Published online Jun 8, 2015. doi: 10.4254/wjh.v7.i10.1427
Peer-review started: December 26, 2014
First decision: January 20, 2015
Revised: March 9, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: June 8, 2015
Processing time: 161 Days and 9 Hours
AIM: To evaluate the association between alpha-1 antitrypsin deficiency (A1ATD) and hepatocellular carcinoma (HCC) in patients with end-stage liver disease (ESLD).
METHODS: Patients with cirrhosis and ESLD referred to the Cleveland Clinic Foundation for liver transplantation between 2003 and 2014 were included in the study (N = 675). ESLD was defined as having histological features of cirrhosis and/or radiological evidence of cirrhosis in the context of portal hypertension (ascites, variceal bleeding, thrombocytopenia, or hepatic encephalopathy). A1ATD was diagnosed using phenotype characterization (MZ or ZZ), liver biopsy detection of PAS-positive diastase-resistant (PAS+) globules, or both. Patients with other causes of liver diseases such as hepatitis C virus (HCV), alcoholic liver disease and non-alcoholic steatohepatitis (NASH) or NASH were also included in the study. HCC was diagnosed by using imaging modalities, biopsy findings, or explanted liver inspection. Follow-up time was defined as the number of years from the diagnosis of cirrhosis to the diagnosis of hepatocellular carcinoma, or from the diagnosis of cirrhosis to the last follow up visit. The rate of HCC was assessed using time-to-interval analysis for interval censored data.
RESULTS: This study included 675 patients. 7% of subjects had A1ATD (n = 47). Out of all subjects who did not have A1ATD, 46% had HCV, 17% had alcoholic liver disease, 19% had NASH and 18% had another primary diagnosis. Of the 47 subjects with A1ATD, 15 had a primary diagnosis of A1ATD (PI*ZZ phenotype and PAS+ globules), 8 had a PI*MZ phenotype alone, 14 had PAS+ alone, and 10 had both the PI*MZ phenotype and PAS+. Median follow-up time was 3.4 (25th, 75th percentiles: 1, 5.2) years. The overall rate of hepatocellular carcinoma in all subjects was 29% (n = 199). In the A1ATD group, the incidence rate of HCC was 8.5% compared to 31% in the group of patients with other causes of cirrhosis (P = 0.001). Patients with ESLD due to A1ATD had the lowest yearly cumulative rate of hepatocellular carcinoma at 0.88% per year compared to 2.7% for those with HCV cirrhosis, 1.5% in patients with NASH and 0.9% in alcohol-induced liver disease (P < 0.001).
CONCLUSION: Within this group of patients with ESLD, there was no significant association between A1ATD and increased risk of HCC.
Core tip: It’s been postulated that alpha-1 antitrypsin deficiency (A1ATD) and the presence of the Z allele increase the risk of hepatocellular carcinoma (HCC) above that attributable to cirrhosis alone. Our study showed that the occurrence of HCC in subjects with cirrhosis due to A1ATD was 0.88%/year. This incidence rate was considerably lower than that among patients with other causes of liver disease including hepatitis C, alcoholic liver disease and non-alcoholic liver steatohepatitis. This challenges the view that A1ATD confers a disproportionate risk of HCC.
- Citation: Antoury C, Lopez R, Zein N, Stoller JK, Alkhouri N. Alpha-1 antitrypsin deficiency and the risk of hepatocellular carcinoma in end-stage liver disease. World J Hepatol 2015; 7(10): 1427-1432
- URL: https://www.wjgnet.com/1948-5182/full/v7/i10/1427.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i10.1427
Alpha-1 antitrypsin deficiency (A1ATD) is an autosomal codominant genetic disease affecting 1 per 3000-5000 individuals[1-3]. Twenty-five million people have been estimated to carry at least one defective gene[4]. A1ATD has been linked to severe pulmonary and liver disease.
The most common, normal allele of the A1AT gene is called M and produces normal levels of alpha-1 antitrypsin protein. The Z mutation is a single base substitution that alters the A1AT molecular structure and promotes intra-hepatocyte polymerization, thereby trapping A1AT in the endoplasmic reticulum of hepatocytes and causing serum A1AT levels to be low[5]. This mechanism of liver disease has been called a “toxic gain of function” and the resulting deficient proteolytic screen in the lung has been called a “toxic loss of function.” The intracellular accumulation of the A1AT molecule in hepatocytes triggers apoptosis of liver cells, instigating chronic hepatocellular death and regeneration[6]. This can manifest in a wide range of symptoms and signs from abnormal liver enzyme tests to liver fibrosis, cirrhosis and the dreaded hepatocellular carcinoma (HCC).
There is marked variability in the degree of liver disease among homozygous individuals for the Z allele (PI*ZZ). Several studies have examined the prevalence of abnormal liver function tests in these individuals and found only 7.7%-10% with mild increases in alanine aminotransferases levels[7,8]. The prevalence of cirrhosis in PI*ZZ individuals varies between 2%-43% and seems to be mostly driven by age[9-11]. Some studies have also reported a similar association between heterozygous PI*MZ individuals and cirrhosis[12-14], though this has been challenged more recently[15]. The most feared complication of A1ATD cirrhosis is hepatocellular carcinoma or HCC. HCC is the sixth most common neoplasm worldwide[16]. It is the third most frequent cause of cancer deaths and is a leading cause of death among patients with cirrhosis[17]. An autopsy study from Sweden demonstrated that homozygotes for the Z allele had increased risk for primary liver cancer, especially in male patients[11]. In addition, it has been suggested in recent studies that heterozygotes for the Z allele or PI*Z (such as PI*MZ) had an increased risk of primary liver carcinoma that developed even in the absence of cirrhosis and was frequently characterized by cholangiocellular differentiation[18,19]. However, the view that A1ATD confers a substantially higher risk for liver cancer remains controversial and studies addressing the occurrence rate of HCC in patients with end-stage liver disease (ESLD) caused by A1ATD are sparse.
In this context, the specific aims of the current study were to: (1) estimate the incidence and cumulative annual risk of HCC in subjects with A1ATD-associated cirrhosis; and (2) compare this incidence rate to that of patients with other causes of liver disease, including hepatitis C virus (HCV), alcoholic liver disease (ALD) and non-alcoholic steatohepatitis (NASH).
The study was approved by the Cleveland Clinic Institutional Review Board. Patients with cirrhosis and ESLD referred to the Cleveland Clinic Foundation for liver transplantation between January 2003 and January 2014 were identified (N = 675) and their medical records were retrospectively reviewed using our electronic medical record (EPIC, Verona, WI). Demographic features such as age, sex and race were extracted.
ESLD was defined as having histologic features of cirrhosis and/or radiologic evidence of cirrhosis in the context of portal hypertension (ascites, variceal bleeding, thrombocytopenia, or hepatic encephalopathy).
The diagnosis of A1ATD was based on phenotype characterization using isoelectric focusing (MZ or ZZ), liver biopsy detection of PAS-positive diastase-resistant (PAS+) globules, or both.
Patients with other causes of liver diseases were also included in the study. The diagnosis of alcohol-induced cirrhosis was based on history and histological findings on biopsy. The diagnosis of hepatitis C was based on detecting hepatitis C antibody and RNA, that of NASH was based on histological features. The diagnosis of hepatitis B was established with seropositivity for hepatitis B surface antigen, hepatitis B core antibody, and hepatitis B DNA. Autoimmune hepatitis was established based on finding serum autoantibodies and consistent histology. Primary biliary cirrhosis was diagnosed based on finding a serum mitochondrial antibody and compatible histology. Hereditary hemochromatosis was established with serum iron studies and genetic testing and if suspected, primary sclerosing cholangitis was diagnosed by cholangiogram.
HCC was diagnosed by using imaging modalities, biopsy findings, or explanted liver inspection.
Follow-up time was defined as the number of years from the diagnosis of cirrhosis to the diagnosis of hepatocellular carcinoma, or from the diagnosis of cirrhosis to the last follow-up visit.
Subjects with A1ATD were recognized and the incidence of HCC was calculated in this group. The incidence of hepatocellular carcinoma in patients with ALD, HCV, NASH and a group of other causes of cirrhosis (which included hepatitis B, hereditary hemochromatosis, primary biliary sclerosis, primary sclerosing cholangitis and autoimmune hepatitis) was assessed using time-to-event analysis for interval-censored data.
Data are presented as mean ± standard deviation or N (%). Differences between subjects with and without A1ATD were determined using univariate analysis. Analysis of variance was used for continuous variables and Pearson’s chi-square tests were used for categorical factors. Time-to-event analysis for interval-censored data was carried out to determine the incidence of hepatocellular carcinoma in each group because only the year of diagnosis was known for many subjects. A cumulative incidence plot was constructed and the generalized log-rank test for interval-censored data was used. Values of P < 0.05 were considered statistically significant. All analyses were performed using R (version 3.0.1, The R Institute for Statistical Computing, Vienna, Austria).
The statistical review of the study was performed by a biomedical statistician.
Demographic features of our patient population are summarized in Table 1. Most (91%) patients were Caucasian. At time of cirrhosis diagnosis, average age was 55 ± 10 years; 31% of cirrhotics were female. The ages and gender distribution were similar between the A1ATD group and the non-A1ATD group (P = 0.64 and P = 0.68 for age and gender, respectively). Furthermore, there was no significant age difference between A1ATD group with or without HCC (53 ± 11 years and 55 ± 10 years respectively) or between HCC patients with A1ATD or without A1ATD (53 ± 11 years and 58 ± 8 years respectively). The overall number of deaths in the cohort was 24.6% (n = 166). Of subjects with HCC, 30.15% were diseased (60/199).
| No A1ATD (n = 628) | A1ATD (n = 47) | ||||
| Factor | n | Summary | n | Summary | P-value |
| Female | 628 | 194 (30.9) | 47 | 13 (27.7) | 0.64c |
| Age at cirrhosis diagnosis | 628 | 54.6 ± 9.5 | 47 | 55.2 ± 10.3 | 0.68a |
| Race | 615 | 47 | 0.14c | ||
| Caucasian | 513 (83.4) | 45 (95.7) | |||
| African-American | 65 (10.6) | 1 (2.1) | |||
| Hispanic | 19 (3.1) | 1 (2.1) | |||
| Other | 18 (2.9) | 0 (0.0) | |||
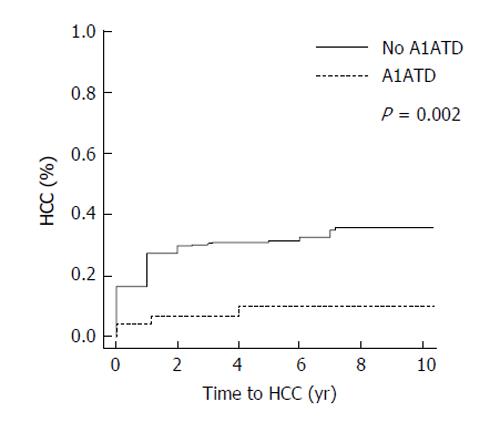
| HCC | 628 | 195 (31.1) | 47 | 4 (8.5) | 0.001c |
| Follow-up (yr, median) | 628 | 3.2 (1.00, 5.1) | 47 | 4.2 (1.9, 5.2) | 0.090b |
Seven percent of subjects had A1ATD (n = 47). Out of all subjects who did not have A1ATD, 46% had HCV, 17% had ALD, 19% had NASH and 18% had other primary diagnoses.
Of the 47 subjects with A1ATD, 15 had severe deficiency of A1ATD (based on a confirmed PI*ZZ phenotype and PAS+ globules), 8 had a PI*MZ phenotype only, 14 had PAS+ globules only, and 10 had a PI*MZ phenotype and PAS+. Overall, 32% had the PI*ZZ phenotype, 38% were PI*MZ, and 83% had PAS+ globules on liver biopsy.
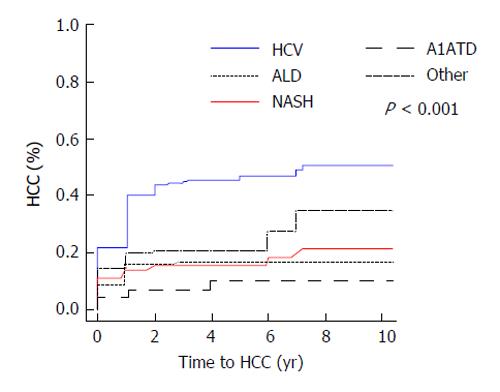
Median follow-up time was 3.4 years (25th, 75th percentiles: 1, 5.2). The overall rate of hepatocellular carcinoma in all subjects was 29% (n = 199). Of the 47 patients with A1ATD, 8.5% (4/47) developed HCC compared to 31.1% (195/628) of patients with other causes of liver disease (P = 0.001, Figure 1). When comparing the incidence rates of HCC in each subgroup of subjects, the highest yearly cumulative incidence of hepatocellular carcinoma was found in HCV subjects (2.7%/year, Figure 2). This was followed by a yearly cumulative incidence rate of 2.3% in patients with other etiologies of cirrhosis, 1.5% in NASH, 0.9% in alcohol-induced liver disease. The rate was lowest (0.88%/year) in patients with A1ATD cirrhosis (P < 0.001). Among those with confirmed PI*ZZ A1ATD, the rate of developing HCC was similar to patients with PI*MZ and those with PAS+ globules only.
The average AFP value at time of diagnosis of HCC in the A1ATD group was 27.6. Of the four patients with A1ATD/HCC, two had a single HCC focal lesion with BCLC stage A3, one subject had bifocal disease and one subject had multi-nodular HCC (BCLC stage B).
The principle finding of this study is that the incidence of HCC among patients with A1ATD was lower than that among patients with other causes of liver disease, thereby challenging the view that A1ATD confers a disproportionate risk of HCC. Our study also confirms that patients with cirrhosis due to hepatitis C have the highest risk of hepatocellular carcinoma compared to patients with NASH or alcohol-induced liver cirrhosis.
In contrast to our findings regarding a low risk of HCC with A1ATD, previous studies have reported a higher risk of HCC in A1ATD cirrhotic patients, with prevalence estimates of 10% to almost 50%. Two autopsy studies from Sweden assessed the prevalence of cirrhosis and hepatocellular carcinoma in PI*ZZ elderly individuals. The first study found that 43% of PI*ZZ patients had liver cirrhosis and 28% had HCC[20]. The second study only found that 16.1% of PI*ZZ patients had HCC at the time of autopsy[21]. A case series of 19 patients with A1ATD-associated liver disease found that 10% of patients had HCC (n = 2, both of whom were PI*ZZ)[22]. Several studies have also reported a similar association between heterozygous PI*MZ individuals and HCC. In a series of 61 patients with A1ATD-associated cirrhosis, most (89%) of whom were PI*MZ, Propst et al[23] reported that 10% had HCC. Several methodological differences could account for the discordance between our findings and prior reports and must be considered. First, our study was aimed at investigating the incidence and annual cumulative risk of developing HCC in A1ATD; whereas the other studies assessed the prevalence of HCC at one time point. Second, HCC was ascertained through imaging studies in our series vs by post-mortem in earlier studies. To the extent that imaging may fail to detect HCC, the frequency would be under-estimated here. Given the sensitivity of computed tomography for HCC, this seems an unlikely source of bias, however. Another possible explanation is that the universe of A1ATD patients in this series includes PI*MZ heterozygotes vs prior observations in all PI*ZZ patients. Earlier series have discounted the risk of cirrhosis in PI*MZ heterozygotes[15]. At the same time, the incidence of HCC among the 15 confirmed PI*ZZ individuals in this series is also low, making this less likely as a source of the discordance with prior reports. While a third potential source of under-recognizing HCC in the patients with A1ATD vs among other patients with other causes of liver disease might be that the duration of follow up was shorter in the A1ATD group or that they were under less close surveillance for the occurrence of HCC, the follow up duration in A1ATD patients was actually longer (median 4.2 years vs 3.2 years) than in others. Other potential limitations of this study include the difficulty of generalizing the findings from this large tertiary care setting to a community context and the relatively small sample size upon which to make the frequency estimates. Knowing the exact risk of occurrence of HCC in this population is important as it can help guide management of these patients.
In summary, our findings suggest that out of all patients with chronic liver disease, HCC incidence was the lowest among patients with A1ATD. Further study is needed to confirm these findings and to enhance generalizability.
Alpha-1 antitrypsin is a molecule that is made in the liver, is then released into the systemic circulation where it travels to the lungs where it has its main function. In alpha-1 antitrypsin deficiency (A1ATD), the molecule is unable to leave the liver, ends up accumulating in the liver cells causing damage, liver fibrosis, cirrhosis and potentially hepatocellular carcinoma (HCC). The view that A1ATD confers a significantly higher risk of liver cancer remains controversial and studies regarding the incidence of HCC in patients with cirrhosis caused by A1ATD are sparse. In this study, the authors compared the rate of developing HCC in patients with liver cirrhosis due to A1ATD to patients with other causes of cirrhosis.
Knowing the exact risk of occurrence of HCC in patients with A1ATD can help guide management of these patients; such as amount of surveillance and screening they would need over their lifetime.
A1ATD was associated with a lower risk of HCC compared to other causes of liver disease. This challenges the view that A1ATD confers a disproportionate risk of HCC.
A1ATD does not confer a higher risk of HCC compared to other causes of liver disease. Therefore, management of these patients should be tailored accordingly. Further research should be carried out to confirm their findings and generalize their data.
Liver disease is characterized by different stages. Injury to liver cells can lead to inflammation followed by liver fibrosis or the development of scar tissue. If the injury continues, the liver can lose its normal function and become permanently cirrhotic.
This retrospective study, examining HCC in cirrhosis, is well written, clear, and appropriately analysed. It also answers an important clinical question.
P- Reviewer: Andreone P, Turner AM, Teschke R
S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | O’Brien ML, Buist NR, Murphey WH. Neonatal screening for alpha1-antitrypsin deficiency. J Pediatr. 1978;92:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Silverman EK, Miletich JP, Pierce JA, Sherman LA, Endicott SK, Broze GJ, Campbell EJ. Alpha-1-antitrypsin deficiency. High prevalence in the St. Louis area determined by direct population screening. Am Rev Respir Dis. 1989;140:961-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Colp C, Pappas J, Moran D, Lieberman J. Variants of alpha 1-antitrypsin in Puerto Rican children with asthma. Chest. 1993;103:812-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | de Serres FJ, Blanco I, Fernández-Bustillo E. Genetic epidemiology of alpha-1 antitrypsin deficiency in North America and Australia/New Zealand: Australia, Canada, New Zealand and the United States of America. Clin Genet. 2003;64:382-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Qu D, Teckman JH, Perlmutter DH. Review: alpha 1-antitrypsin deficiency associated liver disease. J Gastroenterol Hepatol. 1997;12:404-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology. 2005;42:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Clark VC, Dhanasekaran R, Brantly M, Rouhani F, Schreck P, Nelson DR. Liver test results do not identify liver disease in adults with α(1)-antitrypsin deficiency. Clin Gastroenterol Hepatol. 2012;10:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Piitulainen E, Carlson J, Ohlsson K, Sveger T. Alpha1-antitrypsin deficiency in 26-year-old subjects: lung, liver, and protease/protease inhibitor studies. Chest. 2005;128:2076-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Berg NO, Eriksson S. Liver disease in adults with alpha-1 -antitrypsin deficiency. N Engl J Med. 1972;287:1264-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 150] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Eriksson S. Alpha 1-antitrypsin deficiency and liver cirrhosis in adults. An analysis of 35 Swedish autopsied cases. Acta Med Scand. 1987;221:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med. 1986;314:736-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 392] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Hodges JR, Millward-Sadler GH, Barbatis C, Wright R. Heterozygous MZ alpha 1-antitrypsin deficiency in adults with chronic active hepatitis and cryptogenic cirrhosis. N Engl J Med. 1981;304:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 119] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Graziadei IW, Joseph JJ, Wiesner RH, Therneau TM, Batts KP, Porayko MK. Increased risk of chronic liver failure in adults with heterozygous alpha1-antitrypsin deficiency. Hepatology. 1998;28:1058-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Fischer HP, Ortiz-Pallardó ME, Ko Y, Esch C, Zhou H. Chronic liver disease in heterozygous alpha1-antitrypsin deficiency PiZ. J Hepatol. 2000;33:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Regev A, Guaqueta C, Molina EG, Conrad A, Mishra V, Brantly ML, Torres M, De Medina M, Tzakis AG, Schiff ER. Does the heterozygous state of alpha-1 antitrypsin deficiency have a role in chronic liver diseases? Interim results of a large case-control study. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 1:S30-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 17. | Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Zhou H, Fischer HP. Liver carcinoma in PiZ alpha-1-antitrypsin deficiency. Am J Surg Pathol. 1998;22:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Zhou H, Ortiz-Pallardó ME, Ko Y, Fischer HP. Is heterozygous alpha-1-antitrypsin deficiency type PIZ a risk factor for primary liver carcinoma? Cancer. 2000;88:2668-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Eriksson SG. Liver disease in alpha 1-antitrypsin deficiency. Aspects of incidence and prognosis. Scand J Gastroenterol. 1985;20:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Elzouki AN, Eriksson S. Risk of hepatobiliary disease in adults with severe alpha 1-antitrypsin deficiency (PiZZ): is chronic viral hepatitis B or C an additional risk factor for cirrhosis and hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 1996;8:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Rakela J, Goldschmiedt M, Ludwig J. Late manifestation of chronic liver disease in adults with alpha-1-antitrypsin deficiency. Dig Dis Sci. 1987;32:1358-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Propst T, Propst A, Dietze O, Judmaier G, Braunsteiner H, Vogel W. Prevalence of hepatocellular carcinoma in alpha-1-antitrypsin deficiency. J Hepatol. 1994;21:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |