INTRODUCTION: A MULTIDISCIPLINARY FIELD OF INTEREST
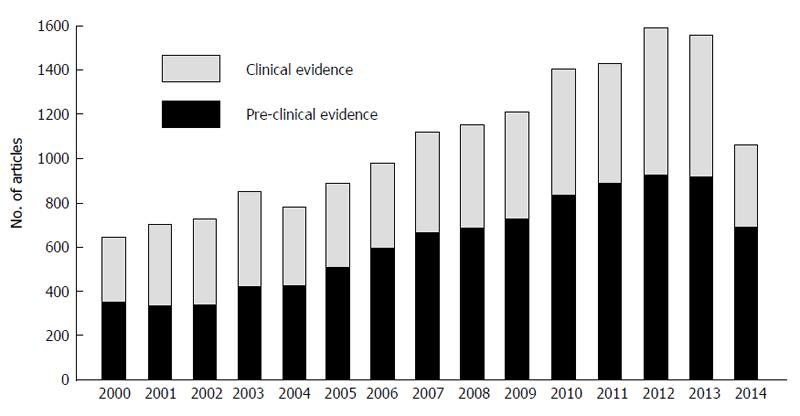
Drug- and herb-induced liver injury (DILI and HILI, respectively) continues to attract interest, as shown by the growing number of publications indexed in PubMed. A broad strategy (i.e., by combining the terms DILI, drug-induced liver injury/damage, HILI/damage, hepatotoxicity), from January 1st to December 31st 2014, yielded 1060 publications, with a mean publication rate of 1440 articles in the last 5-year period (search performed on Jan 13th, 2015) (Figure 1). A publication trend can be easily found in the last 15 years, with remarkable increase since 2010, especially for pre-clinical evidence; as compared to 2013, the apparent decrease in the overall number of publications in 2014 is likely to be related to the delay in publication indexing rather than to an actual decrease in publication rate.
Figure 1 Publication trend over the past 15 years of articles on drug- and herb-induced liver injury, classified in terms of preclinical and clinical evidence.
The search was performed in PubMed on January 13th, 2015, through automatic filters and keywords (see text for details).
The multidisciplinary interest is indicated by the variety of periodicals covering this topic in 2014: apart from dedicated high-ranking Journals (e.g., Seminars in Liver Disease, which entirely devoted an issue to hepatotoxicity, Gastroenterology, Hepatology, Journal of Hepatology and Clinical Gastroenterology and Hepatology), also non-specialized Journals published original research articles, expert opinion and comprehensive reviews for primary care clinicians, who frequently encounter this clinical problem in their daily practice[1,2]. The official Journal of the International Society of Pharmacovigilance Drug Safety published a supplement, called “Liver Safety Assessment in Clinical Drug Development: A Best Practices Workshop report”, describing major achievements and accomplishments for the future (see below for details)[3].
The multifaceted aspects of DILI and its idiosyncratic nature (i.e., unpredictable from the mechanism of drug action) pose a challenge to hepatologists, pharmacologists, toxicologists, clinical investigators and regulators.
From a drug development perspective, DILI caused a number of regulatory actions in the past decades. Very recently, Wang et al[4] reviewed formal reasons for non-approval of 27 drug applications and identified hepatotoxicity in 4 (15%) cases. The oral anticoagulant ximelgatran is a typical example of interruption of drug development for hepatic concern: in 2006, the manufacturer withdrew a pending application to the Food and Drug Administration (FDA). Shah[5] analyzed 38 drugs withdrawn between 1990 and 2006, and found that 14 of them (37%) were removed from the market due to hepatotoxicity. A more recent review highlighted that, among 25 safety-based withdrawals in Europe and United States, ten (40%) were related to cardiovascular issues and seven (28%) to gastrointestinal, primarily hepatic, adverse events, which were not predicted from known pharmacological action[6]. From a historical perspective, in several circumstances, hepatotoxic agents were identified after being used in clinical practice for several months: for instance, this was the case of troglitazone, which was withdrawn after more than 3 years on the United States market.
Form a clinical standpoint, the assessment of DILI risk in individual patients should be performed on a case-by-case basis according to different clinical elements (see below), whereas the evaluation of drug-related liver risk in a population perspective requires integration of data originated from multiple lines of evidence and data sources, including clinical trials, observational studies (cohort and case-control approaches), registries, spontaneous reporting systems, case series/reports[7].
We present this overview to highlight present trends and potential new areas of research: because of the large number of studies in the past year and the non-systematic nature of this review, we selected only articles that, in our opinion, provide key contributions to understand the way forward. After a brief description of key aspects to diagnose and manage drug-related liver disease, the next sections are organized by data source and mainly discuss novel agents associated with DILI in various settings. Specifically, the term “signal” will be used thereafter to indicate any new information/data regarding a possible drug-related association with liver damage (either clinical or statistical), which requires further investigation.
DILI AND HILI: CLUES FOR CLINICIANS
Epidemiology
The list of drugs that have been associated with hepatotoxicity is constantly growing[8]. A collaborative study published in 2010 collected information from different sources to select a unified list of drugs associated with DILI: among 385 agents, 319 compounds were identified in three DILI registries (Spain, Sweden and United States), with notable differences among the different cohorts, depending on the drug marketing access and prescribing patterns[9].
Determining the true incidence of DILI remains difficult. The recent population-based study from Iceland found an incidence of 19 cases per 100000 per year[10], higher than previous findings from France and United Kingdom: 13.9 and 2.4 per 100000, respectively[11,12]. The latest retrospective cohort study, using data from the Kaiser Permanente Northern California healthcare system, calculated an incidence rate of any definite drug-induced acute liver failure (ALF) of 1.61 events/1000000 person-years[13]. For a comprehensive discussion of the current status on epidemiology the reader should refer to Björnsson[14]; in summary, current data consistently identify particular drugs (e.g., amoxicillin-clavulanic acid, isoniazid) and confirms the two drug classes of antibiotics and antiepileptics as most prevalent in causing hepatotoxicity. Notably, recent population-based epidemiologic data on acute liver injury (ALI) found that incidence of ALI in pediatrics is relatively low and broadly comparable with adults, with higher incidence rate in Italy, as compared to the Netherlands (73 vs 21/100000 person-years, respectively) and antibiotics as the drugs most frequently implicated with ALI[15].
As regards HILI, the absence of regulatory guidelines further compromises calculation of true incidence. Notably, complementary and alternative medicines was one of the two most common etiologies reported among 24112 Chinese patients with DILI[16]. Current estimates suggest that 15% of DILI are caused by herbs and a recent tabular compilation of published case reports, including traditional Chinese medicines, established causality for 28 out of 57 different herbs and herbal mixture selected in 77 publications[17].
Risk factors and pathogenesis
The pathogenesis of DILI and HILI is only partially understood, with three intertwined factors: (1) Clinical host-related risk factors. Age and gender are perceived as non-modifiable risk factors[18]; recent studies highlighted age- and gender-related differences in the reporting of DILI that depend on drug and/or drug class (e.g., male were overrepresented in cases associated with antivirals for systemic use, whereas ALF and hepatocellular injury were more frequently reported among children)[19,20]. There is considerable body of evidence (through genome-wide association studies) indicating that susceptibility to DILI is genetically determined, at least for some compounds (e.g., flucloxacillin)[21]; (2) Environment. Our understanding of environmental factors is limited, with coffee, alcohol consumption, and diet that have not been identified as bona fide risk factors for DILI; and (3) Drug-related risk factors. Recent studies have suggested that drugs with high daily dose (> 50 to 100 mg/die), high lipophilicity (known as the “rule-of-two”) and extensive hepatic metabolism are more prone to cause DILI[22,23]. The so-called “damage hypothesis” regards the inadvertent generation of reactive metabolites or parent drug-protein complex that can directly or indirectly mediate intracellular damage via oxidative endoplasmic reticulum stress, mitochondrial damage, inhibition of bile salt export pump. In the “hapten hypothesis”, the drug-protein or metabolite-protein adduct leads to inadvertent activation of the adaptive immune system[24]. At the current state of the art, however, the actual clinical relevance of these pathophysiological mechanisms still requires formal evaluation.
Diagnosis
Patients with DILI pose substantial diagnostic, prognostic, and therapeutic challenges to the gastroenterologist[25]. The presentation of DILI may vary from asymptomatic liver enzyme elevation (which incidentally may come to the attention of clinicians during planned laboratory tests for other medical reasons) to ALF causing hospital admission and potentially requiring transplantation. The thresholds and cutoffs for enzymes elevation has been subject to debate and changes over time for a number of reasons. From one hand, the prevalence of non alcoholic fatty liver disease (NAFLD) is increasing and some subjects are known as “adaptors” (showing transient increase in enzyme levels, which eventually return to baseline despite continuation of the drug); on the other hand, it is crucial to identify early signals of DILI that are predictive of ALF during drug development[26]. Currently, a 3- to 5-fold elevation (x upper limit of normal) in alanine aminotransferase or aspartate aminotransferase represent the most commonly used thresholds. In most of the cases, DILI resolves following drug discontinuation, albeit up to 20% of patients progress to chronic liver damage further challenging the clinicians’ management skills. Although usually the first step in describing DILI is to differentiate “idiosyncratic” (unpredictable) from intrinsic (predictable) type, this distinction is highly debated and, more importantly, it does not affect clinical management. Therefore, diagnosis of DILI first and mostly depends on obtaining a detailed patient’s history and thoughtful use of diagnostic tests[25].
Overall, the clinical assessment focuses on four major areas: (1) timing (exposure or latency; recovery or dechallenge; information about the latest laboratory test before starting treatment can be of great value); (2) pattern of liver biochemistries at presentation (this aspect may influence the request for serological, imaging investigation and liver biopsy); (3) hepatotoxicity profile of suspect agent (some drugs such as telithromycin may have a distinctive clinical signature that may be indicative of high mortality rate[27]); (4) other extra-hepatic signs/symptoms (immune-allergic features such as rash, fever and eosinophilia argue for a drug etiology); and (5) exclusion of competing causes: in particular, acute viral hepatitis caused by less common viruses (type E hepatitis virus, cytomegalovirus, Epstein-Barr virus) and chronic liver diseases (e.g., NAFLD) should be ruled out. Judicious use of blood tests and liver imaging are necessary, but liver biopsy, while often helpful, is not mandatory.
Different clinical algorithms have been published to facilitate diagnosis and management; the reader can refer to the latest recommendations provided by the American College of Gastroenterology[28]. These diagnostic algorithms are based on clinical scoring systems. According to major experts in the field, the Council for International Organizations of Medical Sciences scale has the potential to be a standard scale for DILI and HILI causality assessment and can be adopted by physicians, regulatory agencies, expert panels and the scientific community. Other advantages include its liver specificity and its validation for hepatotoxicity cases, with excellent sensitivity, specificity and predictive validity based on results obtained from cases with a positive re-exposure test[29]. In case of suspect, instead of checking published case reports (which are of varying quality), clinicians should refer to the LiverTox database (http://www.livertox.nih.gov/), a free periodically updated online DILI resource detailing information on more than 600 agents. It should be kept in mind that, causality assessment and actual diagnosis is based on a case-by-case clinical judgment and, in doubtful cases, expert consultation is needed[28,30].
Two clinical issues are particularly challenging for gastroenterologists: HILI and drug-induced autoimmune liver disease. The former may have similar or identical clinical presentation of DILI, raising debate on whether or not HILI needs a separate term. However, major differences exist between DILI and HILI: DILI is usually caused by a single drug (either chemical or biological), whereas HILI is triggered by a chemical mixture from the herbal extract, which often lacks regulatory assessment and surveillance. Herbal product quality varies and is a major issue in HILI, adding to the complexity in evaluating causality for herbs. This may explain why HILI is considered as a poorly defined entity, is a neglected disease, and requires special attention[31]. The latter (drug-induced autoimmune liver disease) is emerging as a poorly defined under-reported and underestimated liver disorder, and poses particular diagnostic dilemma[32]; indeed, overlaps and different clinical scenarios exist among DILI and autoimmune hepatitis. What is clear is that the diagnosis of autoimmune hepatitis is often made in the setting of a patient under poly-pharmacotherapy. Discriminating between true autoimmune hepatitis triggered by drugs and immune-mediated DILI still remains a challenge.
Management
The key treatment of DILI remains withdrawal of the offending medication[28] (hence, the importance of correct differential diagnosis). However, early drug discontinuation does not always prevent the occurrence of ALF. Nonetheless, only a small fraction (10%) of idiosyncratic DILI exceeds in ALF, with coagulopathy and any degree of encephalopathy. Unfortunately, prognostic scores to early predict the clinical outcome for DILI reaching the threshold of ALF are still under development. From a drug development standpoint, the decision on drug discontinuation should be carefully balanced, with stopping rules suggested by the FDA[33].
No definitive therapies are available for idiosyncratic DILI with or without ALF: N-acetylcysteine (NAC), the antidote for acetaminophen overdoses (dose-dependent DILI), may be considered in adults with early-stage ALF, for its good safety profile and some evidence for efficacy in early coma stage patients. A meta-analysis (4 trials selected) concluded that NAC is safe for non-acetaminophen-induced ALF. It can prolong patients’ survival with native liver without transplantation and survival after transplantation, without improvement in the overall survival[34]. Re-exposure to a drug that is thought likely to have caused hepatotoxicity is strongly discouraged, especially if the initial liver injury was associated with remarkable enzyme elevation. Follow-up is also needed until resolution, as chronicity may occur in approximately 14% of those experiencing DILI[28].
SIGNALS EMERGING FROM SPONTANEOUS REPORTING SYSTEMS AND CASE SERIES/REPORTS
Because of limited predictive value of pre-clinical assays[35], the lack of fully validated biomarkers and the limited power of pre-marketing randomized clinical trials to detect rare safety issues, large spontaneous reporting systems of adverse drug reactions are a pivotal source for early identification of safety signals, especially for rare idiosyncratic events such as DILI. Several analyses on spontaneous reporting databases have been published in 2014 (60 out of 369 publications retrieved in PubMed are based on case reports/series and pharmacovigilance databases), thus highlighting the contribution of this tool as a source of clinical evidence. For this reason, we provide here below a few key examples.
Three pharmacological classes of medications were investigated through pharmacovigilance databases: direct oral anticoagulants (DOACs), antimycotics and antidepressants. As regards DOACs, although a recent systematic review on phase III randomized clinical trials failed to demonstrate a significant risk of DILI[36], the experience gained from the history of ximelagatran suggested that caution is needed before considering them free from DILI risk. As a matter of fact, case series have become to accrue and suggested a potential safety signal, especially for rivaroxaban[37,38]. In particular, the assessment of spontaneous reports submitted to the publicly available FDA Adverse Event Reporting System (FAERS) detected a disproportionality signal of DILI for rivaroxaban (including ALF), whereas no association emerged for dabigatran, even when potential competition biases were tested. Notably, a considerable proportion of DILI reports of rivaroxaban (42%) and dabigatran (37%) co-listed possible hepatotoxic and/or interacting drugs, with fatal outcome and very rapid time-to-onset in almost half of ALF reports[39]. These signals should not automatically generate alarm, but certainly prompt comparative population-based studies to characterize and quantify the actual risk, taking into account drug- and patient-related risk factors[40]. Meanwhile, as DILI is unpredictable, these findings strengthen the importance of timely pharmacovigilance to detect post-marketing signals of DILI and underline the role of clinicians in early recognition of signs/symptoms suggestive of severe hepatic damage.
Among the first detected hepatotoxins in 2014, we selected the first post-marketing report by pomalidomide, the latest immunomodulating drugs approved by the FDA for multiple myeloma. The causal association was based on the temporal association with drug exposure and the exclusion of other causes[41]. Notably, DILI occurred despite dose titration and monitoring of liver function. A second biopsy was performed because, within 2 wk of completing steroids, bilirubin markedly increased; this second histological evaluation raised the possibility of acute hepatitis presentation of chronic graft-vs-host disease. Steroids should be considered if hepatotoxicity persists despite discontinuation of pomalidomide.
A case series from Germany highlighted a typical clinical pattern of flupirtine: it almost exclusively occurred in females and was characterized by hepatocellular pattern as a key histological feature and clinical manifestation with jaundice and ALF. In March 2013, the European Medicines Agency (EMA) recommended to limit the duration of flupirtine treatment to 2 wk; however, data by Douros et al[42] suggested that early, severe hepatotoxic symptoms cannot be ruled out.
As regards antimycotics, the 2013 regulatory interventions on ketoconazole for DILI (the oral formulation was withdrawn) posed a prescribing challenge to clinicians, who should now carefully consider safer therapeutic alternatives. Data mining of the publicly available FAERS database highlighted that antimycotics are involved in approximately 3% of DILI cases (including ALF events); as compared to topical-administered antimycotics, virtually all systemic antimycotics (including ketoconazole, newer triazole derivatives voriconazole and posaconazole, as well as terbinafine) generated a significant disproportionality, indicating a post-marketing signal of risk. Thus, clinicians should assume a potential class effect and, in case a therapeutic switch is considered, careful monitoring is recommended, especially in critical poly-treated patients with multiple comorbidities[43]. The worldwide re-appraisal of oral ketoconazole reminds clinicians of the importance of liver safety during oral antifungal treatment and carries implications for future antifungal development[44]. In fact, clinical research on drug-drug interactions is now challenged by the prohibition of ketoconazole, previously used as a prototype CYP3A4 inhibitor in healthy volunteers. Ritonavir and itraconazole have been suggested as possible alternatives, but not clarithromycin[45].
The case of antidepressants carries similar clinical implications. A review of clinical data suggested that duloxetine, bupropion, trazodone, tianeptine, and agomelatine are associated with greater risk, as compared to selective serotonin reuptake inhibitors[46]. Although an infrequent event, DILI from antidepressants may be irreversible, and clinicians should be aware of this. Data from Spanish, French, and Italian spontaneous reporting databases consistently showed a signal of hepatotoxicity for agomelatine[47]. These data and other clinical evidence prompted assessment by the EMA, which confirmed the positive risk-benefit profile of agomelatine, although measures for intense liver function monitoring and new contraindications were introduced: elevation of liver enzymes higher than 3 times as compared to reference values, hepatic impairment (not further specified), parallel use of potent CYP1A2 inhibitors[48].
SIGNALS EMERGING FROM REGISTRIES
During the last decade, data from large registries of DILI patients have been published. Although most of these registries cannot be formally considered population based, they do provide important data on relative prevalence of agents, they may better detect rare hepatoxicity signals in early post-marketing phase and also allow important comparisons between countries[10,14,49].
In 2014, the United States DILI Network (DILIN) continued to publish new analyses from prospective registry, especially by defining clinical signatures of specific agent. Notably, a new syndrome was identified and characterized after a single intravenous dose of cefazolin: 1-3 wk of latency period after exposure (usually following a minor outpatient surgical procedure), marked cholestasis and a self-limited moderate to severe clinical course[50].
New information was provided also for anti-tumor necrosis factor (TNF) agents, among which infliximab was the most frequently implicated (1 in 120 patients): 50% of patients required steroid therapy, but without long-term treatment. Moreover, the addition of methotrexate to anti-TNF therapy might reduce the risk of DILI[51].
The DILIN consortium also addressed the spectrum of statins hepatotoxicity and provided novel previously unknown aspects: DILI with statins is rare and characterized by variable patterns of injury, a range of latencies to onset, autoimmune features in some cases, and persistent or chronic injury in 18% of patients, with an autoimmune phenotype in most of the cases[52].
Herbal and dietary supplements (HDSs) were also under scrutiny[53]. During the 2004-2013 period, it was noted that the proportion of liver injuries attributed to HDSs increased from 7% to 20%, as compared to medications. It is noteworthy that, bodybuilding HDSs are the most commonly implicated class of products and, most importantly, non-bodybuilding HDSs (e.g., products for weight loss) can cause more severe liver injury than conventional medications, as reflected by a higher transplantation rate (13% vs 3%). As discussed below, the relationship between herbal administration and hepatic safety represents a current research question, as demonstrated by some products used to treat liver disease that may also have a detrimental hepatic effect and confounders exist (e.g., multiple ingredients and sometimes undeclared components)[54].
SIGNALS EMERGING FROM OBSERVATIONAL STUDIES
Observational studies, namely population-based case-control and cohort studies, represent key pharmaco-epidemiological tools aimed to assess the likelihood of association. These studies are usually triggered by previous (early) analysis on spontaneous reporting systems highlighting possible drug-event association (signal detection). These analytical approaches may allow risk quantification of adverse events that have a long delay between exposure and clinical manifestations, highlight new risks associated with old drugs, as well as adverse events characterized by high background incidence rates and less likely to carry a drug-induced component[6].
Although these post-marketing studies are highly representative of actual practice (i.e., high external validity as compared to clinical trials), methodological complexity and the need for long-term follow up (for cohort studies) often compromise feasibility and optimal data collection.
In 2014, we identified three publications deserving consideration. The first one regards a comparative hepatic evaluation of antithyroid drugs, for which only anecdotic case reports/series were provided: in a population-based cohort study on Taiwan National Health Insurance Research Database, methimazole/carbimazole showed a dose-dependent increased risk of hepatitis, as compared to propylthiouracil, while the risks were similar for ALF and cholestasis[55].
The second contribution emerged from the hospital-based Berlin Case-Control Surveillance Study: apart from known hepatotoxic drugs (e.g., amiodarone), novel hepatotoxic risk was suggested for biperiden, thus highlighting the need for post-authorization safety studies[56]. These types of researches should be replicated in different settings to highlight possible differences in the pattern of drug use among the different clinical scenarios.
The third study was conducted within the Exploring and Understanding Adverse Drug Reactions by Integrative Mining of Clinical Records and Biomedical Knowledge project and designed as a multi-country cohort study in 7 European healthcare databases with a focus on ALI in children and adolescents. Apart from known signals, three associations (i.e., domperidone, flunisolide and human insulin) were previously undocumented (literature and labels) either in adults or in children, whereas two drugs (citalopram, cetirizine) were not previously described in children but reported in adults[57].
SIGNALS EMERGING FROM CLINICAL TRIALS (INCLUDING META-ANALYSES)
Apart from cardiotoxicity, DILI is a recently recognized safety concern, albeit not formally quantified yet, of oral tyrosine kinase inhibitors (TKIs). The latest meta-analysis of 6 randomized controlled trials on anti-angiogenic TKIs found that hepatotoxicity is a relatively common (occurring in 23%-40% of patients) but non severe event (only 5% of patients experienced high-grade toxicity)[58]. These data corroborate previous findings from an earlier meta-analysis[59] and indicate that TKIs are associated with potentially fatal hepatotoxicity, usually reversible on dose reduction or drug discontinuation. Of note, incidence varies widely among agents, thus suggesting that a class effect is unlikely: the potential for serious hepatotoxicity with lapatinib, pazopanib, ponatinib, regorafenib and sunitinib was believed to be sufficiently high as to require a boxed label warning. Post-marketing surveillance is warranted, especially for newer agents, to assess the actual role of TKIs in the occurrence of DILI, especially in the presence of hepatocellular carcinoma, hepatic metastasis and potential drug interactions[60]. Pre-clinical research should investigate the mechanism of TKI-related DILI; the formation of reactive metabolites has been suggested to play a role in the pathogenesis, at least as a key prerequisite. Current clinical management strategies are based on (1) switching to an alternative TKI with similar mechanism of action (e.g., erlotinib vs gefitinib); (2) using an alternative dosing regimen (reduced doses or dosing frequency); and (3) introduction of steroids for the treatment and prevention of hepatotoxicity (if autoimmune response is present)[61].
Very recently, the debate on alogliptin hepatotoxicity has aroused interest; Scheen reviewed the pharmacokinetics and hepatic profile of incretin-based therapies and concluded that the overall liver safety of dipeptidyl peptidase-4 inhibitors is reassuring, and, in particular, that “no hepatotoxicity has been reported in the development programme of alogliptin”[62]. By contrast, Barbehenn et al[63] pointed out the numerical imbalance, albeit not statistical significant, emerging for alogliptin from the publication of the Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care (EXAMINE) trial. This signal together with other reports from Japan prompted a careful FDA hepatological assessment, which culminated in recommending liver function evaluation before starting alogliptin therapy. Scheen’s rebuttal challenged the FDA measures by providing new pooled data that “indicate that alogliptin is associated with a low risk of hepatic toxicity”[64]. Considering the heterogeneous marketing life, penetration and utilization of the different dipeptidyl peptidase-4 inhibitors, analytical post-marketing studies should be encouraged, especially in the wake of a recent FAERS analysis, showing no signal of liver injury for alogliptin, but statistically significant associations for sitagliptin, saxagliptin and vildagliptin[65].
PERSPECTIVES
The role of biomarkers in drug development
The present multidisciplinary interest in DILI and HILI is well documented by the exponential increase in pre-clinical publications, which suggest a gap in knowledge on the predictivity of in vitro/in vivo assays.
Despite intensive efforts to develop biomarkers sufficiently predictive of DILI risk in earlier phases of drug development, there is still room for improvement in this area as no biomarkers are currently validated for routine use[66]. The role of new serum biomarkers such as glutamate dehydrogenase, high mobility group box protein 1, and microRNA-122 is under scrutiny for possible use in diagnosis and prognosis and provide important insights into the mechanisms of the pathogenesis, which is only partially understood (from bench to bedside)[24]. Thus, new prediction methodologies are needed.
Emerging issues in pre-clinical research
The role of animal studies remains questionable, mainly because of the incomplete understanding of the mechanisms underlying DILI, as well as marked species differences in response to, and in the metabolism of, xenobiotics. As a result, there is currently no universally accepted animal model and no formal approval is granted by Regulatory Agencies.
The use of various techniques involving liver cell cultures for DILI prediction is highly controversial for several reasons. Although it is well accepted the contribution of drug metabolism as initiating step, numerous mechanistic studies have emphasized the fact that DILI may be a multicellular event. Over the past decade, attempts have been made to compile hepatotoxicity data and develop in silico models to be used as a first-line screening of drug candidates[67]. In vitro battery for hepatotoxicity testing comprises a number of cell models, among which 3D cultures, engineered liver-derived cell line and pluripotent stem cell-derived hepatocytes are emerging as promising for toxicological screening of drug candidates[68]. In this context, a chimeric TK-NOG mice model with humanized livers was recently implemented as predictive model to assess cholestatic liver damage induced by fialuridine and bosentan, known to be hepatotoxic from clinical trials. These findings suggested that the use of chimeric mice could improve the pre-clinical drug safety assessment of candidate drugs[69,70].
Novel and current clinical issues
From a clinical standpoint, although progress has been achieved in diagnosis and timely recognition of hepatic damage by drugs and herbs, the idiosyncratic nature of liver toxicity calls for continuing monitoring and vigilance of patients, especially those with comorbidities requiring chronic long-term treatment with multiple agents. In clinical practice, viral hepatitis and NAFLD represent the two most common hepatic disorders that can mimic DILI and should be always considered among the various differential diagnoses. In clinical phases of drug development, DILI prediction and detection relies on Hy’s law and the Evaluation of Drug-Induced Serious Hepatotoxicity (eDISH) plot[71]. Recently, Robles-Diaz et al[72] suggested a new model using liver enzymes to improve prediction of ALF. Moreover, Fontana et al[73] analysed 660 patients with definite, highly likely, or probable DILI and found that, within 6 mo of DILI onset, 9.4% of patients either died or required liver transplantation. However, these results do not convincingly demonstrate that mild liver test abnormalities seen during follow-up are of clinical significance[74]. In populations with underlying liver diseases, such as viral hepatitis, liver safety assessment is particularly challenging, especially because liver enzymes elevation at baseline is quite common, as well as administration of concomitant hepatotoxic drugs and comorbidities such as steato-hepatitis and dyslipidemia. In addition, in oncology, hepatic abnormalities may reflect involvement of the liver in tumor progression[75].
As a general recommendation, clinicians should: (1) be aware and consider DILI and HILI among the various differential diagnoses; (2) inform patients of the potential risk associated with certain drugs, if documented; (3) discontinue suspect offender agent(s); (4) start immunosuppressive agents (e.g., corticosteroids) in case an autoimmune liver disease is considered; (5) overall reconcile drug therapy by paying attention to concomitant medications; (6) consider referral to specialized centers for support in diagnosis and management; and (7) voluntarily report potential drug-related clinical event, especially those with serious life-threatening outcome (e.g., ALF).
Research agenda on HILI
The research agenda of HILI is complicated by a further dimension, as the most effective approach to identify culprit herbal agents requires careful separation of products into their often multiple components, followed by in vitro/in vivo toxicological evaluation[53]. In addition, the precise epidemiology is far from being fully appreciated, mainly because the available evidence is mostly based on case reports/series, which have been systematically collected to create tabular lists[17]. Large registries will be crucial for this purpose; in fact, the DILIN consortium created a repository to explore the hepatotoxic potential of certain ingredients (as of October 1st, 2014, 318 herbal products have been collected) and a workshop took place on May 5-6, 2015 to define opportunities and directions for future research[76].
Among cases of HILI reported worldwide, the following products should be emphasized: herbals containing pyrrolizidine alkaloids, herbal medicine as part of traditional chinese medicine, kava, black cohosh and HDSs (e.g., Herbalife®, Hydroxycut, green tea, anabolic steroids). From one hand, the use of these products, mainly for healthy indications such as weight loss and improvement of physical performance, is extensive and largely uncontrolled by regulatory authorities. On the other hand, their safety and efficacy have not been rigorously tested, thus strengthening the importance of active vigilance, international harmonization and regulatory supervision similar to synthetic drugs, especially in the light of modern globalization[77].
Existing projects
The interest in DILI is also underlined by the number of active research consortia worldwide. Apart from the American DILIN, set up in 2003 and now including retrospective and prospective nationwide registries (https://dilin.dcri.duke.edu/), the International Serious Adverse Events Consortium is a pharmaceutical-industry-led and FDA-supported international research network, focused on identifying and validating DNA variants predictive of the risk of drug-induced serious adverse events. Launched in 2007, by the end of 2015 the consortium expects to have aggregated information for 7500 cases, of which 2500 on DILI phenotype[78]. The DILI-sim Initiative, started in early 2011, is a pre-competitive partnership aiming to develop a computational model (DILIsym® software) for early prediction of DILI (http://www.dilisym.com/). Liver Toxicity Knowledge Base is another FDA-supporter project; it was developed to exploit systems biology analysis for DILI assessment and prediction (http://www.fda.gov/ScienceResearch/BioinformaticsTools/LiverToxicityKnowledgeBase/default.htm). As a first step, a benchmark dataset of 287 drugs with established DILI risk was created using the FDA-approved prescription drug labels[79].
In Europe, there are two initiatives comprising European Federation of Pharmaceutical Industries and Associations members, Academia, Regulatory Agencies and Small Medium Enterprises: the Innovative Medicines Initiative called Safer and Faster Evidence-based Translation (http://www.imi-safe-t.eu/htdocs/), involving 25 members and aiming at qualifying new safety biomarkers for pre-clinical and clinical regulatory decision-making needs; and Mechanism based integrated systems for the prediction of DILI (http://www.mip-dili.eu/), involving 26 participants, aiming to develop and validate novel in vitro assays. The proposal for recent future is to create a Liver Safety Research Consortium comprising representatives from industry, academia and regulatory agencies, a framework similar to the highly successful Cardiac Research Safety Consortium[3].
CONCLUSION
The aforementioned multidisciplinary consortia represent excellent examples to boost innovation and develop collaborative research comprising all stakeholders. The curiosity, expectations and evidence emerging from these multidisciplinary networks are certainly welcome to advance the knowledge on DILI prediction, diagnosis and management.
At the current state of the art, the unpredictable nature of DILI and HILI strongly supports (1) the importance of post-marketing studies to fully characterize the actual liver damage associated with drugs and herbs, in terms of drug- and host-related risk factors (clinical pharmacology perspective) as well as the epidemiological dimension (population perspective); and (2) the timely recognition of signs/symptoms indicative of liver dysfunction by clinicians, who should consider the potential responsibility of drugs/herbs among the differential diagnoses.
In the near future, two key topics should be prioritized for research activities. First, HDSs require better understanding of their actual epidemiological magnitude, which may be achieved by considering international harmonization of their regulatory status. Second, the rapid accrual of clinical evidence on liver injury induced by DOACs calls for well-designed post-authorization safety studies, especially in the light of their potential therapeutic role in a triple antithrombotic therapy after acute coronary syndromes[80]. In this context, specialist prescription event monitoring may be a candidate pharmaco-epidemiological tool to assess the real-world risk in clinical practice and develop proper risk management plans, as recommended by the new pharmacovigilance legislation[81].