents in a subgroup analysis and found no significant difference in survival, although the patients with covered stents had longer patency rates. Perhaps the small number of patients with covered stents in that study led to the non-significant result. It has been reported that patients who receive covered stents have better outcomes than those who receive uncovered stents[48].
To our knowledge, this is the first ever pooled analysis on TIPSS in patients with RHH. By combining data from all available studies, we were able to present the best evidence on the effectiveness of TIPSS in RHH. We showed that TIPSS is a reasonable therapeutic option in patients with RHH. It is associated with a clinically relevant response in close to three-fourths of patients with RHH. The incidence of TIPSS-related complications in RHH is similar to that observed with other established indications for TIPSS. We suggest that TIPSS should be considered relatively early in patients with RHH, given their poor prognosis. However, caution should be exercised in older patients and in those with severe underlying liver or renal dysfunction.
Hepatic hydrothorax (HH) which is the accumulation of “ascitic fluid” in the pleural cavity is an uncommon complication of cirrhosis with poor prognosis. When HH fils to respond to traditional medical management (salt restriction and diuretics), it is referred to as refractory HH (RHH).
Therapeutic options for RHH are limited. Transjugular intrahepatic porto-systemic shunt (TIPSS) has been proposed as an option for RHH. Because HH is rare, studies on the effectiveness of TIPSS in RHH have been restricted to small numbers of patients and findings have varied substantially and are controversial.
The purpose of this study was to evaluate the effectiveness of TIPSS in patients with RHH by pooling all available evidence in a systematic review and cumulative meta-analysis. By combining data from all available studies, the authors generated enough statistical power to study the clinical effectiveness of TIPSS in RHH.
This study shows that TIPSS leads to a clinically relevant response in about three-fourths (73%) of patients with RHH. The 45-d mortality and the 1-year survival in patients with RHH are comparable to those seen in patients with refractory ascites and variceal hemorrhage. The most important predictors of poor outcomes after TIPSS for RHH include older age and severe underlying liver disease and/or associated renal dysfunction. The authors suggest that TIPSS should be considered early in patients with RHH.
HH is the accumulation of fluid in the pleural cavity in patients with cirrhosis. The most widely accepted mechanism for HH is the passage of fluid from the peritoneal to the pleural cavity through a diaphragmatic defect. When HH fails to respond to medical management including salt restriction and maximal tolerated diuretics, it is considered refractory. Transjugular intrahepatic porto-systemic shunt decompresses the portal system, thereby addressing the mechanism of fluid collection in the abdomen and/or chest.
The manuscript is very well written.
P- Reviewer: Chiu KW, Takaki A, Zhang XC S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Morrow CS, Kantor M, Armen RN. Hepatic hydrothorax. Ann Intern Med. 1958;49:193-203. [PubMed] |
| 3. | Strauss RM, Martin LG, Kaufman SL, Boyer TD. Transjugular intrahepatic portal systemic shunt for the management of symptomatic cirrhotic hydrothorax. Am J Gastroenterol. 1994;89:1520-1522. [PubMed] |
| 4. | Alberts WM, Salem AJ, Solomon DA, Boyce G. Hepatic hydrothorax. Cause and management. Arch Intern Med. 1991;151:2383-2388. [PubMed] |
| 5. | Xiol X, Guardiola J. Hepatic hydrothorax. Curr Opin Pulm Med. 1998;4:239-242. [PubMed] |
| 6. | Dumont AE, Mulholland JH. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med. 1960;263:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Johnston RF, Loo RV. Hepatic hydrothorax; studies to determine the source of the fluid and report of thirteen cases. Ann Intern Med. 1964;61:385-401. [PubMed] |
| 9. | Lieberman FL, Hidemura R, Peters RL, Reynolds TB. Pathogenesis and treatment of hydrothorax complicating cirrhosis with ascites. Ann Intern Med. 1966;64:341-351. [PubMed] |
| 10. | Alagiakrishnan K, Patel PJ. Left-sided hepatic hydrothorax with ascites. Int J Clin Pract. 1999;53:225-226. [PubMed] |
| 11. | Benet A, Vidal F, Toda R, Siurana R, De Virgala CM, Richart C. Diagnosis of hepatic hydrothorax in the absence of ascites by intraperitoneal injection of 99m-Tc-Fluor colloid. Postgrad Med J. 1992;68:153. [PubMed] |
| 12. | Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Rubinstein D, McInnes IE, Dudley FJ. Hepatic hydrothorax in the absence of clinical ascites: diagnosis and management. Gastroenterology. 1985;88:188-191. [PubMed] |
| 14. | Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, LoCicero J, Lewis WD, Jenkins RL, Trey C. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366-1369. [PubMed] |
| 15. | Ikard RW, Sawyers JL. Persistent hepatic hydrothorax after peritoneojugular shunt. Arch Surg. 1980;115:1125-1127. [PubMed] |
| 16. | Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3:582-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 519] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 18. | Krok KL, Cárdenas A. Hepatic hydrothorax. Semin Respir Crit Care Med. 2012;33:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Andrade RJ, Martin-Palanca A, Fraile JM, Alcantara R, Carmona C, Medina MC, Muñoz V, Melgarejo F. Transjugular intrahepatic portosystemic shunt for the management of hepatic hydrothorax in the absence of ascites. J Clin Gastroenterol. 1996;22:305-307. [PubMed] |
| 20. | Conklin LD, Estrera AL, Weiner MA, Reardon PR, Reardon MJ. Transjugular intrahepatic portosystemic shunt for recurrent hepatic hydrothorax. Ann Thorac Surg. 2000;69:609-611. [PubMed] |
| 21. | Degawa M, Hamasaki K, Yano K, Nakao K, Kato Y, Sakamoto I, Nakata K, Eguchi K. Refractory hepatic hydrothorax treated with transjugular intrahepatic portosystemic shunt. J Gastroenterol. 1999;34:128-131. [PubMed] |
| 22. | Kidokoro H, Kanazawa H, Nachi T, Narahara Y, Osada Y, Mamiya Y, Kimura Y, Taki Y, Atsukawa M, Nakatsuka Y. [A case of refractory hepatic hydrothorax successfully treated with transjugular intrahepatic portosystemic shunt]. Nihon Shokakibyo Gakkai Zasshi. 2003;100:707-712. [PubMed] |
| 23. | Dhanasekaran R, West JK, Gonzales PC, Subramanian R, Parekh S, Spivey JR, Martin LG, Kim HS. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Jeffries MA, Kazanjian S, Wilson M, Punch J, Fontana RJ. Transjugular intrahepatic portosystemic shunts and liver transplantation in patients with refractory hepatic hydrothorax. Liver Transpl Surg. 1998;4:416-423. [PubMed] |
| 25. | Núñez O, García A, Rincón D, Alonso S, Echenagusía A, Bañares R. [Percutaneous intrahepatic portosystemic shunting as a treatment for refractory hepatic hydrothorax]. Gastroenterol Hepatol. 2002;25:143-147. [PubMed] |
| 26. | Siegerstetter V, Deibert P, Ochs A, Olschewski M, Blum HE, Rössle M. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol. 2001;13:529-534. [PubMed] |
| 27. | Spencer EB, Cohen DT, Darcy MD. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Interv Radiol. 2002;13:385-390. [PubMed] |
| 28. | Wilputte JY, Goffette P, Zech F, Godoy-Gepert A, Geubel A. The outcome after transjugular intrahepatic portosystemic shunt (TIPS) for hepatic hydrothorax is closely related to liver dysfunction: a long-term study in 28 patients. Acta Gastroenterol Belg. 2007;70:6-10. [PubMed] |
| 29. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] |
| 30. | Alonso JC. Pleural effusion in liver disease. Semin Respir Crit Care Med. 2010;31:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Conn HO. Transjugular intrahepatic portal-systemic shunts: the state of the art. Hepatology. 1993;17:148-158. [PubMed] |
| 32. | Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 364] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Kravetz D. Prevention of recurrent esophageal variceal hemorrhage: review and current recommendations. J Clin Gastroenterol. 2007;41 Suppl 3:S318-S322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Lebrec D, Giuily N, Hadengue A, Vilgrain V, Moreau R, Poynard T, Gadano A, Lassen C, Benhamou JP, Erlinger S. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996;25:135-144. [PubMed] |
| 35. | Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, Nicolini A, Salvatori F. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Boyer TD. Transjugular intrahepatic portosystemic shunt: current status. Gastroenterology. 2003;124:1700-1710. [PubMed] |
| 38. | Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 39. | Chalasani N, Clark WS, Martin LG, Kamean J, Khan MA, Patel NH, Boyer TD. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118:138-144. [PubMed] |
| 40. | D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31:468-475. [PubMed] |
| 41. | Helton WS, Belshaw A, Althaus S, Park S, Coldwell D, Johansen K. Critical appraisal of the angiographic portacaval shunt (TIPS). Am J Surg. 1993;165:566-571. [PubMed] |
| 42. | Jalan R, Elton RA, Redhead DN, Finlayson ND, Hayes PC. Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent-shunt for variceal haemorrhage. J Hepatol. 1995;23:123-128. [PubMed] |
| 43. | Jalan R, Redhead DN, Hayes PC. Transjugular intrahepatic portasystemic stent-shunt in the treatment of variceal haemorrhage. Br J Surg. 1995;82:1158-1164. [PubMed] |
| 44. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 474] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 45. | Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825-834. [PubMed] |
| 46. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [PubMed] |
| 47. | Ginès P, Arroyo V, Vargas V, Planas R, Casafont F, Panés J, Hoyos M, Viladomiu L, Rimola A, Morillas R. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med. 1991;325:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 189] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Rössle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |