Published online Jun 8, 2015. doi: 10.4254/wjh.v7.i10.1390
Peer-review started: August 28, 2014
First decision: October 14, 2014
Revised: March 17, 2015
Accepted: April 1, 2015
Article in press: April 7, 2015
Published online: June 8, 2015
Processing time: 278 Days and 20.6 Hours
The chemokine system consists of four different subclasses with over 50 chemokines and 19 receptors. Their functions in the immune system have been well elucidated and research during the last decades unveils their new roles in hepatocellular carcinoma (HCC). The chemokines and their receptors in the microenvironment influence the development of HCC by several aspects including: inflammation, effects on immune cells, angiogenesis, and direct effects on HCC cells. Regarding these aspects, pre-clinical research by targeting the chemokine system has yielded promising data, and these findings bring us new clues in the chemokine-based therapies for HCC.
Core tip: The chemokine system not only serves as the core components in orchestrating the normal immune response but also plays a key role in the microenvironment of hepatocellular carcinoma (HCC). Therefore, the thorough understanding of its role is indispensible for devising effective treatments. During the progress of HCC, the chemokine system boosts aberrant inflammation and angiogenesis through simultaneously affecting different kinds of immune cells and influencing the migration, invasion, growth and survival of tumor cells. Targeting the chemokine system has elicited powerful anti-tumor effects and this indicates an encouraging treatment option in HCC.
- Citation: Liang CM, Chen L, Hu H, Ma HY, Gao LL, Qin J, Zhong CP. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J Hepatol 2015; 7(10): 1390-1402
- URL: https://www.wjgnet.com/1948-5182/full/v7/i10/1390.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i10.1390
The chemokines are a family of small chemotactic molecules about 8-14 kDa which have been well described during the past decades. There are now over 50 chemokines and 19 chemokine receptors, and these chemokines can be divided into four subclasses: CX3C, CXC, CC and (X)C according to the arrangement of the N-terminal two cysteine residues. Corresponding to the four subclasses of chemokines, the chemokine receptors are also subdivided into four families [CX3CR, CXCR, CCR and (X)CR] which are typical G-protein coupled receptors with seven trans-membrane domains[1,2].
The chemokine system is initially found to be critical for immune cells. They orchestrate the migration and localization of immune cells in both lymph organs and other tissues, exerting the “chemotactic effects” which are necessary for the normal immune response in vivo[3]. In addition to chemotactic effects, chemokines can also directly influence the differentiation, survival and functions of immune cells, among which include CCR4, CCR7 and CCR8[4-8]. These observations suggest the chemokine system is not merely the guide signs for the immune cells; instead, they are pleiotropic small molecules with various functions.
The original interest of chemokines in tumor is torched by the observation of immune cells infiltration in tumor tissues. Several groups have speculated that some molecules might be responsible for attracting these immune cells[9]. Although the full spectrum of these molecules is still on the way, some of these important molecules turn out to be chemokines. Since the first chemokine monocyte chemotactic protein 1/CCL2 was detected in the culture media of several different tumor cell lines in 1980s[10,11], more and more chemokines and chemokine receptors have been identified in tumors, including the hepatocellular carcinoma (HCC).
Various studies on the chemokine system have greatly broadened our understanding of its role in HCC, and there are 23 chemokines and 15 chemokine receptors reported in HCC (Table 1). On the one hand, the chemokine system in HCC exerts pleiotropic effects on immune cells and other stroma cells in the microenvironment, and brings both anti- and pro-tumor effects; on the other hand, the HCC cells themselves express chemokine receptors, which allow the chemokines to directly modulate the behaviors of tumor cells including the migration, invasion, growth and survival (Table 2). Data from clinical studies again emphasize the importance of chemokine system in HCC, closely correlating with prognosis. In this review, we will summarize the key roles of the chemokine system in HCC.
| Chemokines | Other names | Chemokine receptors | Subclass | Ref. |
| CXCL1 | GROα | CXCR2 | CXC | [20,21,83] |
| CXCL2 | GROβ | CXCR2 | CXC | [21,83] |
| CXCL5 | ENA78 | CXCR2 | CXC | [21,22] |
| CXCL8 | IL-8 | CXCR1, CXCR2 | CXC | [16,17,21] |
| CXCL9 | MIG | CXCR3 | CXC | [17,18,70] |
| CXCL10 | IP-10 | CXCR3 | CXC | [17,29,51,70] |
| CXCL11 | I-TAC | CXCR3, CXCR7 | CXC | [14,50] |
| CXCL12 | SDF-1 | CXCR4, CXCR7 | CXC | [12,13,15,86,89,92] |
| CXCL14 | BRAK | Unknown | CXC | [23] |
| CXCL16 | SR-PSOX | CXCR6 | CXC | [49,106] |
| CCL2 | MCP-1 | CCR2 | CC | [17,56,81,116] |
| CCL3 | MIP-1α | CCR1, CCR5 | CC | [27,28,81,122] |
| CCL4 | MIP-1β | CCR5 | CC | [29,48] |
| CCL5 | RANTES | CCR1, CCR3, CCR5 | CC | [27,29,100] |
| CCL15 | HCC-2, leukotactin-1 | CCR1, CCR3 | CC | [33,100] |
| CCL17 | TARC | CCR4 | CC | [34,66] |
| CCL19 | ELC, MIP-3β | CCR7 | CC | [37,117-119] |
| CCL20 | MIP-3α | CCR6 | CC | [30-32] |
| CCL21 | SLC | CCR7 | CC | [37,117-119] |
| CCL22 | MDC | CCR4 | CC | [35,66] |
| CCL26 | Eotaxin-3 | CCR3, CX3CR1 | CC | [110] |
| CCL27 | CTACK, ILC | CCR10 | CC | [36] |
| CX3CL1 | Fractalkine | CX3CR1 | CX3C | [39,40] |
| Categories | Chemokines | Chemokine origins | Receptors participated | Functions |
| Inflammation | CXCL8 | HCC cells | Not clarified | Increasing inflammatory cytokines |
| CXCL16 | HCC cells | CXCR6 | (IL-6, IL-8, etc.) and recruiting leukocytes | |
| CCL3 | HCC cells and HSC | CCR3 | (macrophages, neutrophils, etc.) | |
| CCL5 | HCC cells and HSC | CCR5 | ||
| Influences on immune cells | CXCL5 | HCC cells | Not clarified | Chemotaxis of neutrophils |
| CXCL9 | HCC cells and endothelial cells | CXCR3 | Chemotaxis of T cells | |
| CXCL10 | HCC cells, macrophages and TILs | CXCR3 | Chemotaxis of T cells and NK cells | |
| CXCL16 | HCC cells | CXCR6 | Chemotaxis of neutrophils | |
| CCL2 | HCC cells, macrophages and TILs | CCR2 | Chemotaxis of HSC, macrophages, MDSC, and T cells | |
| CCL5 | HCC cells, macrophages and TILs | CCR5 | Chemotaxis of T cells and NK cells | |
| CCL20 | HCC cells | CCR6 | Chemotaxis of Th17 cells and Tregs | |
| CCL22 | HCC cells and DCs | CCR4 | Chemotaxis of Tregs | |
| Angiogenesis | CXCL3, CXCL5 | HCC cells | Not clarified | Promoting angiogenesis via mechanisms not clarified |
| CXCL8 | CSCs | Not clarified | Promoting endothelial cell tube formation | |
| CXCL9 | Not clarified | CXCR3 | Inhibiting angiogenesis by abrogation of VEGF effects | |
| CXCL12 | Endothelia cells | CXCR4, CXCR7 | Enhancing angiogenesis through VEGF | |
| CXCL16 | HCC cells | CXCR6 | Promoting angiogenesis via mechanisms not clarified | |
| CCL2 | HCC cells and endothelial cells | CCR2 | Enhancing the proliferation of endothelial cells | |
| CCL3 | HCC cells and endothelial cells | CCR1, CCR5 | Enhancing the proliferation of endothelial cells | |
| Direct effects on HCC cells | CXCL1, CXCL2, CXCL16 | Not clarified | Not clarified | Enhancing the growth of HCC cells |
| CXCL5 | HCC cells | Not clarified | Enhancing the migration, invasion, and growth of HCC cells | |
| CXCL8 | Not clarified | CXCR2 | Enhancing the migration of HCC cells | |
| CXCL10 | hepatocytes | Not clarified | Enhancing the survival of hepatocytes | |
| CXCL12 | HCC cells, HSC | CXCR4, CXCR7 | Enhancing the migration, invasion, growth and survival of HCC cells | |
| CCL2 | WAT, CAF | CCR2 | Enhancing the migration, invasion, and growth of HCC cells | |
| CCL3, CCL5 | Not clarified | CCR1 | Enhancing the migration and invasion of HCC cells | |
| CCL15 | HCC cells | Not clarified | ||
| CCL20 | HCC cells | CCR6 | Enhancing the migration, invasion, and growth of HCC cells |
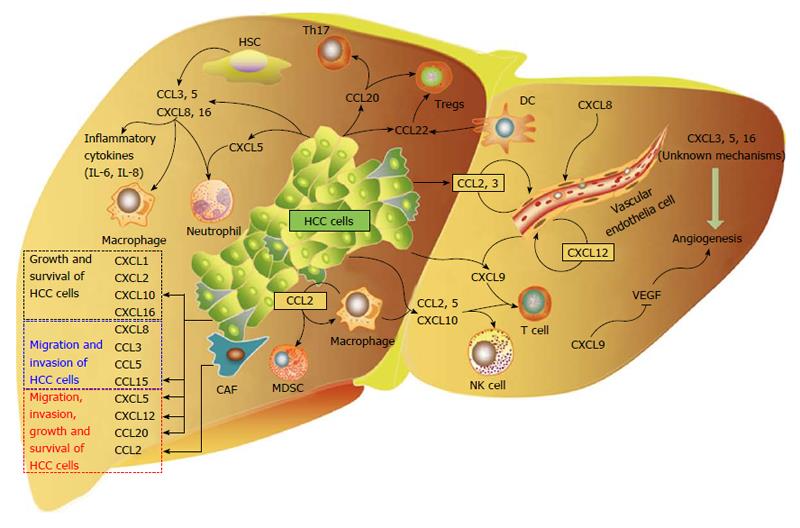
Chemokines in HCC tissues are derived from different sources, including tumor cells, and non-tumor cells such as hepatic stellate cells, T cells, macrophages, neutrophils, etc. Similarly, the chemokine receptors that are involved in the progression of HCC are either expressed on tumor cells or non-tumor cells. This complicated network reflects the mutual interaction between HCC cells and other cells in the microenvironment (Figure 1).
Of all the chemokines and their receptors in HCC, the CXC subclass accounts for the largest group. Among them, the CXCL12-CXCR4/CXCR7 axis is the most documented, and abnormal expression of either CXCL12 or CXCR4/CXCR7 is correlated with clinicopathological characteristics[12-15]. CXCL8 is a potent pro-inflammation chemokine widely studied in other tumors and it is also elevated in serum from HCC patients and represents a risk factor for survival[16]. The CXCL9/CXCL10-CXCR3 axis also shows important influences on prognosis of HCC patients[17-19]. The increase of CXCL1, CXCL2 and their common receptor CXCR2 indicates the increased risk for HCC[20,21]. The possible roles of CXCL5 and CXCL14 are unveiled too in HCC patients[22,23].
The CC subclass constitutes another major part of chemokines in HCC. Expression levels and genetic polymorphisms of CCL2 and CCR2 affect the prognosis of HCC patients[17,24,25]. The CCL5-CCR5 axis is closely correlated with liver chronic inflammation induced by different pathogens and finally participates in the development of HCC[26,27]; meanwhile, CCL3 and CCL4, the other two ligands of CCR5, show a definitive role in accelerating the course HCC[28,29]. The CCL20-CCR6 axis is a prognostic factor for HCC patients and this relates its role to recruiting regulatory T cells (Tregs)[30-32]. The other CC chemokines and receptors have also been found correlated with the clinicopathological parameters of HCC, including CCL15[33], CCL17[34], CCL22[35], CCL27[36], CCR7[37] and CCR9[38]. The CX3C subclass contains only one single member CX3CL1 and this CX3CL1-CX3CR1 axis participates in HCC[39,40].
Cancer related inflammation is the hallmark of HCC, especially for hepatitis B virus (HBV)/HCV-associated HCC, and the chemokine system has dual roles in the inflammation of HCC. On the one hand, chemokines themselves can be induced by different inflammatory cytokines such as interleukin-1 (IL-1) and IL-6, and exist as mediators for inflammation by recruiting different immune cells (details will be discussed in EFFECTS ON IMMUNE CELLS); on the other hand, chemokines can trigger the secretion of various other inflammatory cytokines from tumor cells and non-tumor cells in the microenvironment of HCC. Both of the two facets are indispensible in the inflammation of HCC.
CXCL8 is a well-defined pro-inflammatory chemokine. It is produced by HCC cells through activation of several different pathways including JNK, nuclear factor-kappa B (NF-κB), and PI3K-AKT pathways[41,42], and the elevated CXCL8 in turn induces multiple inflammatory cytokines and recruits various immune cells, all of which promotes the development of the inflammation microenvironment in HCC[43].
CCR5 mediated inflammation is also important in the development of HCC. CCL3, one ligand for CCR5, is remarkably increased in different HCC cell lines when stimulated with IL-1α or IL-1β, which consequently attracts large amount of macrophages and neutrophils into the inflammation sites[44]. The hepatic stellate cells are capable of producing a group of inflammatory cytokines including IL-6 and transforming growth factor alpha (TGF-β); blocking the CCR5 signals with maraviroc, a CCR5 antagonist, effectively abrogates the intracellular signal transduction and inhibits the progression of HCC in vivo[45]. Likewise, in the CCR5-knockout mice (Mdr2:CCR5 DKO), the oval cells, which are the putative liver progenitor cells that proliferate and differentiate in response to liver damage[46,47], show decreased levels of insulin-like growth factor-binding protein 1, secreted phosphoprotein 1, CD24, keratin 19, and epithelial cell adhesion molecule, concomitant with reduced risk of HCC[48]. Besides, activation of the CXCR6 signal in HCC cells results in increased expression of IL-6 and IL-8, while disturbing the CXCL16-CXCR6 axis can potently abrogate this effect[49].
During the infection of HCV, CXCL10 and CXCL11 play a key role in the HCV-related inflammation. Either interferon (IFN)-α or IFN-γ stimulation results in a significant increase of CXCL11, and IFN-γ shows potent synergy with TNF-α in promoting the expression of CXCL11 in vitro[50]. Resembling this phenomenon, TLR3 and RIG-1 also potentiate the induction of CXCL10 in the course of HCV infection in hepatocytes, and IFN-α/IFN-β and IFN-γ boost this induction synergistically[51].
The liver is a very special organ containing huge amount of immune cells in normal physiological state, and these immune cells consist of T cells, natural killer cells (NK cells), Kupffer cells, macrophages, neutrophils, etc. Therefore, it is considered to be a lymph organ[52,53]. During the development of HCC, the numbers and ratios of different immune cells have changed specifically, which exerts profound influences in the course of HCC, either promoting or inhibiting the tumor progression[54].
Regarding this issue, the first question is how these immune cells abnormally aggregate in HCC tumor tissues or peri-tumor tissues. The chemokines in the microenvironment have surely played a critical role[55]. In a CCR2-knockout mice model, intraportal injected colon cancer cells exhibit obvious delayed growth in liver; the reduced accumulation of macrophages and hepatic stellate cells, relying on the CCL2-CCR2 signal for effective migration to the liver, accounts for this inhibitory effects[56]. Besides, the HCC cells secrete high levels of CCL2 upon up-regulation of Forkhead box Q1, and conduct a direct chemotactic effect on macrophages, which again deteriorates the progression of HCC[57]. In addition to macrophages, the CCL2-CCR2 signal also recruits myeloid derived suppressor cells (MDSCs) into tumor tissues, and maintains the immunosuppression in the microenvironment. The HCC cell line H22 produces CCL2 constitutionally and induces the migration of MDSCs significantly in vitro[58]. Following experiments in vivo confirm this observation that the increased expression of CCL2 in tumor tissues correlates with the accumulation of MDSCs in different HCC models, either DEN-induced HCC or subcutaneously implanted HCC model[59]. However, the roles of CCL2 might be both harmful and beneficial, as suggested by the finding that the reduction of intratumoral CCL2, due to nitration by reactive nitrogen species, inhibits the infiltration of tumor specific T cells and traps these T cells in the peri-tumor stroma, contributing to the immune suppression in tumor tissues[60]. Indeed in the human HCC tissues, the CCL2 produced by both tumor cells and immune cells also correlates significantly with intratumoral CD4+ Th1 cells, CD8+ cells and NK cells, indicating a chemotactic role for these cells that favor an anti-tumor repertoire[61]. Therefore, the thorough understanding of CCL2 in HCC needs further experiments taking into account both the models and tumor stages.
Regulatory T cells (Tregs) are key modulators in tumor-induced immune suppression and the aggregation of Tregs in HCC inevitably influences the progression of HCC[62]. Different chemokines have been found to attract Tregs into the HCC tissues. In patients infected by HCV, intrahepatic levels of CCL17 and CCL22 are significantly up-regulated, correlating with the increased number of Tregs; the in vitro system identifies that dendritic cells (DCs) derived CCL17 and CCL22 leads to the enhanced aggregation of Tregs[63]. Interestingly, in HBV-positive HCC, CCL22 also recruits Tregs into tumor tissues via the TGF-β-miR-34a-CCL22 axis[64]. The CCL20-CCR6 axis is another chemokine signal that recruits Tregs into the tumor tissues. The CCL20 is highly expressed in tumor tissues and correlates with the increased number of Tregs, and the migration experiments also confirm the direct chemotactic effects of CCL20 on Tregs[31]. In concordance with these observation, our recent results also indicate a key role of chemokine system in Tregs from peripheral blood of HCC from the perspective of microRNAs[65]. However, the highly expressed CCL20 is also an important signal for Th17 cells infiltration into HCC[66]. Because Tregs and Th17 cells are two representative T cells with relatively opposite functions in most immune milieu, it is worth elucidating how the two subpopulations work in the same HCC microenvironment.
The CXCL16-CXCR6 and CXCL5-CXCR2 axes have a major effect on neutrophils in HCC. HCC cell lines and tumor tissues contain high levels of CXCL16 and CXCR6, and the latter correlates with increased neutrophils in tumor tissues and with a worsen prognosis of HCC patients[49]. It should be noted that the evidence for direct chemotaxis of neutrophils towards CXCL16 is still lacking, and it is not clear what and how this axis affects neutrophils. In contrast, CXCL5 shows an obvious chemotactic effect on neutrophils in vitro, and the level of CXCL5 in HCC tissues significantly associates with the increased neutrophils in the tumor tissues and promotes the progression of tumor[22]. CXCR3 and CCR5 are reported to facilitate different T cells traffic to the HCC, either inhibiting or promoting the progression of HCC. CXCL9 and CXCL10, the ligands for CXCR3, are produced by HCC cells and show potent attraction of CD4+ and CD8+ T cells[17,67]; CCL5 produced by tumor tissues has closely correlated with infiltration of CCR5 positive T cells and macrophages[48,61].
Although macrophages can be efficiently recruited into the tumor sites in HCC via CCL2/CCR2 and CCR5[48,56,57], their functions tightly rely on their phenotypes. Upon activation by stimuli such as antigens and cytokines, macrophages undergo different polarization into either M1 or M2 or M2-like phenotype. M1 phenotype (classical activation, stimulated by TLR ligands and IFN-γ) shows potent anti-tumor functions via production of large amount of proinflammatory cytokines, while M2 and M2-like phenotype (alternative activation, stimulated IL-4/IL-13) promote tumor progression via tissue remodeling and immunoregulation[68,69]. Therefore, it is worth exploring exactly which phenotypes dominate in the microenvironment and its roles in the progress of HCC.
The abundant chemokines in the HCC milieu contribute greatly to the aberrant infiltration of immune cells; in the meantime, some chemokine receptors on these immune cells also alter significantly, which synergistically contributes to the abnormal migration. To gain a better understanding of the chemokine system in immune cells, these alterations of chemokine receptors should not be neglected.
The initial research finds that CCR6 is reduced significantly in CD4+ and CD8+ T cells from peripheral blood in HCC patients, indicating a possible role in the recruitment of lymphocytes from peripheral blood to HCC[70]; however, following studies in delineated T cell subpopulation find that CCR6 expression is not altered significantly on Th17 cells from either tumor tissues or peripheral blood[66]. In contrast, the expression of CCR6 is significantly higher on IL-17-producing CD8+ T cells (Tc17 cells, derived from HCC tissues) and Tregs (derived from peripheral blood), suggesting a role of CCR6 facilitating Tc17 cells and Tregs infiltration into tumor tissues[31,71]. The origins of immune cells might affect the expression pattern of CCR6[72], but this need more evidence. Similarly, the expression of CCR5 is reduced significantly in CD4+ and CD8+ T cells from peripheral blood in HCC patients[70], but increased on intrahepatic CD4+ and CD8+ T cells, NKT cells, NK cells, and B cells in chronic HCV infection[73]. The detailed comparison of expression levels of CCR5 on CD4+ and CD8+ T cells from different sources yields interesting results: T cells from both tumor infiltrating leukocytes and non-tumor liver-infiltrating lymphocytes show increased levels of CCR5 compared with those from peripheral blood lymphocytes[72]. The expression of other chemokine receptors (CCR2, CCR4, CXCR3, CXCR4 and CXCR6) also exhibits certain alterations on T cells, neutrophils, NK cells, NK T cells[34,35,66,71-74].
HCC is the typical tumor with hypervascular behaviors and different anti-angiogenic treatments have been utilizing in clinical practices[75,76]. In addition to the traditional angiogenic factors including vascular endothelial growth factor (VEGF) and angiopoietins, the chemokine system is also involved in this process during the development of HCC.
The CXCL12-CXCR4/CXCR7 axis exhibits a direct pro-angiogenic effect in HCC. The initial findings in the rat model demonstrate that AMD3100 (a specific CXCR4 antagonist) simultaneously decreases the size and number of blood vessels in vivo[77]. In support of this result, experiments in vitro detect large amount of VEGF produced by the HCC cell line SMCC7721 in the presence of CXCL12; the elevated CXCL12 is responsible for tube formation of endothelial cells in vitro and in vivo[14]. Importantly, cancer stem cells or tumor-initiating cells (TICs) also utilize chemokines to facilitate the angiogenesis. Previous studies have identified CD133 as a marker of TICs in HCC; the CD133+ cells only account for 1.3%-13.6% of the cells in human primary HCC, whereas they have great potentials to self-renew and differentiate, and constitute the indispensible core for HCC cells[78,79]. The sorted CD133+ TICs secrete high levels of CXCL8, and this chemokine consequently promotes the growth and capillary tube formation of HUVECs in vitro; blocking the CXCL8 signal by neutralizing antibodies or RNA interfering in HUEVCs leads to reduction of their ability to proliferate and form capillary tubes in vitro, and animal experiments validate the angiogenic functions of CXCL8 in vivo[80].
CCL2 and CCL3, which are significantly up-regulated in endothelial cells from HCC tissues, also enhance the proliferation of endothelial cells strikingly[81]; further studies facilitated by the CCR1-knockout mice demonstrate that CCR1, the putative receptor for CCL3, directly induces the growth of endothelial cells in HCC[82]. In the CCR2-knockout mice, the reduced microvessel density is correlated with decreased number of macrophages and hepatic stellate cells which are important components during the angiogenesis[56].
Utilizing a different mechanism, CXCL3, CXCL5, CXCL8, and CXCL16 are found to recruit neutrophils into the HCC tissues, and the neutrophils have a well-defined pro-angiogenic role in hepatocarcinogenesis; disrupting these signals either by antibodies or virus-mediated silencing yields potent anti-angiogenic effects[49,83].
Not all chemokines induce angiogenesis in the context of HCC. For example, in the CXCR3-knockout mice, the microvessels in the liver are much higher than the wild type counterpart; in contrast, stimulation of chemical carcinogen carbon tetrachloride leads to the increased levels of the ligand CXCL9 that efficiently ameliorates the angiogenesis in the liver[84].
As the scenarios in the immune system[1,85], chemokines not only exert chemotaxis effects on HCC cells but also directly influence the properties of tumor cells. Now it is well demonstrated that chemokines directly affect the migration, invasion, growth and survival of tumor cells, which plays a critical role in the development of HCC.
Among the chemokines and receptors, the CXCL12-CXCR4 axis is of great importance. The first study of CXCR4 in HCC demonstrates that in the presence of CXCL12 HCC cell lines show peri-nuclear translocation of CXCR4 and increase the invasion ability significantly[86], and the increased expression level of CXCR4 in HCC tissues also correlates with the tumor size, metastasis, and survival[86-88]. The binding of CXCL12 to CXCR4 on HCC cells triggers reorganization of cytoskeleton and activates matrix metalloproteinase-9 (MMP-9) and MMP-2, both of which give rise to increased migration and invasion[89-91]. In the cancerous ascitic fluid, CXCL12 is up to 8364 pg/mL, and this concentration effectively induces the migration of HCC cells[87]. During the epithelial-mesenchymal transition (EMT), the CXCL12-CXCR4 signal also plays an important role. On the one hand, in the TGF-β induced EMT system, CXCR4 is highly expressed and required for the enhanced migration and invasion of HCC cells, and further immunostaining in tumor tissues finds that CXCR4 concentrates at the tumor border and perivascular areas[92,93]; on the other hand, CXCL12 derived from hepatic stellate cells induces EMT of HCC cells in vitro, coinciding with the increased migration[94]. The findings that CXCR4 can be modulated by several other molecules in the microenvironment complicate its roles. TGF-β, osteopontin and astrocyte elevated gene-1 significantly up-regulate the expression of CXCR4 via NF-κB, PI3K-Akt and JNK pathways[91,93,95]. Glycosaminoglycans also compete with cellular heparan sulfate chains to bind CXCL12, which finally causes inhibition of CXCL12 mediated chemotaxis of HCC cells[96].
The other receptor for CXCL12, CXCR7, also has profound effects on the migration and invasion of HCC cells. Increased expression of CXCR7 is found in HCC tumor tissues and highly invasive cell lines; knockdown of its expression in different invasive cell lines results in reduced migration and invasion abilities both in vitro and in vivo, and this reduction are partially caused by decreased levels of MMP-2 and MMP-9[14,97]. However, in a large cohort of 408 HCC samples, up-regulation of CXCR7 in HCC tissues is confirmed specifically on endothelial cells, but neither human primary hepatocytes nor HCC cell lines. Furthermore, the expression level of CXCR7 on endothelial cells is regulated by hypoxia and low pH which is the typical microenvironment in HCC[15]. These controversial results need to be verified by more experiments in future.
The effects of the CXCL12-CXCR4 axis on proliferation and survival of HCC cells are examined in different cell lines. Because of the intrinsic heterogeneity of these cell lines, the data seem a little paradoxical. Therefore, when reach the conclusions, we should be more cautious. CXCL12 stimulates the proliferation of Huh7 cells[86], possibly through activation of JNK[89]; analysis of the cell cycle demonstrates that CXCL12 triggers the transition of Huh7 cells from G0 into cycle phase, and also drives those cells in G1 phase into S, G2-M phase[89]. In contrast, in other HCC cell lines such as HepG2, there exist no or subtle such effects. Although HepG2 cells express CXCR4, the binding of CXCL12 does not trigger the Ca2+ influx, phosphorylation or internalization of CXCR4, and finally fails to activate the following cascade signals[86,98]. Consequently, blocking this axis by other molecules, such as fucoidan, obviously prevents the growth of HCC cells induced by CXCL12[99]. In another HCC cell line FaO induced by TGF-β, CXCL12 efficiently activates extracellular signal-regulated protein kinases (ERK) pathway and enhances the survival in the absence of serum; however, the proliferation and cell cycle of FaO cells is not affected[93]. Recent data also suggest that CXCL12-CXCR7 axis, another ligation of CXCL12, has the same function on proliferation of HCC cells. Silencing CXCR7 by small interfering RNAs in HCCLM3, a highly invasive HCC cell line with abundant expression of CXCR7, decreases the growth of tumor cells both in vitro and in vivo[97].
The binding of CCL5 and CCL3 to HCC cells depends on CCR1 expression. After the ligation, CCL5 stimulates the tyrosine phosphorylation of focal adhesion kinase, activates PI3K, MAPK, and Rho kinase, leading to increased migration and invasion of HCC cells[100,101]; in contrast, knocking down the expression of CCR1 on HCC cells or disrupting the binding of CCL5 to CCR1 via monoclonal antibodies against SDC-1 or SDC-4 effectively abrogates this effect[101,102]. Once binding to the CCR1, CCL3 also induces the potent influx of Ca2+ in HCC cells and consequently stimulates the formation of various pseudopodia. These downstream effects directly enhance the migration of tumor cells[103]. Other recent reports also identify a direct effect of CXCL5, CXCL8, CCL15 and CCL20 on the migration and invasion of HCC cells[22,33,104,105], which sheds more light on this field.
CXCR2, along with its ligands CXCL1, CXCL2, and CXCL5, exhibits potent functions in promoting growth of HCC cells. CXCL5 efficiently promotes the proliferation of HCC cells by activating the PI3K-Akt and ERK1/2 pathways via the receptor CXCR2[22]. In another experiment in vitro, the addition of CXCL1, CXCL2 as well as CXCL16 significantly increases the proliferation of different HCC cell lines[106]. In addition, other chemokines belonging to the CXC family potently drive the growth and survival of hepatocytes under certain pathophysiological conditions such as toxic liver injury which increases the risk of HCC. CXCL10 is largely secreted by hepatocytes treated with CCL4 in the acute toxic liver injury model, and this increased chemokine efficiently rescues the injured hepatocytes from death in an autocrine manner[107].
CCL2 secreted by white adipose tissue induces lipid accumulation in both the primary hepatocytes and Huh7 cells, suggesting a direct role of CCL2 in the pathogenesis of liver steatosis[108]. In other studies, treating HCC cells with apigenin or co-culture them with cancer-associated fibroblasts significantly inhibits or promotes the proliferation of HCC cells, accompanied by the increase of CCR2/CCL2[109,110]. Nevertheless, direct evidence demonstrating the effects of CCL2-CCR2 axis on HCC cells is needed. In contrast, CCL20 has a definitive role and directly enhances the growth of HCC cells through activating the p44/42 MAPK pathway[111].
HCC is among one of the most refractory tumors resistant to chemotherapies, and now there exist very few drugs available for systemic chemotherapies[112]. The important roles the chemokine system played pave new roads to solve this problem, albeit there are no chemokine-based therapies approved in clinical practices at present.
The roles of each single chemokine and the corresponding receptor in the development of HCC are well documented, and disruption of the signal axis indeed hinders the invasive behaviors of HCC; however, due to the complex of microenvironment in HCC, targeting the chemokines alone might not be enough for successful treatments. In contrast, many studies have already found that the chemokine-based combination therapies are promising.
The bicistronic recombinant adenovirus vector expressing HSV thymidine kinase, a suicide gene, and CCL2 on HCC cells has shown remarkable anti-tumor effects in different HCC models. The apoptosis of HCC cells and abundantly accumulated CCL2 synergistically elicits enhanced infiltration of M1 macrophages and NK cells, as well as elevated IL-12 and IL-18 in tumor tissues[113-115]. In the following work, an improved system with adenovirus vector expressing membrane-bound form of CCL2 manifests more powerful anti-tumor effects with increased intratumoral Mac-1+ macrophages, CD4+ and CD8+ T cells[116].
Although the role of CCL21/CCL19-CCR7 axis in the development of HCC has not been clearly elucidated, the powerful chemotactic effects of this axis and the specific immune milieu in the liver prompt us to explore the therapeutic effects of the CCL21-CCR7 axis. Over-expression of CCL21 either in HCC cells or in DCs shows potent anti-tumor effects in HCC models. Within the tumors containing high level of CCL21, the number of CD4+ and CD8+ T cells and DCs significantly increases, along with elevated levels of IL-12 and IFN-γ and reduced microvessels[117,118]. To further enforce the anti-tumor effects, we devise the new treatment policy by combination of CCL21 and depleting the immunosuppressive Tregs. The combination therapy manifests better anti-tumor effects with increased intratumoral CD4+ and CD8+ T cells and decreased Tregs not only in the local tumor tissues but also in peripheral lymph organs; in addition, the profiles of cytokines and MMPs are also optimized in tumor tissues[119].
Combination therapies based on IL-12 treatment are very effective in different HCC models. CXCL10 is utilized and the co-transfer of IL-12 and CXCL10 yields a very powerful anti-tumor effect, in which the tumor specific cytotoxic lymphocytes and NK cells both play a key role[120]. With the same inspiration, combination of CXCL10/IL-12 expression vector with α-fetoprotein DNA vaccination also achieves better anti-tumor effects and significantly prolongs the survival of the model mice of HCC[121].
In addition, the traditional therapies of HCC can benefit from chemokine-based treatments. Radiofrequency ablation (RFA) is used to locally eradicate HCC, but the recurrence is relatively high. In the HCC mice model, RFA in combination with injection of CCL3 significantly enhances the number of CD4+, CD8+ T cells and CD11c+ DCs in a CCR1-dependent manner, which finally leads to an obvious inhibition of tumor growth[122]. Cisplatin (cis-diamminedichloroplatinum) reduces the tumor burden by 52%, while the combination of cisplatin and G31P (the CXCL8 antagonist) remarkably enhances the suppression effects; meanwhile, the side effects of cispaltin are also released obviously[123].
During the carcinogenesis of HCC, the tumor itself needs pivotal mediators to efficiently modulate the microenvironment. These mediators should simultaneously fulfill the basics of tumor cells and steer or disable the functions of immune cells; the chemokines and their receptors are the ideal mediators. Firstly, the Morse code applied by immune cells for routine surveillance is the most effective way to patrol the body, but this code is unfortunately spied by the tumor cells, by which the tumor cells learn and gain effective invasion and dissemination. Secondly, the mechanisms and weapons gifted by the chemokine system, which are originally authorized to the normal immune cells, are excessively utilized by tumor cells in a pro-tumor way, such as modulating the cell cycle and survival, recruiting other immune cells, and secretion of MMPs, etc. Thirdly, the bidirectional influences between the tumor cells and the immune cells are bridged by the chemokine system, and this mutual interaction stabilizes the immunosuppression in the microenvironment of HCC. Taking advantages of the strength rooting in the chemokine system, the HCC cells achieve quick progression even when confronted with the host immune system.
Although we have not succeeded in managing HCC through targeting the chemokine system, the more we understand this system in the context of tumor, the more treatment options we will have. It is likely that the future translational research will give us more answers in verifying the therapeutic value of this complicated system in HCC.
P- Reviewer: Fabris L, Solinas A S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 907] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 2. | Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 4. | Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 525] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 5. | Molon B, Gri G, Bettella M, Gómez-Moutón C, Lanzavecchia A, Martínez-A C, Mañes S, Viola A. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Sun J, Zhang Y, Yang M, Zhang Y, Xie Q, Li Z, Dong Z, Yang Y, Deng B, Feng A. Hypoxia induces T-cell apoptosis by inhibiting chemokine C receptor 7 expression: the role of adenosine receptor A(2). Cell Mol Immunol. 2010;7:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kim JW, Ferris RL, Whiteside TL. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin Cancer Res. 2005;11:7901-7910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Coghill JM, Fowler KA, West ML, Fulton LM, van Deventer H, McKinnon KP, Vincent BG, Lin K, Panoskaltsis-Mortari A, Cook DN. CC chemokine receptor 8 potentiates donor Treg survival and is critical for the prevention of murine graft-versus-host disease. Blood. 2013;122:825-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1-17. |
| 10. | Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210-212. [PubMed] |
| 11. | Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449-1459. [PubMed] |
| 12. | Shibuta K, Begum NA, Mori M, Shimoda K, Akiyoshi T, Barnard GF. Reduced expression of the CXC chemokine hIRH/SDF-1alpha mRNA in hepatoma and digestive tract cancer. Int J Cancer. 1997;73:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF. Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res. 2002;93:789-797. [PubMed] |
| 14. | Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Monnier J, Boissan M, L’Helgoualc’h A, Lacombe ML, Turlin B, Zucman-Rossi J, Théret N, Piquet-Pellorce C, Samson M. CXCR7 is up-regulated in human and murine hepatocellular carcinoma and is specifically expressed by endothelial cells. Eur J Cancer. 2012;48:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Ren Y, Poon RT, Tsui HT, Chen WH, Li Z, Lau C, Yu WC, Fan ST. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res. 2003;9:5996-6001. [PubMed] |
| 17. | Yoong KF, Afford SC, Jones R, Aujla P, Qin S, Price K, Hubscher SG, Adams DH. Expression and function of CXC and CC chemokines in human malignant liver tumors: a role for human monokine induced by gamma-interferon in lymphocyte recruitment to hepatocellular carcinoma. Hepatology. 1999;30:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Hirano S, Iwashita Y, Sasaki A, Kai S, Ohta M, Kitano S. Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes. J Gastroenterol Hepatol. 2007;22:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Zhang P, Chan DW, Zhu Y, Li JJ, Ng IO, Wan D, Gu J. Identification of carboxypeptidase of glutamate like-B as a candidate suppressor in cell growth and metastasis in human hepatocellular carcinoma. Clin Cancer Res. 2006;12:6617-6625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Ueda S, Basaki Y, Yoshie M, Ogawa K, Sakisaka S, Kuwano M, Ono M. PTEN/Akt signaling through epidermal growth factor receptor is prerequisite for angiogenesis by hepatocellular carcinoma cells that is susceptible to inhibition by gefitinib. Cancer Res. 2006;66:5346-5353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Liu Z, Yang L, Xu J, Zhang X, Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res. 2011;166:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 23. | Wang W, Huang P, Zhang L, Wei J, Xie Q, Sun Q, Zhou X, Xie H, Zhou L, Zheng S. Antitumor efficacy of C-X-C motif chemokine ligand 14 in hepatocellular carcinoma in vitro and in vivo. Cancer Sci. 2013;104:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Shin EC, Choi YH, Kim JS, Kim SJ, Park JH. Expression patterns of cytokines and chemokines genes in human hepatoma cells. Yonsei Med J. 2002;43:657-664. [PubMed] |
| 25. | Yeh CB, Tsai HT, Chen YC, Kuo WH, Chen TY, Hsieh YH, Chou MC, Yang SF. Genetic polymorphism of CCR2-64I increased the susceptibility of hepatocellular carcinoma. J Surg Oncol. 2010;102:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Lin YL, Liu CC, Chuang JI, Lei HY, Yeh TM, Lin YS, Huang YH, Liu HS. Involvement of oxidative stress, NF-IL-6, and RANTES expression in dengue-2-virus-infected human liver cells. Virology. 2000;276:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Nahon P, Sutton A, Rufat P, Simon C, Trinchet JC, Gattegno L, Beaugrand M, Charnaux N. Chemokine system polymorphisms, survival and hepatocellular carcinoma occurrence in patients with hepatitis C virus-related cirrhosis. World J Gastroenterol. 2008;14:713-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Yang X, Lu P, Fujii C, Nakamoto Y, Gao JL, Kaneko S, Murphy PM, Mukaida N. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer. 2006;118:1869-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Uchida H, Iwashita Y, Sasaki A, Shibata K, Matsumoto T, Ohta M, Kitano S. Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 32. | Ding X, Wang K, Wang H, Zhang G, Liu Y, Yang Q, Chen W, Hu S. High expression of CCL20 is associated with poor prognosis in patients with hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2012;16:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Li Y, Wu J, Zhang W, Zhang N, Guo H. Identification of serum CCL15 in hepatocellular carcinoma. Br J Cancer. 2013;108:99-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Tsuda Y, Fukui H, Asai A, Fukunishi S, Miyaji K, Fujiwara S, Teramura K, Fukuda A, Higuchi K. An immunosuppressive subtype of neutrophils identified in patients with hepatocellular carcinoma. J Clin Biochem Nutr. 2012;51:204-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, Loh M, Bolze A, Quek R, Lee VK. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 2010;52:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 36. | Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, Scala S, Izzo F, Castello G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw. 2010;21:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 37. | Schimanski CC, Bahre R, Gockel I, Junginger T, Simiantonaki N, Biesterfeld S, Achenbach T, Wehler T, Galle PR, Moehler M. Chemokine receptor CCR7 enhances intrahepatic and lymphatic dissemination of human hepatocellular cancer. Oncol Rep. 2006;16:109-113. |
| 38. | Zhang Z, Qin C, Wu Y, Su Z, Xian G, Hu B. CCR9 as a prognostic marker and therapeutic target in hepatocellular carcinoma. Oncol Rep. 2014;31:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Efsen E, Grappone C, DeFranco RM, Milani S, Romanelli RG, Bonacchi A, Caligiuri A, Failli P, Annunziato F, Pagliai G. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J Hepatol. 2002;37:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Matsubara T, Ono T, Yamanoi A, Tachibana M, Nagasue N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J Surg Oncol. 2007;95:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, McClain CJ. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 42. | Wang Y, Wang W, Wang L, Wang X, Xia J. Regulatory mechanisms of interleukin-8 production induced by tumour necrosis factor-α in human hepatocellular carcinoma cells. J Cell Mol Med. 2012;16:496-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Yu J, Ren X, Chen Y, Liu P, Wei X, Li H, Ying G, Chen K, Winkler H, Hao X. Dysfunctional activation of neurotensin/IL-8 pathway in hepatocellular carcinoma is associated with increased inflammatory response in microenvironment, more epithelial mesenchymal transition in cancer and worse prognosis in patients. PLoS One. 2013;8:e56069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Lu P, Nakamoto Y, Nemoto-Sasaki Y, Fujii C, Wang H, Hashii M, Ohmoto Y, Kaneko S, Kobayashi K, Mukaida N. Potential Interaction between CCR1 and Its Ligand, CCL3, Induced by Endogenously Produced Interleukin-1 in Human Hepatomas. Am J Pathol. 2003;162:1249-1258. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Ochoa-Callejero L, Pérez-Martínez L, Rubio-Mediavilla S, Oteo JA, Martínez A, Blanco JR. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One. 2013;8:e53992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M. Surface markers for the murine oval cell response. Hepatology. 2008;48:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Barashi N, Weiss ID, Wald O, Wald H, Beider K, Abraham M, Klein S, Goldenberg D, Axelrod J, Pikarsky E. Inflammation-induced hepatocellular carcinoma is dependent on CCR5 in mice. Hepatology. 2013;58:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, Yi Y, Shi JY, Shi GM, Ding ZB. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. 2012;72:3546-3556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Brownell J, Wagoner J, Lovelace ES, Thirstrup D, Mohar I, Smith W, Giugliano S, Li K, Crispe IN, Rosen HR. Independent, parallel pathways to CXCL10 induction in HCV-infected hepatocytes. J Hepatol. 2013;59:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Nemeth E, Baird AW, O’Farrelly C. Microanatomy of the liver immune system. Semin Immunopathol. 2009;31:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 743] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 54. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 55. | Oo YH, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. J Autoimmun. 2010;34:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Yang X, Lu P, Ishida Y, Kuziel WA, Fujii C, Mukaida N. Attenuated liver tumor formation in the absence of CCR2 with a concomitant reduction in the accumulation of hepatic stellate cells, macrophages and neovascularization. Int J Cancer. 2006;118:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Xia L, Huang W, Tian D, Zhang L, Qi X, Chen Z, Shang X, Nie Y, Wu K. Forkhead box Q1 promotes hepatocellular carcinoma metastasis by transactivating ZEB2 and VersicanV1 expression. Hepatology. 2014;59:958-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 58. | Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 60. | Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949-1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 61. | Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 62. | Byrne WL, Mills KH, Lederer JA, O‘Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71:6915-6920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Riezu-Boj JI, Larrea E, Aldabe R, Guembe L, Casares N, Galeano E, Echeverria I, Sarobe P, Herrero I, Sangro B. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, Deng Y, Zhao J, Jiang S, Yuan Y. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 65. | Chen L, Ma H, Hu H, Gao L, Wang X, Ma J, Gao Q, Liu B, Zhou G, Liang C. Special role of Foxp3 for the specifically altered microRNAs in Regulatory T cells of HCC patients. BMC Cancer. 2014;14:489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 67. | Liu YQ, Poon RT, Hughes J, Li QY, Yu WC, Fan ST. Desensitization of T lymphocyte function by CXCR3 ligands in human hepatocellular carcinoma. World J Gastroenterol. 2005;11:164-170. [PubMed] |
| 68. | Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3696] [Cited by in RCA: 4732] [Article Influence: 364.0] [Reference Citation Analysis (1)] |
| 69. | Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (1)] |
| 70. | Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. Am J Gastroenterol. 2004;99:1111-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol. 2010;185:1544-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Liu Y, Poon RT, Hughes J, Feng X, Yu WC, Fan ST. Chemokine receptors support infiltration of lymphocyte subpopulations in human hepatocellular carcinoma. Clin Immunol. 2005;114:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Wang J, Holmes TH, Cheung R, Greenberg HB, He XS. Expression of chemokine receptors on intrahepatic and peripheral lymphocytes in chronic hepatitis C infection: its relationship to liver inflammation. J Infect Dis. 2004;190:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Zhang PJ, Wei R, Wen XY, Ping L, Wang CB, Dong ZN, Deng XX, Bo W, Bin C, Tian YP. Genes expression profiling of peripheral blood cells of patients with hepatocellular carcinoma. Cell Biol Int. 2012;36:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Sun HC, Tang ZY. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Cancer Res Clin Oncol. 2004;130:307-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Jain RK, Carmeliet P. SnapShot: Tumor angiogenesis. Cell. 2012;149:1408-1408.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Abe H, Ina K, Kitamura H, Sumiyoshi H, Tatsukawa S, Yoshioka H, Fujikura Y. Role of the CXCL12/CXCR4 axis in milky spots of rats bearing ascitic-type hepatoma. Anat Sci Int. 2009;84:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 79. | Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, Ng I, Man K, Wong N, To KF. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7:694-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 80. | Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 81. | Ryschich E, Lizdenis P, Ittrich C, Benner A, Stahl S, Hamann A, Schmidt J, Knolle P, Arnold B, Hämmerling GJ. Molecular fingerprinting and autocrine growth regulation of endothelial cells in a murine model of hepatocellular carcinoma. Cancer Res. 2006;66:198-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Rodero MP, Auvynet C, Poupel L, Combadière B, Combadière C. Control of both myeloid cell infiltration and angiogenesis by CCR1 promotes liver cancer metastasis development in mice. Neoplasia. 2013;15:641-648. [PubMed] |
| 83. | Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 84. | Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, Nellen A, Schmitz P, Heinrichs D, Berres ML. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 85. | Bachmann MF, Kopf M, Marsland BJ. Chemokines: more than just road signs. Nat Rev Immunol. 2006;6:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Schimanski CC, Bahre R, Gockel I, Müller A, Frerichs K, Hörner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 87. | Liu H, Pan Z, Li A, Fu S, Lei Y, Sun H, Wu M, Zhou W. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Li N, Guo W, Shi J, Xue J, Hu H, Xie D, Wu M, Cheng S. Expression of the chemokine receptor CXCR4 in human hepatocellular carcinoma and its role in portal vein tumor thrombus. J Exp Clin Cancer Res. 2010;29:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Sutton A, Friand V, Brulé-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poiré A, Saffar L, Kraemer M, Vassy J. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007;39:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Zhang R, Pan X, Huang Z, Weber GF, Zhang G. Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS One. 2011;6:e23831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Bertran E, Crosas-Molist E, Sancho P, Caja L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E. Overactivation of the TGF-β pathway confers a mesenchymal-like phenotype and CXCR4-dependent migratory properties to liver tumor cells. Hepatology. 2013;58:2032-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 93. | Bertran E, Caja L, Navarro E, Sancho P, Mainez J, Murillo MM, Vinyals A, Fabra A, Fabregat I. Role of CXCR4/SDF-1 alpha in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-beta. Cell Signal. 2009;21:1595-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Li X, Li P, Chang Y, Xu Q, Wu Z, Ma Q, Wang Z. The SDF-1/CXCR4 axis induces epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Cell Biochem. 2014;392:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Zhou Z, Deng H, Yan W, Luo M, Tu W, Xia Y, He J, Han P, Fu Y, Tian D. AEG-1 promotes anoikis resistance and orientation chemotaxis in hepatocellular carcinoma cells. PLoS One. 2014;9:e100372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Friand V, Haddad O, Papy-Garcia D, Hlawaty H, Vassy R, Hamma-Kourbali Y, Perret GY, Courty J, Baleux F, Oudar O. Glycosaminoglycan mimetics inhibit SDF-1/CXCL12-mediated migration and invasion of human hepatoma cells. Glycobiology. 2009;19:1511-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Xue TC, Chen RX, Han D, Chen J, Xue Q, Gao DM, Sun RX, Tang ZY, Ye SL. Down-regulation of CXCR7 inhibits the growth and lung metastasis of human hepatocellular carcinoma cells with highly metastatic potential. Exp Ther Med. 2012;3:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Mitra P, De A, Ethier MF, Mimori K, Kodys K, Shibuta K, Mori M, Madison JM, Miller-Graziano C, Barnard GF. Loss of chemokine SDF-1alpha-mediated CXCR4 signalling and receptor internalization in human hepatoma cell line HepG2. Cell Signal. 2001;13:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 99. | Nagamine T, Hayakawa K, Kusakabe T, Takada H, Nakazato K, Hisanaga E, Iha M. Inhibitory effect of fucoidan on Huh7 hepatoma cells through downregulation of CXCL12. Nutr Cancer. 2009;61:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Sutton A, Friand V, Papy-Garcia D, Dagouassat M, Martin L, Vassy R, Haddad O, Sainte-Catherine O, Kraemer M, Saffar L. Glycosaminoglycans and their synthetic mimetics inhibit RANTES-induced migration and invasion of human hepatoma cells. Mol Cancer Ther. 2007;6:2948-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Charni F, Friand V, Haddad O, Hlawaty H, Martin L, Vassy R, Oudar O, Gattegno L, Charnaux N, Sutton A. Syndecan-1 and syndecan-4 are involved in RANTES/CCL5-induced migration and invasion of human hepatoma cells. Biochim Biophys Acta. 2009;1790:1314-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Wu X, Fan J, Wang X, Zhou J, Qiu S, Yu Y, Liu Y, Tang Z. Downregulation of CCR1 inhibits human hepatocellular carcinoma cell invasion. Biochem Biophys Res Commun. 2007;355:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Yuan Y, Liu J, Liu Z, He Y, Zhang Z, Jiang C, Qian Q. Chemokine CCL3 facilitates the migration of hepatoma cells by changing the concentration intracellular Ca. Hepatol Res. 2010;40:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Wu LH, Shi BZ, Zhao QL, Wu XZ. Fucosylated glycan inhibition of human hepatocellular carcinoma cell migration through binding to chemokine receptors. Glycobiology. 2010;20:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Du D, Liu Y, Qian H, Zhang B, Tang X, Zhang T, Liu W. The effects of the CCR6/CCL20 biological axis on the invasion and metastasis of hepatocellular carcinoma. Int J Mol Sci. 2014;15:6441-6452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Vansaun MN, Mendonsa AM, Lee Gorden D. Hepatocellular proliferation correlates with inflammatory cell and cytokine changes in a murine model of nonalchoholic fatty liver disease. PLoS One. 2013;8:e73054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 107. | Chan CC, Lee KC, Huang YH, Chou CK, Lin HC, Lee FY. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J Gastroenterol Hepatol. 2014;29:603-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Clément S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, Meier CA, Negro F. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology. 2008;48:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 109. | Cai J, Zhao XL, Liu AW, Nian H, Zhang SH. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine. 2011;18:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Lin ZY, Chuang YH, Chuang WL. Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells. Biomed Pharmacother. 2012;66:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 111. | Fujii H, Itoh Y, Yamaguchi K, Yamauchi N, Harano Y, Nakajima T, Minami M, Okanoue T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res Commun. 2004;322:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 113. | Tsuchiyama T, Kaneko S, Nakamoto Y, Sakai Y, Honda M, Mukaida N, Kobayashi K. Enhanced antitumor effects of a bicistronic adenovirus vector expressing both herpes simplex virus thymidine kinase and monocyte chemoattractant protein-1 against hepatocellular carcinoma. Cancer Gene Ther. 2003;10:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Tomoya T, Yasunari N, Yoshio S, Yohei M, Masaaki K, Naofumi M, Shuichi K. Prolonged, NK Cell-Mediated Antitumor Effects of Suicide Gene Therapy Combined with Monocyte Chemoattractant Protein-1 against Hepatocellular Carcinoma. JI. 2007;178:574-583. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Tsuchiyama T, Nakamoto Y, Sakai Y, Mukaida N, Kaneko S. Optimal amount of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy against hepatocellular carcinoma by M1 macrophage activation. Cancer Sci. 2008;99:2075-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Marukawa Y, Nakamoto Y, Kakinoki K, Tsuchiyama T, Iida N, Kagaya T, Sakai Y, Naito M, Mukaida N, Kaneko S. Membrane-bound form of monocyte chemoattractant protein-1 enhances antitumor effects of suicide gene therapy in a model of hepatocellular carcinoma. Cancer Gene Ther. 2012;19:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Liang CM, Zhong CP, Sun RX, Liu BB, Huang C, Qin J, Zhou S, Shan J, Liu YK, Ye SL. Local expression of secondary lymphoid tissue chemokine delivered by adeno-associated virus within the tumor bed stimulates strong anti-liver tumor immunity. J Virol. 2007;81:9502-9511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Liang CM, Ye SL, Zhong CP, Zheng N, Bian W, Sun RX, Chen J, Li RL, Zhou S, Liu YK. More than chemotaxis: a new anti-tumor DC vaccine modified by rAAV2-SLC. Mol Immunol. 2007;44:3797-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 119. | Chen L, Zhou S, Qin J, Hu H, Ma H, Liu B, Wang X, Ma J, Ye S, Zhong C. Combination of SLC administration and Tregs depletion is an attractive strategy for targeting hepatocellular carcinoma. Mol Cancer. 2013;12:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, Melero I, Prieto J. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol. 2000;164:3112-3122. [PubMed] |
| 121. | Rodríguez MM, Ryu SM, Qian C, Geissler M, Grimm C, Prieto J, Blum HE, Mohr L. Immunotherapy of murine hepatocellular carcinoma by alpha-fetoprotein DNA vaccination combined with adenovirus-mediated chemokine and cytokine expression. Hum Gene Ther. 2008;19:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Iida N, Nakamoto Y, Baba T, Nakagawa H, Mizukoshi E, Naito M, Mukaida N, Kaneko S. Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC Chemokine ligand 3/macrophage inflammatory protein-1alpha. Cancer Res. 2010;70:6556-6565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 123. | Wei J, Chen X, Li Q, Chen J, Khan N, Wang B, Cheng JW, Gordon JR, Li F. ELR-CXC chemokine antagonism and cisplatin co-treatment additively reduce H22 hepatoma tumor progression and ameliorate cisplatin-induced nephrotoxicity. Oncol Rep. 2014;31:1599-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |