Peer-review started: August 20, 2014
First decision: October 14, 2014
Revised: October 30, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: January 27, 2015
Processing time: 143 Days and 16.7 Hours
Hepatitis C is a strong prognostic factor for patients with hepatocellular carcinoma (HCC). Although liver resection and liver transplantation offer the chance of a cure for HCC, adequate management of co-existing infection with hepatitis C virus (HCV) is important to enable better long-term outcomes after surgery for HCV-related HCC. For patients undergoing liver resection, perioperative anti-viral treatment is recommended, since a decreased HCV viral load itself is reportedly associated with a lower tumor recurrence rate and a longer overall survival. For patients undergoing transplanatations for HCC complicated by end-stage liver disease, the post-transplant management of HCV infection is also necessary to prevent progressive graft injury caused by active hepatitis under the immunosuppressive condition that is needed after liver transplantation. Although only a few lines of solid evidence are available for postoperative antiviral treatment because of the limited indication and frequent adverse events caused by conventional high-dose combination interferon therapy, new direct acting anti-viral agents would enable interferon-free anti-viral treatment with a higher virologic response and minimal side effects.
Core tip: Hepatitis C infection is associated with a poor survival outcome after curative surgical resection or liver transplantation in patients with hepatocellular carcinoma (HCC). For patients undergoing liver resection, the adequate perioperative management of hepatitis C is vital for reducing the carcinogenic potential of the liver remnant and obtaining a longer disease-free interval. For patients undergoing transplantations for HCC with end-stage liver disease, the control of hepatitis C is also needed to avoid progressive graft dysfunction because of active hepatitis under immunosuppressive condition.
- Citation: Shindoh J, Hashimoto M, Watanabe G. Surgical approach for hepatitis C virus-related hepatocellular carcinoma. World J Hepatol 2015; 7(1): 70-77
- URL: https://www.wjgnet.com/1948-5182/full/v7/i1/70.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i1.70
Primary liver cancer is one of the most common solid tumors and is the second leading cause of cancer-related deaths worldwide[1]. Despite recent developments in the prevention and treatment of viral hepatitis, approximately 746000 deaths were reported in 2012 among patients with primary liver cancer. Nowadays, various treatment options are available for the most common type of primary liver cancer, hepatocellular carcinoma (HCC), including surgical resection, ablation therapies, arterial chemoembolization, radioembolization, and systemic therapies. However, the presence of chronic liver disease in the underlying liver and a high tumor recurrence rate even after curative-intent treatment make the management of HCC difficult[2,3].
HCC usually arises in liver tissue that has been injured because of chronic hepatitis or cirrhosis; accordingly, the impaired hepatic functional reserve often precludes curative treatment options. Therefore, several clinical algorithms have been proposed for the optimal selection of HCC treatments, with consideration given to (1) the size and number of tumors; (2) the presence of extrahepatic disease; (3) the hepatic functional reserve; and (4) the performance status of the patient[4,5]. Currently, surgical resection and liver transplantation are the two mainstays of surgical treatment for patients with a limited number of HCC lesions. These approaches may offer a higher chance of a cure through the eradication of micrometastases surrounding the main tumor, thereby improving the recurrence-free survival rate compared with those after ablation therapies[6-10]. However, the presence of hepatitis C virus (HCV) is significantly related to a poor survival outcome, compared with other etiologies of HCC, among patients undergoing liver resection[11] or liver transplantation[10]. Therefore, careful perioperative management is required for patients with HCV-related HCC.
Because persistent viremia and active hepatitis after surgical resection are thought to be potent risk factors for progressive histopathologic injury and multicentric carcinogenesis in the underlying liver, adjuvant antiviral therapy is theoretically preferable after curative treatment for HCV-related HCC. Nevertheless, only a few lines of evidence regarding the efficacy of postoperative antiviral therapy are available[12,13] partially because of the limited indication for conventional high-dose antiviral therapy among elderly or cirrhotic patients and the genetic variability of HCV, which determines its refractoriness to interferon (IFN)-based combination therapies. In the present era of new direct-acting antiviral agents (DAAs), however, the virologic response rate has been dramatically improved[14-16] and these new drugs may change the current perioperative management of HCV-related HCC. The purpose of this review was to summarize the clinical features of HCV-related HCC, to clarify the current clinical problems, and to discuss optimal surgical approaches based on reported evidence.
The MEDLINE electronic database for English-language articles was searched for reports published between January 1991 and October 2014 by using the keywords “hepatocellular carcinoma”, “hepatitis C”, and “surgery”. The reference lists of the relevant articles were also scanned for additional studies.
Differences in the etiologies and pathogenic mechanisms of carcinogenesis may reflect the clinical characteristics of HCC[17-19]. Hepatitis C is one of the leading causes of HCC[20] and a Japanese nationwide survey performed by the Liver Cancer Study Group of Japan (LCSGJ) reported that 67.7% of patients with HCC were positive for hepatitis C[21]. Patients infected with HCV reportedly have a higher risk of HCC compared with those infected with HBV[22-24]. This situation can probably be explained by the fact that no effective treatment for hepatitis C that can be used for all patients with minimal adverse events has been available until recently.
When a patient is diagnosed as having HCC, the current clinical algorithms[4,5,25] offer similar therapeutic options irrespective of the etiology of the disease. However, several studies have reported that HCC emerging on a background of viral hepatitis was associated with a poorer long-term survival, compared with HCC without viral hepatitis[26-29]. A recent large cohort study from LCSGJ has shown that hepatitis C infection is a significant prognostic factor and that HCV-related HCC was associated with poorer survival outcomes in terms of both the recurrence-free survival rate and the overall survival rate after surgical resection, compared with those of HCC patients without viral hepatitis[11].
Given these natural history and clinical features of HCV-related HCC, the management of patients should be considered in terms of the following 3 steps: (1) treatment BEFORE emerging HCC; (2) treatment FOR HCC; and (3) adjuvant management AFTER treatment for HCC.
A recent meta-analysis has reported that a sustained virologic response (SVR) after treatment for HCV is associated with a reduced incidence of HCC at any stage of fibrosis[30]. Conventionally, combination therapy using IFN and ribavirin has been used for patients with chronic active hepatitis C. However, the SVR rate associated with the conventional IFN-based treatment was not satisfactory because of the high prevalence of HCV genotype 1b which is associated with a poor response to anti-viral therapy. Also, adverse effects such as fever, pancytopenia, interstitial pneumonia, and depression, frequently preclude treatment with an adequate intensity and duration in elderly and cirrhotic patients.
However, with the recent introduction of DAAs, the SVR rate of HCV has changed dramatically, and adverse events caused by conventional IFN-based therapies have been avoided using these new IFN-free regimens[14-16]. Although the efficacy of these new drugs with regard to the incidence of HCC needs to be clarified in the near future, anti-viral treatment is recommended for all patients with serologically positive hepatitis C based on the expected reduction in the carcinogenic potential of the underlying liver tissue.
When HCC is diagnosed incidentally or during a follow-up examination for chronic liver disease, the optimal treatment options are selected according to the oncologic and physical status of the patient. In the Barcelona Clinic Liver Cancer (BCLC) algorithm[5,25], surgical resection is indicated for patients with a good performance status and solitary HCC when there is no evidence of portal hypertension. Liver transplantation is recommended for patients with HCC who meet the Milan criteria (solitary tumor ≤ 5 cm or ≤ 3 tumors with each tumor ≤ 3 cm)[31], regardless of the hepatic functional reserve. In the guideline for liver cancer treatment proposed by the Japan Society of Hepatology[4], surgical resection is currently indicated for Child-Pugh class A or class B patients with HCC less than or equal to 3 nodules irrespective of the size of each tumor, while liver transplantation is limited to only Child-Pugh class C patients who meet the Milan criteria.
Liver resection is usually indicated for patients with oligonodular HCC and a preserved hepatic functional reserve[4,5,25]. Portal hypertension is basically considered to be a contraindication for surgery. However, favorable surgical outcomes have also been reported in a carefully selected population with portal hypertension who received meticulous perioperative management[32]. The basic principles of liver resection are not influenced by the etiologies of HCC. However, careful preoperative assessment and surgical planning are needed to maximize both the surgical curability and the safety of the surgery.
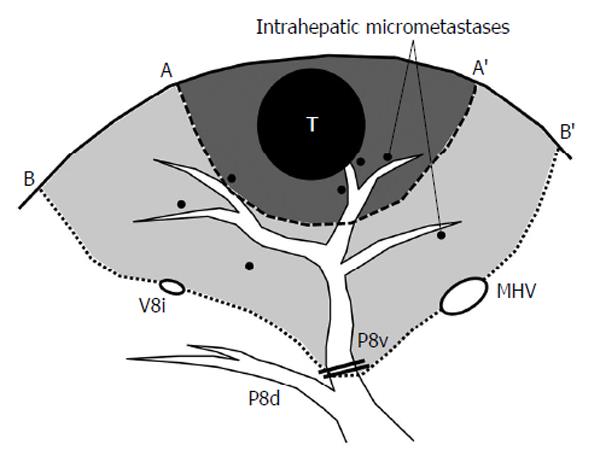
To secure the curability of surgery, an accurate preoperative assessment of the tumor extent is important. Dynamic computed tomography (CT) and enhanced magnetic resonance imaging (MRI) can sensitively delineate HCCs, and ultrasonography allows surgeons to confirm the three-dimensional (3-D) relationship between the tumors and the surrounding major vascular structures. In these imaging studies, the location of the tumor should be described according to the Couinaud’s classification of liver segments for the adequate selection of surgical maneuvers. Because HCC tends to spread via portal veins, the “anatomic resection” of the tumor-bearing portal territory (Figure 1) is a theoretically reasonable approach for HCC. Although the true efficacy of anatomic resection remains uncertain, various studies have reported a superior outcome after anatomic resection with an apparently lower rate of local recurrence, compared with non-anatomic limited resections of the liver[33-35].
Next to these accurate assessments of tumor distribution and the selection of a surgical maneuver for a cure, the risk of resection should be evaluated using systematic volumetry and hepatic functional tests such as the indocyanine green clearance test or 99mTc-GSA scintigraphy. Because the excessive removal of the hepatic parenchyma increases the risk of postoperative hepatic insufficiency according to the quality of the underlying liver[36-39], the extent of the resection should be balanced between the surgical curability and the hepatic functional reserve[40].
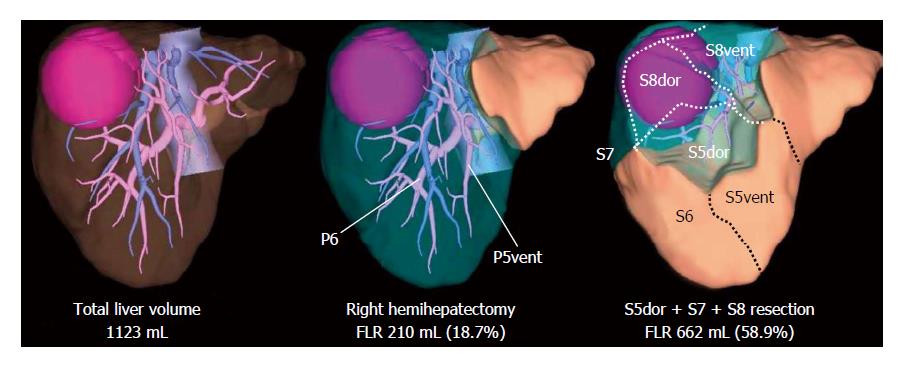
With recent developments in 3-D simulation techniques, surgical planning procedures have become easier through the simulation of various hepatic resections on a computer prior to surgery. This technique offers accurate anatomic confirmation and automatic calculation of the absolute volume of an interested part of the liver. When planning a complex anatomic resection, preoperative 3-D liver simulation is mandatory and volume estimation using the simulation software is helpful in determining the surgical indications, especially for patients with a marginal hepatic functional reserve (Figure 2).
Liver transplantation is a reasonable approach with a theoretically higher chance of tumor eradication, especially in patients with severe hepatic dysfunction. The clinical outcomes of liver transplantation for HCC were initially poor in the early era of this treatment[41,42]. However, since the publication of the landmark study by Mazzaferro et al[31] it has become widely recognized that preferable survival outcomes can be expected in a selected population with a limited tumor size and number of HCCs (Milan criteria). Nowadays, these criteria have been extended including tumor markers or biopsy findings in several high-volume transplant centers[43-50].
Several studies have suggested that HCV infection has an additional negative impact on the outcomes of patients undergoing transplantations for HCC[51-54], and other studies have reported similar survival outcomes in HCV and non-HCV patients with HCC[55,56]. Small sample sizes, heterogeneous populations, and non-adjustments for multiple confounders in liver transplant recipients may explain these variations in observations. However, both HCC and HCV seem to have a deleterious impact on long-term patient and graft survival. Dumitra et al[57] reviewed 601 liver transplant recipients and reported that the coexistence of HCC and HCV had the largest deleterious impact on the long-term survival rate, doubling the risk of mortality after liver transplantation.
With the introduction of DAAs, however, the safety and efficacy of protease inhibitors compared with conventional combination therapy with IFN and ribavirin have been reported in patients undergoing liver transplantation for hepatitis C[58]. Because the deleterious effect of HCV can be attributed to uncontrollable viremia after transplantation, these new effective drugs could improve the long-term outcomes of liver transplant recipients complicated with HCC and end-stage liver disease.
Although adequate surgical intervention contributes to an improvement in long-term survival, the postoperative management of hepatitis C is also important for patients with HCV-related HCC. After liver resection, HCC shows two modes of recurrence: recurrence from residual intrahepatic micrometastases, and neocarcinogenesis in the underlying liver. The former type of recurrence can be reduced by adequate surgical maneuvers and the complete removal of the hepatic parenchyma, including both the main tumor and the surrounding latent micrometastases, while the latter type of recurrence is closely associated with the carcinogenic potential of the underlying liver itself. Because sustained viremia is associated with chronic histopathological injury and an increased risk of tumor recurrence and a poor survival outcome[59], adjuvant anti-viral therapy may be preferable for patients with a positive serology for HCV.
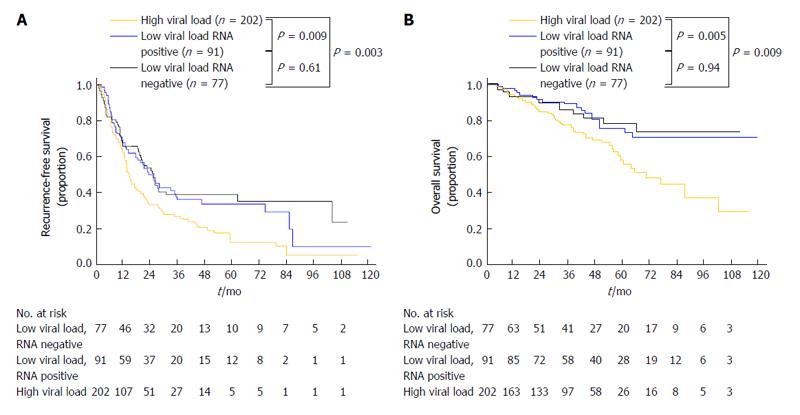
Recent meta-analyses have revealed that postoperative IFN treatment for HCV-related HCC prevents HCC recurrence and improves survival[12,13]. Conventionally, the eradication of HCV and a sustained status of undetectable HCV-RNA have been regarded as the most important factors for obtaining better clinical outcomes. However, a recent study based on a prospective population has revealed that a lower HCV viral load itself predicts better long-term surgical outcomes in patients with HCC regardless of the serologic eradication of HCV (Figure 3)[60,61]. Therefore, postoperative antiviral therapy with individually adjusted intensities might be advantageous for reducing HCC recurrence, even among patients who cannot tolerate the currently used standard high dose anti-viral therapy.
After liver transplantation, the control of HCV infection is also necessary for the protection of the liver graft. Although the risk of tumor recurrence is relatively low as long as the patient meets the Milan criteria, re-infection with HCV is inevitable, and the rapid progression of graft fibrosis toward cirrhosis is sometimes observed because of the active HCV infection that occurs under the immunosuppressive conditions. As for the efficacy of prophylactic anti-viral therapy during the early posttransplant period, a recent multicenter randomized study denied its efficacy in terms of patient/graft survival rates[62]; therefore, most Western surgeons do not support the routine use of preemptive antiviral therapy. However, this study was performed prior to recent effective combination therapies with DAAs, and the reported SVR rate was only 22%. The University of Tokyo has recently reported that preemptive antiviral therapy is feasible, with acceptable tolerance and an end-of-treatment response rate of 56% and SVR rates of 44% under a strict treatment protocol[63]. Several recent studies have also reported that patients who achieved an SVR after antiviral therapy showed significantly better patient/graft survival rates[64-66]. Given the improved SVR rates that have been achieved in the era of DAAs, prophylactic treatment during the early post-transplant period may be able to reduce the HCV viral load effectively and to suppress histopathological injury to grafted livers.
Hepatitis C infection is associated with a poor survival outcome after surgical resection and liver transplantation for the treatment of HCC. Because chronic infection with HCV and increased carcinogenic potential of the underlying liver are the main reasons for the poor survival outcome after liver resection, adjuvant anti-viral therapy at an individually adjusted intensity may be important for achieving a longer survival period after surgery. Liver transplantation offers a higher chance of cure for HCC, regardless of the hepatic functional reserve. However, post-transplant re-infection with HCV is troublesome and sometimes causes the rapid progression of liver damage, resulting in graft failure. Although only a few lines of solid evidence have been available for postoperative antiviral treatment because of limited indications and frequent adverse events caused by the conventional high-dose combination interferon therapy, new DAAs enable interferon-free anti-viral treatment with a higher virologic response and minimal side effects.
P- Reviewer: Bianchini M, Chetty R, Sertoglu E, Waisberg J S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | World Cancer Report 2014. Lyon, France: The International Agency for Research on Cancer 2014; . |
| 2. | Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991;214:114-117. [PubMed] |
| 3. | Imamura H, Matsuyama Y, Miyagawa Y, Ishida K, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Kudo M. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75 Suppl 1:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 6. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 540] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 7. | Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 8. | Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, Okita K, Omata M, Kudo M, Kojiro M. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Akamatsu N, Sugawara Y, Kokudo N. Living donor liver transplantation for patients with hepatocellular carcinoma. Liver Cancer. 2014;3:108-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Akamatsu N, Sugawara Y, Kokudo N, Eguchi S, Fujiwara T, Ohdan H, Nagano H, Taketomi A, Kitagawa Y, Shimada M. Outcomes of living donor liver transplantation for hepatitis C virus-positive recipients in Japan: results of a nationwide survey. Transpl Int. 2014;27:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, Takayama T, Kokudo N; for the Liver Cancer Study Group of Japan. A Comparison of the Surgical Outcomes Among Patients With HBV-Positive, HCV-Positive, and Non-B Non-C Hepatocellular Carcinoma: A Nationwide Study of 11,950 Patients. Ann Surg. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Müllhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Singal AK, Freeman DH, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 15. | Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 17. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 633] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 18. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2506] [Article Influence: 192.8] [Reference Citation Analysis (2)] |
| 19. | Schlaeger C, Longerich T, Schiller C, Bewerunge P, Mehrabi A, Toedt G, Kleeff J, Ehemann V, Eils R, Lichter P. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 795] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 22. | Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, Yano M, Ishikawa O, Imaoka S. Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg. 2006;244:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Takenaka K, Yamamoto K, Taketomi A, Itasaka H, Adachi E, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. A comparison of the surgical results in patients with hepatitis B versus hepatitis C-related hepatocellular carcinoma. Hepatology. 1995;22:20-24. [PubMed] |
| 25. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 26. | Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, Del Gaudio M, Pinna AD. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Kaneda K, Kubo S, Tanaka H, Takemura S, Ohba K, Uenishi T, Kodai S, Shinkawa H, Urata Y, Sakae M. Features and outcome after liver resection for non-B non-C hepatocellular carcinoma. Hepatogastroenterology. 2012;59:1889-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 28. | Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Differences in long-term outcome and prognostic factors according to viral status in patients with hepatocellular carcinoma treated by surgery. J Gastrointest Surg. 2008;12:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Li Q, Li H, Qin Y, Wang PP, Hao X. Comparison of surgical outcomes for small hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a Chinese experience. J Gastroenterol Hepatol. 2007;22:1936-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 652] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 31. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5305] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 32. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 582] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 33. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [PubMed] |
| 34. | Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013;15:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 363] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 37. | Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 38. | Shindoh J, Tzeng CW, Aloia TA, Curley SA, Zimmitti G, Wei SH, Huang SY, Mahvash A, Gupta S, Wallace MJ. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013;20:2493-2500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 40. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [PubMed] |
| 41. | Iwatsuki S, Gordon RD, Shaw BW, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401-407. [PubMed] |
| 42. | Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285. [PubMed] |
| 43. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [PubMed] |
| 44. | Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1570] [Article Influence: 92.4] [Reference Citation Analysis (1)] |
| 47. | Silva M, Moya A, Berenguer M, Sanjuan F, López-Andujar R, Pareja E, Torres-Quevedo R, Aguilera V, Montalva E, De Juan M. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14:1449-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 49. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1693] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 50. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 51. | Bozorgzadeh A, Orloff M, Abt P, Tsoulfas G, Younan D, Kashyap R, Jain A, Mantry P, Maliakkal B, Khorana A. Survival outcomes in liver transplantation for hepatocellular carcinoma, comparing impact of hepatitis C versus other etiology of cirrhosis. Liver Transpl. 2007;13:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Moya A, Berenguer M, Aguilera V, Juan FS, Nicolás D, Pastor M, López-Andujar R, Rayón M, Orbis F, Mora J. Hepatocellular carcinoma: Can it be considered a controversial indication for liver transplantation in centers with high rates of hepatitis C? Liver Transpl. 2002;8:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S268-S276. [PubMed] |
| 54. | Shimoda M, Ghobrial RM, Carmody IC, Anselmo DM, Farmer DG, Yersiz H, Chen P, Dawson S, Durazo F, Han S. Predictors of survival after liver transplantation for hepatocellular carcinoma associated with Hepatitis C. Liver Transpl. 2004;10:1478-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Figueras J, Ibañez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, Mir J, Loinaz C, Herrera L, López-Cillero P. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl. 2001;7:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R, Kerlan R, Venook A, Ascher NL, Emond JC, Roberts JP. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Dumitra S, Alabbad SI, Barkun JS, Dumitra TC, Coutsinos D, Metrakos PP, Hassanain M, Paraskevas S, Chaudhury P, Tchervenkov JI. Hepatitis C infection and hepatocellular carcinoma in liver transplantation: a 20-year experience. HPB (Oxford). 2013;15:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, Si-Ahmed SN, Guillaud O, Antonini TM. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 59. | Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: a meta-analysis of observational studies. World J Surg Oncol. 2011;9:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Shindoh J, Hasegawa K, Matsuyama Y, Inoue Y, Ishizawa T, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. Low hepatitis C viral load predicts better long-term outcomes in patients undergoing resection of hepatocellular carcinoma irrespective of serologic eradication of hepatitis C virus. J Clin Oncol. 2013;31:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Shindoh J, Hasegawa K, Takemura N, Omichi K, Ishizawa T, Aoki T, Sakamoto Y, Suagawara Y, Kokudo N. Hepatitis C viral load predicts tumor recurrence after curative resection of hepatocellular carcinoma regardless of the genotype of hepatitis C virus. Hepatol Int. 2014;In press. |
| 62. | Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, Prabhakar A, Charlton MR. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011;17:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Sugawara Y, Tamura S, Yamashiki N, Kaneko J, Aoki T, Sakamoto Y, Hasegawa K, Kokudo N. Preemptive antiviral treatment for hepatitis C virus after living donor liver transplantation. Transplant Proc. 2012;44:791-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, Machicao VI, Chaoru C, Nelson DR. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, Rosen CB, Heimbach JK, Charlton MR. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Berenguer M, Palau A, Aguilera V, Rayón JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |