Published online Aug 27, 2014. doi: 10.4254/wjh.v6.i8.601
Revised: March 26, 2014
Accepted: July 17, 2014
Published online: August 27, 2014
Processing time: 199 Days and 13 Hours
AIM: To inform clinicians on the level of hepatotoxic risk among antimycotics in the post-marketing setting, following the marketing suspension of oral ketoconazole for drug-induced liver injury (DILI).
METHODS: The publicly available international FAERS database (2004-2011) was used to extract DILI cases (including acute liver failure events), where antimycotics with systemic use or potential systemic absorption were reported as suspect or interacting agents. The reporting pattern was analyzed by calculating the reporting odds ratio and corresponding 95%CI, a measure of disproportionality, with time-trend analysis where appropriate.
RESULTS: From 1687284 reports submitted over the 8-year period, 68115 regarded liver injury. Of these, 2.9% are related to antimycotics (1964 cases, of which 112 of acute liver failure). Eleven systemic antimycotics (including ketoconazole and the newer triazole derivatives voriconazole and posaconazole) and terbinafine (used systemically to treat onychomicosis) generated a significant disproportionality, indicating a post-marketing signal of risk.
CONCLUSION: Virtually all antimycotics with systemic action or absorption are commonly reported in clinically significant cases of DILI. Clinicians must be aware of this aspect and monitor patients in case switch is considered, especially in critical poly-treated patients under chronic treatment.
Core tip: The recent regulatory interventions (United States restriction and Europe suspension) concerning ketoconazole for drug-induced liver injury (DILI) poses a prescribing challenge to clinicians, who should now carefully consider safer therapeutic alternatives. Data mining of FAERS database (2004-2011) highlighted that: (1) antimycotics are involved in approximately 3% of DILI cases (including acute liver failure events); (2) virtually all systemic antimycotics (e.g., azole derivatives), are associated with disproportionality signals; careful monitoring is therefore recommended, especially in critical poly-treated patients with multiple comorbidities; and (3) topical antimycotics, as expected, do not generate a post-marketing signal of DILI, thus indicating the accuracy of our approach.
- Citation: Raschi E, Poluzzi E, Koci A, Caraceni P, Ponti FD. Assessing liver injury associated with antimycotics: Concise literature review and clues from data mining of the FAERS database. World J Hepatol 2014; 6(8): 601-612
- URL: https://www.wjgnet.com/1948-5182/full/v6/i8/601.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i8.601
Following the recent interest and regulatory measures on ketoconazole-related liver injury, the present manuscript provides: (1) a concise overview of the literature on drug-induced liver injury (DILI), namely a practical guide for clinicians to realize the need for active post-marketing vigilance; and (2) a case study on antimycotics, based on key results obtained from our original analysis of the international publicly available Food and Drug Administration (FDA) database, to advance the reporting pattern of DILI in the real-world practice. This approach can provide physicians with practical clues to assign the level of DILI risk among antimycotics.
Definition of DILI: Hepatotoxicity and DILI are interchangeable terms adopted by clinicians, researchers as well as drug developers to describe a broad spectrum of liver manifestations. Indeed, DILI can be defined as a liver injury induced by a drug or herbal medicinal products leading to liver test abnormalities or liver dysfunction with reasonable exclusion of other competing etiologies. More than 1100 medicines, natural products, vitamins, dietary supplements, recreational and illicit compounds have been reported to cause DILI, and the list is constantly growing. Antibiotics, anticonvulsants, and antidepressant therapy remain the commonest causes of DILI in the Western Hemisphere[1]. Among others, tumor necrosis factor-alpha antagonists, fluoroquinolones, tyrosine kinase inhibitors, statins and food supplements are gaining appreciation[2].
Issues in clinical practice: A number of classifications have been proposed to facilitate diagnoses and research in the field. From a pharmacological standpoint, DILI can be divided into “intrinsic” (i.e., dose-related) and “idiosyncratic” (i.e., dose-independent)[3]; however, the validity of this classification remains controversial and debated[4]. The term idiosyncratic was first used to identify a reaction occurring in susceptible individuals, usually associated with variable or prolonged latency (several weeks to 1 year) and generally unexpected on the basis of the pharmacological action of the drug. Idiosyncratic DILI is linked to the vast majority of compounds, although a common misconception of idiosyncratic DILI regards its non relation with the dose. As a matter of fact, recent data suggested that a daily dose > 50 mg (and especially when the dose exceeds 100 mg daily) for drugs with high lipophilicity undergoing extensive hepatic metabolism is associated with a higher risk of idiosyncratic DILI[5-7]. The latest evidence revealed that (1) drugs which are substrates of CYP enzymes would have the higher likelihood of causing DILI (which is dose-independent); (2) drugs which are cytochrome P450 (CYP) inhibitors would have the higher likelihood of generating DILI only when they are administered at a high daily dose; and (3) drugs which are CYP inducers are not observed to be associated with DILI[8].
From a clinical standpoint, DILI can be classified into acute and chronic persistent (i.e., evidence of continue liver injury after discontinuation of causative agent beyond 12 mo of follow-up). Paracetamol (acetaminophen in the United States) represents the leading cause of acute dose-dependent DILI. More precisely, an international DILI working group of clinicians and scientists developed uniform consensus criteria to standardize the phenotypes of DILI[9]. Although liver enzymes such as transaminases lack specificity, liver injury in the context of DILI has been defined decades ago as an elevation in the serum concentration of alanine aminotransferase (ALT), conjugated bilirubin or alkaline phosphatase exceeding 2 × the upper limit of normal (ULN)[10]. The expert working group proposed a cut-off level of 5 × ULN for ALT as better threshold to exclude clinically unimportant and self-limited drug-related hepatic events as well as nonalcoholic steatohepatitis not related to DILI.
The most common clinical presentations of DILI are hepatocellular, cholestatic and mixed, which should be defined on the basis of biochemical criteria. The R value is use for this purpose [R = (ALT/ULN)/(ALP/ULN)]; when R ≥ 5 the pattern is considered hepatocellular, whereas if R < 2 is cholestatic. Usually, the damage induced by amoxicillin-clavulanate is considered to be cholestatic, as compared to the hepatocellular pattern caused by methotrexate.
Diagnosis is a major challenge for clinicians and is based on a combination of factors, which are influenced by the expertise: (1) exclusion of other causes that may elevate hepatic biochemical tests (in primis hepatitis viruses); (2) causality assessment methods, which may be based on expert opinion or on standardized liver-specific scoring instruments such as the roussel uclaf causality assessment method (RUCAM), endorsed by the Council of International Organization of Medical Sciences[11]; and (3) the presence of a signature pattern indicative of a specific causative agent (e.g., typical features of DILI by telithromycin include short latency and abrupt onset of fever, abdominal pain, and jaundice, sometimes with the presence of ascites even in cases that resolved)[12]. In this context, a clinical research workshop took place to review and attempt to standardize the current nomenclature and terminology used in DILI research. Because DILI is a diagnosis of exclusion, selected elements of the medical history, laboratory tests, and previous reports were proposed to improve causality assessment[13]. The role of liver biopsy is still controversial, especially histological features and their relationship with biochemical parameters, although a systematic approach has been recently proposed as a foundation to correlate DILI pathology with its causality and outcome[14]. Efforts have been also directed to identify a list of essential elements (minimum requirements) when submitting case reports for publication[15]. Recently, a dedicated website was finally created as an aid for clinicians and researchers to quickly extract updated information on DILI (http://livertox.nih.gov/).
Issues in drug development: The regulatory impact of DILI is appreciably expanding, as demonstrated by the fact that specific Consortia have been established, especially in the United States, where the DILI Network was created in 2003 to prospectively identify a large number of patients with bona fide DILI that will allow for collection of epidemiological data and biological samples for future mechanistic studies[16].
DILI has been a major cause of drug withdrawals, non approval and variegate regulatory actions in the past 50 years. In one study of 38 drugs withdrawn from the market between 1990 and 2006, 14 (37%) were related to hepatotoxicity[17]. A more recent review highlighted that of the 25 safety-based withdrawals in Europe and United States, ten (40%) were for cardiovascular events and seven (28%) for gastrointestinal, primarily hepatic, adverse events. It is clear that the majority of these regulatory measures concerned rare events with delayed onset and were not predicted based on known pharmacological action[18]. It is also remarkable that spontaneous reporting systems have been a primary source of information that mainly contributed to regulatory actions, especially for newly marketed drugs and events that are likely to be drug- induced.
From a historical perspective, in several circumstances, hepatotoxic agents were identified after being approved by regulators and marketed for some time; this was the case, for instance, of bromfenac (withdrawn 11 mo after its approval) and more recently troglitazone, which was marketed in 1997 and withdrawn worldwide in March 2000. In other cases, DILI led to non approval or interruption of late phases of drug development (e.g., in 2006, the manufacturer of the oral anticoagulant ximelagatran withdrew a pending application to the FDA).
In this context, potential safety issues on DILI should be recognized as early as possible during drug development in order to accurately predict the actual risk in the post-marketing phase[19]. There are currently a number of challenges and controversies in exploiting predictive pre-clinical studies, especially for animal models, where ethical issues pose important limitations[20].
In line with the clinical situation, there are no specific biomarkers that may be used for optimal prediction of the risk. In fact, the frequency of asymptomatic rise in serum ALT in the pre-approval phase does not correlate per se with the frequency of symptomatic liver injury in the post-marketing period. The quest for highly predictive biomarkers has been underway for years and remains an ongoing challenge[21]. Although some genetic associations (e.g., flucloxacillin and HLA-B15701) have been identified, the clinical utility of genetic polymorphisms associated with drug-specific DILI appears still limited[1]. In addition, there are at least 3 groups of individuals showing different pattern of hepatic response: tolerates (the vast majority of patients without significant changes in liver biochemical tests), susceptibles (showing progressive increase in ALT level that continues to increase and evolves into clinically significant liver damage with signs and symptoms) and adaptors (showing transient increase in enzyme levels, which eventually return to baseline despite continuation of the drug)[19].
So far, regulatory authorities and drug developers have mainly relied on the so-called Hy’s law or rule[22], coined following Zimmerman’s observations, to predict post-marketing risk of serious hepatic events and for making recommendations on whether treatment should be continued, stopped or its dose reduced, following biochemical abnormalities with the suspect drug. To define a clinical trial subject as a Hy’s law case, the following components must be present: (1) ALT increase to a level ≥ 3 times ULN; (2) total bilirubin increase ≥ 2 times ULN; (3) no significant increase in ALP (initial Alp values does not reach 2 times ULN); and (4) no other cause is found to explain liver injury.
When the above criteria are met, Hy’s rule predicts a mortality rate that can exceed 10%. In the last decade, two population-based studies further support the validity of this rule by demonstrating a mortality rate of 9%-12%[23,24]. Based on the 2009 FDA guidance, finding two cases is considered highly predictive that the drug has the potential to cause severe DILI when given to a larger population[25]. Thus, Hy’s law calls for enhanced vigilance on the patient so that the drug is discontinued before the patient crosses the threshold of hepatotoxic irreversibility. The correct timing of discontinuation and the exact level of ALT elevation is still a matter of debate; an eight-fold increase from baseline is generally considered a standard cut-off, although even levels greater that 3 time the ULN may be sufficient if accompanied by additional symptoms. Given the importance of monitoring thousands of laboratory parameters during clinical phases, the eDISH method was developed to visualize, assess and summarize hepatic safety data during clinical trials[26]. As regards causality assessment, pharmaceutical companies prefer to rely on a 3-category scale instead of using the 5-category scale adopted by the DILIN consortium or the RUCAM score[19].
Antimycotics and DILI: clinical and regulatory aspects: The true incidence of DILI associated with antimycotics in the post-marketing phase challenges precise estimation, especially as compared to other antimicrobials[27]. Previous studies, mostly based on data derived from clinical trials, reported a 2%-23% range, depending on the drug of interest and especially the severity of the disease[28]. Clinical evidence on ketoconazole emerged in early 80’, when 54 reports of alleged ketoconazole-induced liver injury submitted to the FDA from the time of initial marketing in 1980, of which 33 were labeled as likely. The incidence of symptomatic, potentially serious hepatic injury appeared to be very low, perhaps 1 in 15000 exposed individuals, whereas the incidence of mild, asymptomatic, reversible elevations in serum transaminases has been estimated to be 5%-10%[29]. Also García Rodríguez et al[30] found that ketoconazole was associated with significant risk. Very recently, a systematic review and meta-analysis documented an overall incidence of 3.6%-4.2%, which may be considered as common[31]. Data on other antifungals such as terbinafine are more scant and mostly based on individual case reports[32-34]. The latest population-based study in Taiwanese concluded that oral antifungal agents are associated with a low incidence if DILI, which may be fatal (especially in the elderly) and increased for longer treatment[35].
On July 26th, 2013, the European Medicines Agency (EMA) and the FDA issued important safety announcements: the EMA’s Committee on Medicinal Products for Human Use (CHMP) has recommended that the marketing authorisations of oral ketoconazole-containing medicines should be suspended throughout the European Union (the CHMP concluded that the risk of liver injury is greater than the benefits in treating fungal infections) (http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/07/news_detail_001855.jsp&mid=WC0b01ac058004d5c1), whereas the FDA limits usage of Nizoral® (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems (http://www.fda.gov/drugs/drugsafety/ucm362415.htm). The FDA has revised the Boxed Warning, added a strong recommendation against its use (contraindication) in patients with liver disease, and included new recommendations for assessing and monitoring patients for liver toxicity. The FDA has also approved a new patient Medication Guide containing information on the potential risks associated with Nizoral® tablets, which must be dispensed with every prescription for the drug. In the revised US drug label, indications for dermatophyte and Candida infections have been removed and the indications for treatment of blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis have been retained only for patients in whom other antifungal treatments have failed or are not tolerated.
These regulatory measures were driven by the following reasons: (1) suspension of the drug in France (June 2011), which asked the EMA to carry out a full assessment of the benefit-risk balance of oral ketoconazole-containing medicines; (2) the incidence and severity of liver injury with oral ketoconazole were higher than with other antifungals; (3) reports of liver injury occurred early after starting treatment with recommended doses, and it was not possible to identify measures to adequately reduce this risk; and (4) the widespread off-label use of oral ketoconazole for treating patients with Cushing’s syndrome; and (5) currently available alternative treatments are deemed to be safer.
Possible contribution of pharmacovigilance in DILI research: Pharmacovigilance, namely post-marketing phase of drug development, represents the mainstay to evaluate the safety of recently marketed drugs or to monitor high-priority adverse events[36]. On one hand, spontaneous reports and data mining of large spontaneous reporting systems still represent the traditional source for safety surveillance; on the other hand, exploiting electronic healthcare records. Both approaches are important and can be viewed to complement each other. However, it is a common opinion (corroborated by original research) that spontaneous reporting systems are more suited for newly approved drugs (as they aim at early signal detection) and rare events with suggestive drug-related component[18]. It is noteworthy that the diagnostic potential of commonly applied signal-detection algorithms is reasonably efficient and accurate to discriminate true drug-event associations from those that are likely to be spurious[37].
In this context, the aim of this study is to show that spontaneous reporting systems can contribute in characterizing the hepatotoxic potential of systemic antifungals. As a matter of fact, a recent systematic review highlighted that regulatory measures on drug risk (e.g., dear doctor letters, safety warnings) may differently but substantially impact prescribers’ attitudes, thus causing a switch towards therapeutic alternatives, perceived as safe[38]. This could be the case for some systemic antifungals, especially following marketing suspension of oral ketoconazole, both in US and Europe, for risk of DILI.
The publicly available international FDA Adverse Event Reporting System, now termed FAERS database (2004-2011), was used for data mining of DILI associated with antimycotics. FAERS serves as repository of potential adverse events and medication errors associated with chemical and biological agents spontaneously submitted by healthcare professionals, patients and manufacturers. Since 2003, more than 7 million reports have been accumulated into FAERS, with more than 700000 records entered in 2011. In the light of its large catchment area (including also European reports potentially related to serious events) and public availability (since 2004), FAERS plays a leading role in signal detection and characterization, especially for rare events with high drug-attributable risk such as torsade de pointes (TdP)[39-41]. DILI shares different clinical, pharmacological and regulatory issues with TdP and, remarkably, the accuracy of FAERS to investigate DILI cases has been very recently demonstrated, especially as an important aid to systematically track emerging signals of DILI for newly marketed drug[42].
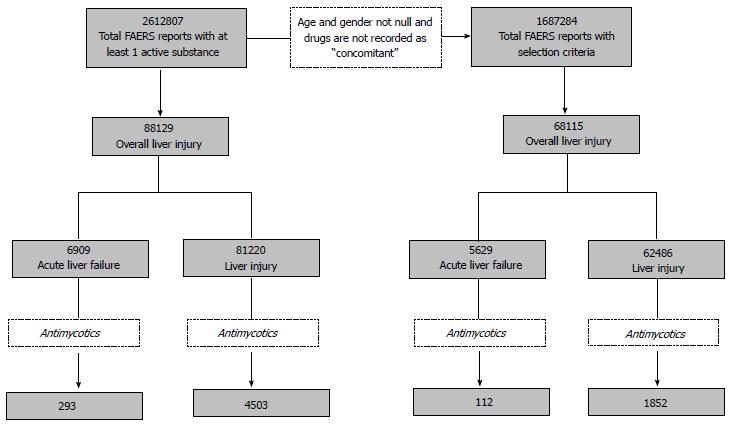
Data processing and management (e.g., duplicate detection and removal, handling of missing data) were performed according to previous methodology, extensively described in a dedicated book chapter[43]. In this study, main selection criteria regards the role code assigned to the drug (i.e., only “Primary Suspect”, “Secondary Suspect” or “Interacting”, whereas cases where antimycotics were reported as “Concomitant” were not included) and information on age and gender (records with missing data in these fields were not considered). Drugs of interest were represented by antimycotics with systemic use, including those agents used for topical indications that may have systemic absorption (e.g., terbinafine and griseofulvine). These compounds were mapped through an ad hoc created archive of drug names and converted into relevant active substances. Events of interest were selected according to a pre-specified search strategy (see below) and based on the standard MedDRA (Medical Dictionary for Regulatory Activities) terminology for codification.
In line with a previous multidisciplinary collaborative work[44], a two-fold approach was carried out to identify any type of liver damage, namely acute and chronic injuries as well as acute liver failure events. To this aim, different MedDRA Preferred Terms (PTs) were identified to define two patterns of liver event with different severity and clinical implication: “liver injury” (LI, including both chronic and acute liver events) and “acute liver failure” (ALF, a severe liver injury potentially reversible in nature and with onset of encephalopathy[45]) (Table 1). In order to assign a certain report to a precise group, at least one pre-specified PT should be present. A mutually exclusive approach was performed; a single case report of interest was classified only in one group based on the following priority: ALF>LI. Therefore, LI does not include cases with ALF; this allowed us the identification of a so-called “overall liver injury” (OLI).
| PT | Liver injury | Acute hepatic failure |
| Acute hepatic failure | X | |
| Alanine aminotransferase abnormal | X | |
| Alanine aminotransferase increased | X | |
| Ammonia increased | X | |
| Aspartate aminotransferase abnormal | X | |
| Aspartate aminotransferase increased | X | |
| Bilirubin conjugated increased | X | |
| Bilirubin urine | X | |
| Blood bilirubin abnormal | X | |
| Blood bilirubin increased | X | |
| Blood bilirubin unconjugated increased | X | |
| Cholestasis | X | |
| Coma hepatic | X | |
| Cytolytic hepatitis | X | |
| Hepatic encephalopathy | X | |
| Hepatic enzyme abnormal | X | |
| Hepatic enzyme increased | X | |
| Hepatic failure | X | |
| Hepatic function abnormal | X | |
| Hepatic necrosis | X | |
| Hepatitis | X | |
| Hepatitis acute | X | |
| Hepatitis cholestatic | X | |
| Hepatitis fulminant | X | |
| Hepatitis toxic | X | |
| Hepatocellular damage | X | |
| Hepatotoxicity | X | |
| Hyperammonaemia | X | |
| Hyperbilirubinaemia | X | |
| Jaundice | X | |
| Jaundice cholestatic | X | |
| Jaundice hepatocellular | X | |
| Liver function test abnormal | X | |
| Liver injury | X | |
| Liver transplant | X | |
| Mixed hepatocellular-cholestatic injury | X | |
| Subacute hepatic failure | X | |
| Transaminases abnormal | X | |
| Transaminases increased | X | |
| Urine bilirubin increased | X |
Description of the overall number of cases was first provided, both in terms of ALF, LI and OLI; demographic information (age and gender) were also analysed. Then, a case/non case disproportionality approach was carried out by calculating the Reporting Odds Ratio (ROR) with relevant Confidential Interval (CI). Statistically significant disproportionality was formally defined when the lower limit of the 95%CI was > 1, with at least 3 cases[46]. Cases were represented by reports of DILI according the aforementioned pre-specified MedDRA PTs, whereas non cases were defined as all other reports (i.e., those without such PTs). The ROR was first calculated by comparing a given antimycotic against all remaining drugs recorded in the entire FAERS database (i.e., comprising all spontaneous reports from all drugs), by considering ALF and LI both separately and cumulatively (i.e., by providing the ROR for OLI). Finally, where appropriate, the ROR was calculated within antimycotics by using a subset of data (i.e., reports associated only with antimycotics). Restricting the analysis among agents belonging to the same pharmaco-therapeutic class may be useful as a sensitivity approach to test whether any potential intraclass differences exist in terms of risk. Cumulative time-trend disproportionality analysis was also performed, when appropriate[47].
Over the 8-year period, 2612807 reports with at least one mapped active substance were processed; in 3.4% of these, at least one pre-selected PT of interest was recorded. The precise allocation of reports is provided in Figure 1, according to case definition and drugs of interest. Based on our selection criteria, 68,115 DILI cases (OLI) were identified, corresponding to 4% of overall FAERS reports; the majority (91.7%) regarded LI. Antimycotics were reported in 3% of LI and 2% of ALF cases.
In the entire FAERS database, 52% of cases of DILI occurred in females, whereas only in 44% of cases associated with antimycotics female gender was recorded. The analysis on age distribution showed a peak occurring in patients with 50-60 years of age (all FAERS database, no matter the drug under scrutiny), both for ALF and LI; only 26% and 27% of DILI cases occurred in patients aged > 65 years (ALF and LI, respectively). Concerning antimycotics, a peak was found for patients aged 60 (number of OLI cases = 48), with only 30% of OLI cases occurring in subjects aged > 65 years. The analysis on the outcome of the events revealed that: (1) in the entire FAERS database, death was recorded in 37% and 17% of cases, whereas hospitalization or life-threatening conditions were reported in 52% and 49% of cases (ALF and LI, respectively); and (2) as regards antimycotics, death occurred in 51% and 24% of events, hospitalization or life-threatening conditions in 39% and 47% of events (ALF and LI, respectively).
As a first screening to test the sensitivity of the approach in detecting drug-event associations, we generated a list of top-10 drugs based on their reporting frequency for DILI (Table 2). Paracetamol ranked first both for ALF and LI and also generated statistically significant disproportionality; it was followed by a number of drugs with known hepatotoxic potential such as statins, duloxetine, amoxicillin clavulanate, bosentan. Except for interferon, all these agents showed statistically significant ROR. Sorafenib ranked second in terms of ALF.
| ATC code | Active substance | Cases LI | Cases ALF | Cases OLI | ROR (95%CI) LI | ROR (95%CI) ALF | ROR (95%CI) OLI |
| N02BE01 | Paracetamol | 2780 | 645 | 3425 | 3.69 (3.54-3.84)1 | 9.58 (8.82-10.41)1 | 4.31 (4.16-4.48)1 |
| C10AA05 | Atorvastatin | 1763 | 131 | 1894 | 3.46 (3.29-3.64)1 | 2.62 (2.20-3.12)1 | 3.43 (3.27-3.60)1 |
| C10AA01 | Simvastatin | 1516 | 80 | 1596 | 2.62 (2.49-2.77)1 | 1.44 (1.15-1.80)1 | 2.54 (2.41-2.67)1 |
| N06AX21 | Duloxetine | 1456 | 59 | 1515 | 3.34 (3.15-3.53)1 | 1.42 (1.10-1.83)1 | 3.32 (3.14-3.50)1 |
| L03AB07 | Interferon beta-1 | 1377 | 46 | 1423 | 0.73 (0.70-0.78) | 0.27 (0.20-0.36) | 0.69 (0.66-0.73) |
| J05AF05 | Lamivudine | 1162 | 130 | 1292 | 4.68 (4.40-4.98)1 | 5.22 (4.38-6.22)1 | 4.86 (4.57-5.16)1 |
| L01BA01 | Methotrexate | 1200 | 86 | 1286 | 2.89 (2.72-3.07)1 | 2.15 (1.74-2.66)1 | 2.85 (2.69-3.02)1 |
| J01CR02 | Amoxicillin/clavulanic acid | 1164 | 94 | 1258 | 5.86 (5.49-11.36)1 | 4.50 (3.67-5.53)1 | 5.89 (5.54-6.27)1 |
| C02KX01 | Bosentan | 1177 | 49 | 1226 | 9.53 (8.91-10.19)1 | 3.35 (3.53-4.45)1 | 9.20 (8.61-9.83)1 |
| L01XE05 | Sorafenib | 920 | 271 | 1191 | 4.98 (4.64-5.35)1 | 15.39 (13.58-17.44)1 | 6.22 (5.83-6.63)1 |
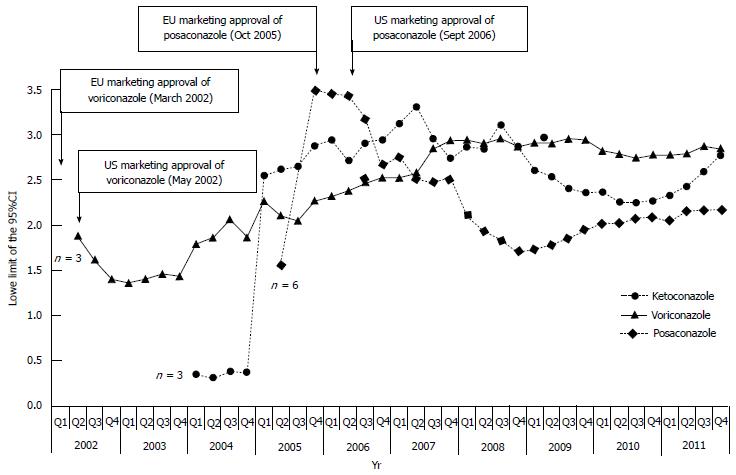
The analysis on antimycotics revealed a total of 1964 DILI cases; 35% were submitted by European Countries, 21% by Japan and 18% by United States. Terbinafine ranked first in terms of number of cases (422), followed by fluconazole and voriconazole (Table 3). Except griseofulvin, and miconazole, all remaining compounds generated a significant disproportion for ALF or LI or OLI. The intraclass analysis on OLI among azole-derivate agents showed the following ranking: ketoconazole (ROR = 1.70; 95%CI: 1.34-2.15) > voriconazole (1.47; 1.29-1.68) > posaconazole (1.36; 1.04-1.77) > fluconazole> itraconazole> miconazole (no statistical significance obtained). The time-trend cumulative analysis of the ROR (performed on the two most recently marketed compounds and ketoconazole, the only antimycotic receiving recent regulatory intervention on DILI) showed that: (1) ketoconazole reached significant ROR in the first quarter of 2005 (i.e., well before the recent safety measure); (2) posaconazole (marketed in October 2005 by EMA and in September 2006 by the FDA) showed significant disproportion in the second quarter of 2005 (i.e., before its actual marketing approval), which persisted throughout the period of analysis, although fluctuations were recorded during the first 2-year period; and (3) voriconazole (marketed in March 2002 by EMA and May 2002 by FDA) showed significant disproportion in the second quarter of 2002, a striking correspondence with its regulatory approval (Figure 2).
| Pharmacological class | Activesubstance | Cases LI | CasesALF | CasesOLI | ROR (95%CI) LI | ROR (95%CI)ALF | ROR (95%CI)OLI |
| Antibiotics | Amphotericin B | 251 | 14 | 265 | 5.33 (4.65-6.10)1 | 2.86 (1.69-4.84)1 | 5.20 (4.55-5.94)1 |
| Imidazole derivatives | Miconazole2 | 16 | - | 16 | 0.33 (0.20-0.54) | - | 0.30 (0.18-0.50) |
| Ketoconazole2 | 88 | 6 | 94 | 6.68 (5.28-8.44)1 | 4.22 (1.88-9.45)1 | 6.64 (5.28-8.34)1 | |
| Triazole derivatives | Fluconazole | 381 | 31 | 412 | 4.25 (3.81-4.74)1 | 3.46 (2.42-4.93)1 | 4.26 (3.83-4.73)1 |
| Itraconazole | 178 | 4 | 182 | 3.73 (3.19-4.37)1 | 0.84 (0.32-2.25) | 3.50 (2.99-4.09)1 | |
| Voriconazole | 342 | 19 | 361 | 5.61 (4.99-6.31)1 | 2.97 (1.89-4.67)1 | 5.48 (4.89-6.14)1 | |
| Posaconazole | 65 | 5 | 70 | 5.39 (4.12-7.04)1 | 4.00 (1.65-9.66)1 | 5.39 (4.16-6.99)1 | |
| Other antimycotics for systemic use | Flucytosine | 6 | - | 6 | 3.06 (1.31-7.13)1 | - | 2.80 (1.20-6.52)1 |
| Caspofungin | 161 | 7 | 168 | 7.03 (5.90-7.37)1 | 2.79 (1.32-5.87)1 | 6.78 (5.71-8.05)1 | |
| Micafungin | 48 | 2 | 50 | 6.90 (5.02-9.49)1 | - | 6.64 (4.86-9.09)1 | |
| Anidulafungin | 13 | 1 | 14 | 4.97 (2.75-9.00)1 | - | 4.97 (2.79-8.84)1 | |
| Antimycotics used for topical indications (dermatological use) with systemic absorption | Griseofulvin3 | 3 | - | 3 | 2.00 (0.62-6.47) | - | 1.83 (0.57-5.92) |
| Terbinafine3 | 395 | 27 | 422 | 5.11 (4.58-5.69)1 | 3.39 (2.32-4.96)1 | 5.06 (4.55-5.62)1 | |
| Antimycotics for topical use with potential systemic absorption (e.g., gynecological and intestinal use) | Nystatin | 12 | - | 12 | 2.01 (1.12-3.62)1 | - | 1.84 (1.02-3.31)1 |
| Econazole | 6 | - | 6 | 3.25 (1.39-7.60)1 | - | 2.97 (1.27-6.94)1 | |
| Ciclopirox | 3 | - | 3 | 3.39 (1.02-11.30)1 | - | 3.10 (0.93-10.33) |
This study shows that virtually all antimycotics for systemic use are associated with clinically significant risk of DILI in the post-marketing setting, where comorbidities and poly-pharmacotherapy exist. Although the summary of product characteristics of all highlighted compounds and the livertox database already addressed this safety issue (e.g., by mentioning liver monitoring in the warning section), clinicians should be aware of this aspect, especially following the marketing suspension of oral ketoconazole. This means that switching prescription towards therapeutic alternatives of ketoconazole does not sufficiently protect patients from risk and active vigilance is still needed.
Although actual incidence cannot be inferred from spontaneous reporting systems, the fact that antimycotics are reported in approx 3% of the total cases in FAERS suggests that the risk could be common. Our study also confirmed that miconazole and griseofulvin are not associated with clinically significant risk of DILI, a finding in line with recent data from EU-ADR and OMOP Consortia (Europe and United States projects, respectively), which labeled these drugs as “negative controls” in drug safety studies investigating DILI, based on the existing scientific literature and expert opinion[48,49]. As a matter of fact, miconazole is mainly used as topical preparation, whereas griseofulvin has specific tropism for keratin tissues with only low plasma concentrations. This kinetic features could explain the lack of a signal of liver injury. On the other hand, one recent population-based study in Taiwanese detected 8 cases of DILI associated with griseofulvin, although the cumulative exposure dose and the concomitant co-prescription of antibiotics and antinflammatory drugs (which have hepatotoxic potential) may have contributed to the occurrence of liver events[35].
Clinicians should interpret our data in the light of limitations affecting pharmacovigilance data. In particular, apart from well-described bias inherent to all spontaneous reporting systems (e.g., underreporting, causality assessment, influence of media attention/safety alerts[50]), it should be kept in mind that these analyses, although formally quantitative, do not allow firm quantification of risk, especially because the role of concomitant drugs as culprit agents in the occurrence of the event cannot be ruled out with certainty. However, they allow early identification of alert signals, which require further confirmation through pharmacoepidemiological approaches. In our case, the evaluation of “old” drugs may be useful to understand the reporting pattern over time and contribute to the general picture of perceived risk in a consolidated clinical setting. In any case, it should be recognized that DILI cases cannot be fully validated and precisely classified because laboratory values and liver biopsy are not available.
Nonetheless, our analysis has some strengths which can be summarized as follows. First, the validity of FAERS in early detection of DILI risk has been recently shown in a study allowing discrimination of a broad range of hepatotoxic drugs with clinically significant serious DILI, including those with only clinically apparent risk (e.g., amlodipine)[42]. In this context, our top-10 ranking drugs have already known hepatotoxic risk (e.g., paracetamol), a finding in line with similar pharmacovigilance study recently performed on Vigibase[44], and can be considered our positive controls. Second, it should be noticed that: (1) sorafenib (a novel tyrosine kinase inhibitor for which liver toxicity is under scrutiny and requires diligent surveillance[51]) is ranked second in terms of ALF (after paracetamol); and (2) in our analysis, voriconazole and posaconazole are associated with significant disproportion already in their first year of marketing life (Figure 2). Although the indication for unresectable hepatocellular carcinoma complicates the causality assessment for sorafenib, EMA and FDA amended the prescribing information to add the hepatotoxic potential in the label (2010 and 2012, respectively)[52,53].
An incidental observation of this study is that female gender does not seem to be associated with a reporting pattern of DILI for antimycotics, which differs from current data showing that females predominated in liver cases associated with a number of drug class[54].
The large number of cases associated with triazole antifungals and terbinafine can be partially explained by the underlying clinical conditions of patients (critically ill patients, usually with systemic infections, cancer and other diseases that may increase the likelihood of adverse drug reactions). Moreover, when discussing and interpreting pharmacovigilance results, drug consumption data should be also taken into account can provide an additional perspective to regulators and clinicians in assessing the possible consequences of side effects of drugs (e.g., by mapping the level of risk in a population standpoint)[55,56]. Notably, the first ESAC survey on 2007 consumption data found that terbinafine, ketoconazole, itraconazole and fluconazole represented > 94% of the total outpatient antimycotic and antifungal use in 20 European Countries. Terbinafine use represented > 50% of the total systemic antimycotic and antifungal use in 16 out of 20 Countries[57]. The updated analysis on 2009 data showed an increased outpatient use and confirms terbinafine and fluconazole as the most used compounds[58]. Two point-prevalence ESAC surveys in European hospitals revealed that the most used antifungal was fluconazole (60.5%) followed by caspofungin (10.5%). Notably, antifungal-antibacterial combinations were frequently used (77.5%)[59]. As clearly emerged from these drug utilization studies, widely used antimycotics are also frequently reported in DILI cases extracted from FAERS. Therefore, not only active monitoring is warranted, but also appropriateness of prescriptions should be carefully considered by clinicians.
Another important aspect regards the issue of potential drug interactions. As a matter of fact, several antimycotics are metabolized or even inhibit crucial liver cytochromes for drug metabolism (e.g., CYP3A4) (Table 4). Although the clinical relevance of drug interactions is still a matter of debate, concomitant administration of drugs and/or herb (which are recognized to have hepatotoxic potential) should be assessed for potential interactions or interference with hepatic metabolism. Remarkably, the clinical implications of our data are also influenced by the different therapeutic indications of antimycotics (Table 4). In this context, griseofulvin, terbinafine, fluconazole and itraconazole share onicomycosis as main indication, whereas micafungin, voriconazole, posaconazole, caspofungin and amphotericin are used for systemic invasive infections, implying different baseline patients’ conditions that may contribute to increase their hepatotoxic potential. In particular, voriconazole and posaconazole are the most recently marketed compound and are indicated as second-line treatment for invasive fungal infections (e.g., following treatment failure with fluconazole); therefore, a potential channeling bias should be considered (i.e., the possibility that drugs may be differently prescribed in relation to the severity of disease).
| Drug | Approval (EMA-FDA) | Indication(s) | Main hepatic issues | Drug interactions |
| Amphotericin B | 1995 (FDA)1 | Empirical therapy for presumed fungal infection in febrile, neutropenic patients Cryptococcal Meningitis in HIV infected patients Visceral leishmaniasis | No detailed information provided (general statement in the section on side effects) | Unclear from the label (metabolic pathway unknown) |
| Miconazole | No centralized approval | Onychomycosis (topical) Local candida infections (topical and systemic) | Not reported | CYP3A4 and CYP2C9 inhibitor (when administered systemically) |
| Fluconazole | 1990 (FDA) | Acute vaginal candidiasis when local therapy is not appropriate Candidal balanitis when local therapy is not appropriate Mucosal and invasive candidiasis, genital candidiasis (trush), cryptococcal meningitis, dermatomycosis, coccidiodomycosis and onychomycosis (EMA revision in 2011) | Hepatic injury (warning FDA and EMA) | Potent CYP2C9 inhibitor; moderate CYP3A4 inhibitor; CYP2C19 inhibitor |
| Itraconazole | 1992 (FDA) | Onychomycosis of the toenail caused by Trichophyton rubrum or T. mentagrophytes (FDA) | Hepatic injury (FDA) | Potent CYP3A4 inhibitor (drug interactions in warnings) |
| Voriconazole | 2002 (EMA and FDA) | Invasive aspergillosis Candidemia in non-neutropenic patients Esophageal candidiasis (FDA) Fluconazole-resistant serious invasive Candida infections (including C. krusei) (EMA) Serious fungal infections caused by Scedosporium spp. and Fusarium spp. | Hepatic toxicity and monitoring of hepatic function (EMA and FDA) | CYP2C9, 2C19 and 3A4 inhibitor (several contraindicated drugs) |
| Posaconazole | 2006 (FDA) 2005 (EMA) | Refractory IFI/Patients with IFI intolerant to first line therapy Oropharyngeal candidiasis Prophylaxis of IFI | Hepatic toxicity and monitoring of hepatic function (EMA and FDA) | Potent CYP3A4 inhibitor (drug interactions in contraindications) |
| Caspofungin | 2001(EMA and FDA) | Empirical therapy for presumed fungal infections in febrile, neutropenic patients Invasive candidiasis Invasive aspergillosis (patients refractory or intolerant) | Hepatic effects | No CYP3A4 inhibition; No P-gp induction and poor substrate |
| Micafungin | 2005 (only FDA) | Treatment of invasive candidiasis Treatment of esophageal candidiasis Prophylaxis of Candida infection | Hepatic effects | No P-gp induction or substrate |
| Anidulafungin | 2006 (FDA) 2007 (EMA) | Invasive candidiasis in adult non-neutropenic patients (EMA) Esophageal candidiasis (FDA) | Hepatic effects (warning FDA and EMA) | No CYP substrate, inhibitor or inducer |
| Terbinafine | 19952 | Fingernail onychomycosis Toenail onychomycosis | Hepatotoxicity | CYP2D6 inhibitor |
| Griseofulvin | 1975 (FDA) | Various forms of tineas (corporis, pedis, cruris, barbae, capitis, unguium) | Hepatotoxicity | Unclear from the label |
In a conclusion, we have used the recent regulatory case of ketoconazole to provide clues to clinicians needing practical guidance to assign the level of DILI risk among antimycotics.
From our analysis, it clearly emerges that it is not possible to identify a safe systemic antimycotic because all agents show a disproportionality signal for DILI. Although this safety issue was already formally mentioned in the label, it is still possible that clinicians do not fully appreciate this aspect.
The recent marketing restrictions of ketoconazole by regulatory Agencies should not lead physicians to overlook hepatotoxicity due to other systemic antifungals. Careful monitoring is therefore recommended, especially in critical poly-treated patients, keeping in mind that treatment usually requires drug exposure for a significant period of time.
It is clear that drug developers and clinicians face a challenging task in early recognition of DILI, a rare but potentially serious event for which drug discontinuation remains the key aspect of management, provided that diagnosis is correct. Therefore, the creation of novel multidisciplinary projects and the implementation of existing consortia (e.g., the DILI network) is desirable to achieve the best risk prediction in the preclinical phase and make pharmacovigilance a reliable indicator of the post-marketing risk. This is also in line with new European pharmacovigilance legislation (in force since July 2012), which advocates the need for global risk-benefit assessment.
The recent regulatory interventions (United States restriction and Europe suspension) concerning ketoconazole for drug-induced liver injury (DILI) poses a prescribing challenge to clinicians, who should now carefully consider safer therapeutic alternatives.
Eleven systemic antimycotics (including ketoconazole and the newer triazole derivatives voriconazole and posaconazole) and terbinafine (used systemically to treat onychomicosis) generated a significant disproportionality, indicating a post-marketing signal of risk.
Authors have used the recent regulatory case of ketoconazole to provide clues to clinicians needing practical guidance to assign the level of DILI risk among antimycotics.
This is a well-written manuscript that provides valuable information about drug hepatotoxicity, particularly those induced by antimycotics.
P- Reviewer: Julie NL, Neil L S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an update on the 2007 overview. Expert Opin Drug Saf. 2014;13:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 2. | Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 3. | Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 736] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 4. | Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity--two villains or one? J Pharmacol Exp Ther. 2010;332:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Lammert C, Einarsson S, Saha C, Niklasson A, Bjornsson E, Chalasani N. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Yu K, Geng X, Chen M, Zhang J, Wang B, Ilic K, Tong W. High daily dose and being a substrate of cytochrome P450 enzymes are two important predictors of drug-induced liver injury. Drug Metab Dispos. 2014;42:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 720] [Article Influence: 51.4] [Reference Citation Analysis (1)] |
| 10. | Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 772] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6:17-32. [PubMed] |
| 12. | Brinker AD, Wassel RT, Lyndly J, Serrano J, Avigan M, Lee WM, Seeff LB. Telithromycin-associated hepatotoxicity: Clinical spectrum and causality assessment of 42 cases. Hepatology. 2009;49:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52:730-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V, Reddy R. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661-670. [PubMed] |
| 15. | Agarwal VK, McHutchison JG, Hoofnagle JH. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010;8:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 16. | Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 384] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 17. | Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics. 2006;7:889-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Coloma PM, Trifirò G, Patadia V, Sturkenboom M. Postmarketing safety surveillance : where does signal detection using electronic healthcare records fit into the big picture? Drug Saf. 2013;36:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Regev A. How to avoid being surprised by hepatotoxicity at the final stages of drug development and approval. Clin Liver Dis. 2013;17:749-67, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Corsini A, Ganey P, Ju C, Kaplowitz N, Pessayre D, Roth R, Watkins PB, Albassam M, Liu B, Stancic S. Current challenges and controversies in drug-induced liver injury. Drug Saf. 2012;35:1099-1117. [PubMed] |
| 21. | Hawkins MT, Lewis JH. Latest advances in predicting DILI in human subjects: focus on biomarkers. Expert Opin Drug Metab Toxicol. 2012;8:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Durán JA. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 689] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 24. | Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 25. | Björnsson E; FDA. Guidance for Industry. Drug-Induced Liver Injury: Premarketing Clinical Evaluation (2009). Available from: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf. |
| 26. | Watkins PB, Desai M, Berkowitz SD, Peters G, Horsmans Y, Larrey D, Maddrey W. Evaluation of drug-induced serious hepatotoxicity (eDISH): application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf. 2011;34:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Andrade RJ, Tulkens PM. Hepatic safety of antibiotics used in primary care. J Antimicrob Chemother. 2011;66:1431-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Cronin S, Chandrasekar PH. Safety of triazole antifungal drugs in patients with cancer. J Antimicrob Chemother. 2010;65:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy. Analysis of 33 cases. Gastroenterology. 1984;86:503-513. [PubMed] |
| 30. | García Rodríguez LA, Duque A, Castellsague J, Pérez-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Yan JY, Nie XL, Tao QM, Zhan SY, Zhang YD. Ketoconazole associated hepatotoxicity: a systematic review and meta- analysis. Biomed Environ Sci. 2013;26:605-610. [PubMed] |
| 32. | Fernandes NF, Geller SA, Fong TL. Terbinafine hepatotoxicity: case report and review of the literature. Am J Gastroenterol. 1998;93:459-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Ajit C, Suvannasankha A, Zaeri N, Munoz SJ. Terbinafine-associated hepatotoxicity. Am J Med Sci. 2003;325:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Anania FA, Rabin L. Terbinafine hepatotoxicity resulting in chronic biliary ductopenia and portal fibrosis. Am J Med. 2002;112:741-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Kao WY, Su CW, Huang YS, Chou YC, Chen YC, Chung WH, Hou MC, Lin HC, Lee FY, Wu JC. Risk of oral antifungal agent-induced liver injury in Taiwanese. Br J Clin Pharmacol. 2014;77:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Trifirò G, Pariente A, Coloma PM, Kors JA, Polimeni G, Miremont-Salamé G, Catania MA, Salvo F, David A, Moore N. Data mining on electronic health record databases for signal detection in pharmacovigilance: which events to monitor? Pharmacoepidemiol Drug Saf. 2009;18:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Harpaz R, DuMouchel W, LePendu P, Bauer-Mehren A, Ryan P, Shah NH. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 38. | Dusetzina SB, Higashi AS, Dorsey ER, Conti R, Huskamp HA, Zhu S, Garfield CF, Alexander GC. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care. 2012;50:466-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA Adverse Event Reporting System. Drug Saf. 2010;33:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Poluzzi E, Raschi E, Koci A, Moretti U, Spina E, Behr ER, Sturkenboom M, De Ponti F. Antipsychotics and torsadogenic risk: signals emerging from the US FDA Adverse Event Reporting System database. Drug Saf. 2013;36:467-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Brinker AD, Lyndly J, Tonning J, Moeny D, Levine JG, Avigan MI. Profiling cumulative proportional reporting ratios of drug-induced liver injury in the FDA Adverse Event Reporting System (FAERS) database. Drug Saf. 2013;36:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data Mining Techniques in Pharmacovigilance: Analysis of the Publicly Accessible FDA Adverse Event Reporting System (AERS). Data Mining Applications in Engineering and Medicine. InTech: Rijeka, Croatia 2012; 267-301. |
| 44. | Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, Hunt CM, Freston JW. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Saf. 2010;33:503-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 846] [Article Influence: 70.5] [Reference Citation Analysis (2)] |
| 46. | van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 863] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 47. | Raschi E, Piccinni C, Poluzzi E, Marchesini G, De Ponti F. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol. 2013;50:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Ryan PB, Schuemie MJ, Welebob E, Duke J, Valentine S, Hartzema AG. Defining a reference set to support methodological research in drug safety. Drug Saf. 2013;36 Suppl 1:S33-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Coloma PM, Avillach P, Salvo F, Schuemie MJ, Ferrajolo C, Pariente A, Fourrier-Réglat A, Molokhia M, Patadia V, van der Lei J. A reference standard for evaluation of methods for drug safety signal detection using electronic healthcare record databases. Drug Saf. 2013;36:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 599] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 51. | Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 52. | Shah RR; NEVAXAR. EMA: procedural steps taken and scientific information after the authorization (2010). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Procedural_steps_taken_and_scientific_information_after_authorisation/human/000690/WC500027709.pdf. |
| 53. | Shah RR; NEVAXAR. FDA: Labeling Revision (2010). Available from: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/021923Origs013ltr.pdf. |
| 54. | Petronijevic M, Ilic K. Associations of gender and age with the reporting of drug-induced hepatic failure: data from the VigiBase™. J Clin Pharmacol. 2013;53:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Raschi E, Poluzzi E, Zuliani C, Muller A, Goossens H, De Ponti F. Exposure to antibacterial agents with QT liability in 14 European countries: trends over an 8-year period. Br J Clin Pharmacol. 2009;67:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Raschi E, Poluzzi E, Godman B, Koci A, Moretti U, Kalaba M, Bennie M, Barbui C, Wettermark B, Sturkenboom M. Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe. PLoS One. 2013;8:e81208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Adriaenssens N, Coenen S, Muller A, Vankerckhoven V, Goossens H. European Surveillance of Antimicrobial Consumption (ESAC): outpatient systemic antimycotic and antifungal use in Europe. J Antimicrob Chemother. 2010;65:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Adriaenssens N, Coenen S, Versporten A, Goossens H. Outpatient systemic antimycotic and antifungal use in Europe: new outcome measure provides new insight. Int J Antimicrob Agents. 2013;42:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Zarb P, Amadeo B, Muller A, Drapier N, Vankerckhoven V, Davey P, Goossens H. Antifungal therapy in European hospitals: data from the ESAC point-prevalence surveys 2008 and 2009. Clin Microbiol Infect. 2012;18:E389-E395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |