Published online Aug 27, 2014. doi: 10.4254/wjh.v6.i8.549
Revised: February 9, 2014
Accepted: June 18, 2014
Published online: August 27, 2014
Processing time: 313 Days and 7 Hours
The present study aims to review the evolution of surgical management of portal (PVT) and splanchnic venous thrombosis (SVT) in the context of liver transplantation over the last 5 decades. PVT is more commonly managed by endovenous thrombectomy, while SVT requires more complex technical expedients. Several surgical techniques have been proposed, such as extensive eversion thrombectomy, anastomosis to collateral veins, reno-portal anastomosis, cavo-portal hemi-transposition, portal arterialization and combined liver-intestinal transplantation. In order to achieve satisfactory outcomes, careful planning of the surgical strategy is mandatory. The excellent results that are obtained nowadays confirm that, even extended, splanchnic thrombosis is no longer an absolute contraindication for liver transplantation. Patients with advanced portal thrombosis may preferentially be referred to specialized centres, in which complex vascular approaches and even multivisceral transplantation are performed.
Core tip: The present study aims to review the evolution of surgical management of portal and splanchnic venous thrombosis in the context of liver transplantation.
- Citation: Lai Q, Spoletini G, Pinheiro RS, Melandro F, Guglielmo N, Lerut J. From portal to splanchnic venous thrombosis: What surgeons should bear in mind. World J Hepatol 2014; 6(8): 549-558
- URL: https://www.wjgnet.com/1948-5182/full/v6/i8/549.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i8.549
Portal vein thrombosis (PVT) has been described as a multi-factorial condition resulting from the combination of both inherited and acquired factors[1]. Cirrhosis represents the most common etiologic factor, accounting for up to 24%-32% of cases[2]. Other common causes include cancer, infection, inflammation and thrombophilic disorders.
The incidence of PVT also correlates with the severity of cirrhosis[3], thus being a common problem during liver transplantation (LT). PVT usually arises within the liver and extends downwards into the extra-hepatic portion of the portal vein (PV). In some cases the thrombosis further extends to the mesenteric branches resulting in a splanchnic venous thrombosis (SVT).
Until the late 1980’s, PVT and SVT were considered contra-indications for LT due to concerns about compromised portal allograft inflow.
The first successful LT in a patient with PVT was reported by the Pittsburgh group in 1985 using a free iliac vein allograft[4]. Two years later, the same group presented the first large series of LT in patients with PVT (n = 22), representing a landmark paper in this field[5]. Since that seminal experience, several new techniques have been proposed to overcome this problem. The present study reviews the surgical evolution in this field of LT over the last five decades.
Despite progress in preoperative and cross-sectional imaging, a substantial number of cases of PVT or SVT are still discovered at the time of LT[6,7]. Doppler-ultrasound examination remains the most common initial diagnostic tool. However, it has limitations in detecting thrombosis due to (spontaneous or medical) recanalization and because of thrombus extension to the mesenteric veins, which cannot always be visualised clearly. Therefore, computed tomography and magnetic resonance angiography have an important role in diagnosing this condition[8]. The presence of arterial enhancement in contrast-enhanced ultrasound may help differentiate between malignant and benign thromboses[9].
The sensitivity in detecting complete venous thrombosis ranges from 92% to 100%, decreasing to 14%-50% in partial thrombosis[10]. The preoperative identification of PVT enables surgical planning and the exclusion of patients with malignant thrombosis from listing for LT.
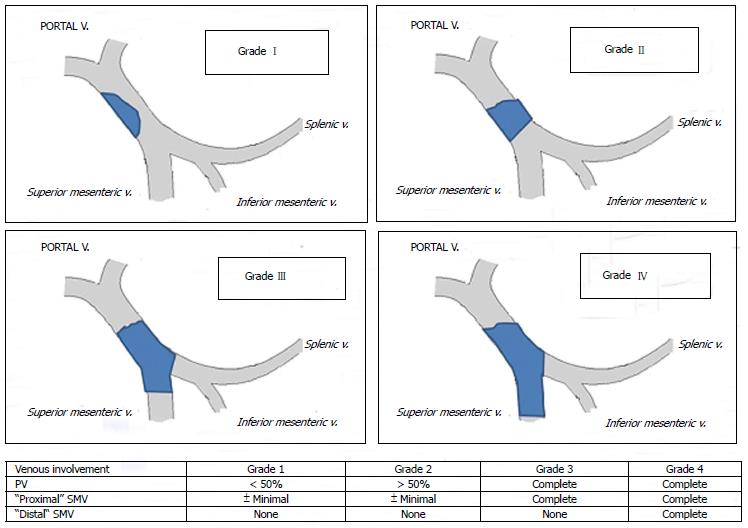
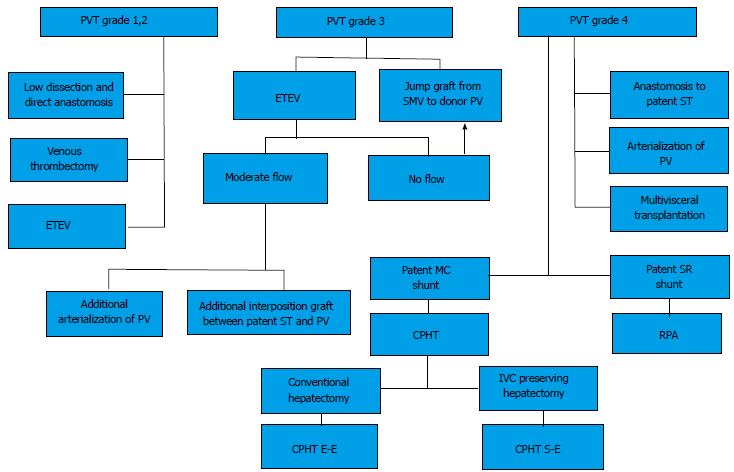
Several classifications have been proposed so far; the Yerdel classification gained the greatest acceptance and widespread clinical application[3] (Figure 1). Grade I and II PVT can almost always be managed by portal vein resection with or without thrombectomy; grade III and IV PVT require a more complex technique (Figure 2).
Management of grade I-IIPVT
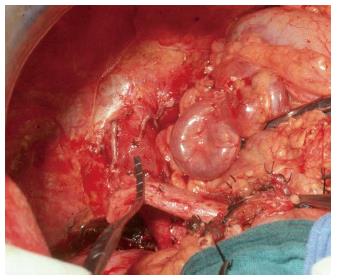
The initial strategy for grades I-II PVT is the removal of the thrombus. This is best done by removing it together with the innermost layer of the vessel (thromboendovenectomy)[3]. If the thrombosis involves a short segment of the PV, this can be resected; the residual part of the thrombus can also be fixed to the vessel wall[3]. The thrombus is separated from the PV wall using an endarterectomy spatula and the thrombus is freed under direct vision whilst everting the vessel wall[11-13]. Thrombi extending up to the mesenteric vein can be extracted successfully with this technique. Blind extraction using vascular clamps should be avoided as it can rip the vessel, which may result in uncontrollable bleeding, especially at the level of the pancreatic head. The completeness of the thrombectomy can be verified by restoration of an adequate portal blood flow (Figure 3).
Eversion thromboendovenectomy (ETEV) is another surgical technique applicable to type I-III thromboses. Type IV thrombosis can only be occasionally treated with this technique, but typically requires more complex procedures. With ETEV, the clot is progressively and circumferentially freed with the aid of a tonsil clamp by everting the venous wall, and clamping the free edge of the clot with a tonsil. Some authors consider ETEV a risky technique, as a piece of diseased venous wall with thrombogenic potential is left in place[14].
Pan et al[15] described a modification of ETEV, called improved eversion thrombectomy, in which 1 cm of the anterior wall of the PV is cut, with the final removal of the smooth wall of PT after clot removal. This technique was reported in 23 type I-III cases, with no PVT recurrences or post-operative deaths.
Several single-centre series have been reported in relation to the treatment of grade I-II PVT[14-19]. A large review of 1957 LT recipients with PVT[10] showed that thrombectomy and/or thromboendovenectomy with end-to-end portal anastomosis was the most frequently used technique (75% of cases) with a very low risk of PVT recurrence and complications.
Management of grade IIIPVT
In the case of type III PVT, ETEV alone can be insufficient, due to involvement of the distal portion of the SMV[15]. If portal flow is insufficient, different options can be considered in order to establish an adequate portal flow (> 600 mL/min). Porto-systemic shunt collaterals can be suture-ligated; in the case of spontaneous or surgical spleno-renal shunt, the left renal vein can be divided[20]. Sometimes a reno-portal anastomosis using a free iliac vein graft between the left renal vein and the PV (end-to-side or end-to-end anastomosis) can provide adequate portal inflow[21]. Another technique in grade III PVT may consist of anastomosing (eventually with a venous graft) the PV to recipient collaterals (coronary or choledochal veins). All these techniques can be considered when the PV is found to be a small fibrotic vessel.
A jump graft can be used in cases in which a low dissection of the retro-pancreatic PV or distal SMV part is required. This method avoids hazardous dissection with potential fatal bleeding and risk of pancreatitis[5,22].
Rodríguez-Castro et al[10], adopted venous interposition grafting between donor and recipient PV in 158 cases (8.4%), which represents the second most commonly used surgical technique after thrombectomy/thromboendovenectomy.
Kim reported 50 cases of living donor LT with PVT: in one case (2.4%) of partial PVT, the PV was reconstructed using a cryopreserved interposition graft after resection of a thrombosed segment; in 3/7 cases of total PVT, the distal SMV or coronary vein were used for the inflow using a jump graft; two patients with SVT, both needed jump grafts[17].
Management of grade IV PVT
Up to 15 years ago, patients with diffuse SVT were not considered for LT. More recently, 5 different surgical techniques to restore portal inflow have been suggested: (1) anastomosis to a patent splanchnic tributary (APST); (2) PV arterialization (PVA); (3) reno-portal anastomosis (RPA); (4) cavo-portal hemitransposition (CPHT); (5) hepato-intestinal or (6) multi-visceral transplantation (MVT).
APST represents, when feasible, the preferred approach in the case of SVT as it is the “easiest” to perform. This technique was initially described by Lerut et al[5] and Hiatt et al[23]. In the review by Rodríguez-Castro et al[10], 49 (2.4%) cases of APST were described; the reported series rarely contain more than 5 cases[3,24]. Virtually any large collateral (2 cm of diameter or more) can suffice to supply the graft; these are mostly a bile duct varix or a middle colic or coronary (left gastric) veins (Figure 4). The venous flow must be tested before implanting the graft to ensure adequate inflow. An interposition graft is sometimes necessary[25]. Particular care must be taken when suturing these variceal structures to the donor portal vein.
PVA is a simple method to restore the portal blood flow into the graft, anastomosing the PV of the graft to the hepatic or gastro-duodenal artery or aorta using an iliac interposition graft. This revascularisation procedure is well documented in surgery for portal hypertension[26] and post-LT arterial thrombosis[27] or, more commonly in the setting of PVT during LT[28-35]. It is occasionally used to deal with early PVT complicating LT. Here, PVA is usually associated with PV thrombectomy. PVA has been reported once in a case of auxiliary heterotopic LT[36].
The PV can be directly anastomosed to the recipient hepatic artery[28,29,32,33], or anastomosed to the supra- or infra-renal aorta with an interposition graft from a segment of donor iliac artery[28-31]. In one case, PV was anastomosed to the accessory right hepatic artery originating from the superior mesenteric artery[34]. However, this is associated with significant mortality due to haemorrhage, right heart failure, acute[28,32] and secondary PVT[28,30-34]. Some patients developed graft fibrosis due to modified hepatic microcirculation[28,32], right-sided heart decompensation[29] as well as persistent portal hypertension due to ‘‘over-arterialization’’[28,32]. Experimental syngenic rat models confirmed that PV ‘‘over-arterialization’’ during LT can lead to liver fibrogenesis[37]. A possible solution for this problem is the surgical modulation of the arterialized portal inflow. There is, however, no agreement regarding the ‘‘ideal’’ flow to aim at in this setting; some authors propose 0.6-0.8 L/min[33], others 1 L/min[30] or even 1.5-1.8 L/min[32]. The calibration of arterio-portal anastomosis can also be done either surgically or radiologically using coil embolization of the artery anastomosed to the PV[29,36]. In the 3 cases in which calibration of PVA was performed either surgically or radiologically favourable outcome were observed, with a follow-up up to 36 mo[29,36]. However, the progressive aneurysmal dilatation of intrahepatic portal branches and the possible risk of liver fibrosis suggest that PVA should be used only exceptionally.
RPA was originally reported by Sheil et al[38], and subsequently modified with a venous interposition graft by Bhangui et al[39] and Kato et al[40]. This approach represents a good option in grade IV PVT when engorged collateral vessels are unavailable or when their blood flow is inadequate. However, the liver pathophysiological consequences of this reconstruction are not yet clearly elucidated[41,42]. This method, when possible to adopt, is safe[43]. Until now, RPA has been reported in about 50 cases worldwide; however, only a handful of series contain more than five cases[39,40].
After identification and control of the renal vein, a free iliac vein can be anastomosed either end-to-end or side-to-end to the renal vein. Next, the renal vein itself or the interposition graft will be anastomosed to the donor PV. Kocherisation of the duodenum can be useful when the renal vein has a lower location.
This technique also has some complications such as ascites, renal dysfunction, GI-bleeding, deep venous thrombosis and oedema of lower limbs. These events are all due to a persistent portal hypertension that is resolved only partially by this technique[44].
CPHT represents an exceptional technique to overpass an extensive splanchnic venous thrombosis. The inflow from IVC is used to perfuse the PV of the allograft. CPHT was developed in animal models for the treatment of some metabolic diseases[45-47]. The first human series were performed by Starzl et al[48] and Riddell et al[49] for glycogen storage disease. In 1998, Tzakis et al[50] reported a series of nine cases of CPHT performed during LT due to diffuse PVT. To date, 107 cases have been reported worldwide (Table 1) with the largest series described by Tzakis (n = 23)[14,15,24,50-80].
| Ref. | Year | Country | Pts | Post-LT | Mortality | Survival (mo)1 | ||
| Varicealbleeding | Cavo-portalthrombosis | Severe renal failure | ||||||
| Weeks et al[52] | 2000 | United States | 1 | 0 | 1 | 0 | 0 | 20 |
| Varma et al[53] | 2000 | United States | 1 | 0 | 0 | 0 | 0 | 12 |
| Olausson et al[55] | 2001 | Sweden | 6 (12) | 1 | 2 | 2 | 1 | 13 |
| Santaniello et al[56] | 2001 | Italy | 1 | 1 | 0 | 1 | 0 | 9 |
| Bakthavatsalam et al[58] | 2001 | United States | 12 | 0 | 0 | 0 | 0 | 12 |
| Urbani et al[59] | 2002 | Italy | 6 (22) | 1 | 1 | 1 | 1 | 23 |
| Gerunda et al[60] | 2002 | Italy | 2 | 2 | 0 | 2 | 1 | 12 |
| Azoulay et al[61] | 2002 | France | 8 | 2 | 0 | 1 | 3 | 37 |
| Shrotri et al[62] | 2003 | United Kingdom | 1 | 0 | 1 | 0 | 0 | 12 |
| Kumar et al[63] | 2003 | United Kingdom | 1 | 0 | 0 | 0 | 0 | 24 |
| Verran et al[64] | 2004 | Australia | 12 | 0 | 0 | 0 | 0 | 6 |
| Bertelli et al[66] | 2005 | Italy | 1 | 1 | 0 | 0 | 0 | 84 |
| Wang et al[67] | 2005 | China | 1 | 0 | 0 | 0 | 0 | 6 |
| Ozden et al[68] | 2006 | Turkey | 12 | 0 | 0 | 0 | 0 | 13 |
| Lipshutz et al[69] | 2006 | United States | 7 (12) | 0 | 0 | 0 | 2 | 96 |
| Egawa et al[70] | 2006 | Japan | 1 | 0 | 0 | 1 | 1 | 0 |
| Lladó et al[24] | 2007 | Spain | 1 | 0 | 0 | 0 | 1 | 1 |
| Selvaggi et al[71] | 2007 | United States | 23 | 7 | 6 | 3 | 13 | 112 |
| Li et al[72] | 2008 | China | 1 | 0 | 1 | 0 | 0 | 18 |
| Yan et al[73] | 2008 | China | 3 | 1 | 0 | 0 | 1 | 48 |
| Pan et al[15] | 2009 | China | 1 | 0 | 0 | 0 | 1 | - |
| Tao et al[16] | 2009 | China | 2 | 0 | 1 | 0 | 0 | - |
| Gao et al[74] | 2009 | China | 2 | 1 | 0 | 0 | 2 | 6 |
| Campsen et al[75] | 2010 | United States | 10 | 0 | 0 | 0 | 0 | - |
| Suarez et al[76] | 2010 | Spain | 4 | - | - | - | 2 | - |
| Ravaioli et al[77] | 2011 | Italy | 6 | 0 | 0 | 0 | 1 | - |
| Shi et al[78] | 2011 | China | 1 | 0 | 0 | 0 | 0 | - |
| Lai et al[79] | 2012 | Belgium | 8 | 3 | 4 | 2 | 5 | 139 |
| Chmurowicz et al[80] | 2013 | Poland | 12 | 0 | 0 | 0 | 0 | - |
| Total | 103 (72) | 20 | 17 | 13 | 35 | |||
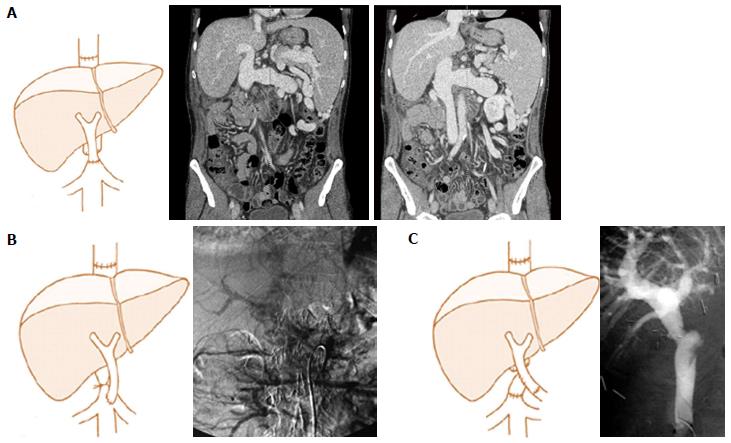
CPHT can be performed either as an end-to-end or an end-to-side anastomosis between IVC and PV; with the latter carried out in the case of IVC sparing hepatectomy (Figure 5). Both connections may require the use of an interposition graft. In the end-to-side anastomosis, the IVC is best ligated in order to redirect the systemic venous blood flow to the allograft. This procedure carries a high mortality [36 (33.6%) patients] mainly due to sepsis and multiple organ failure. The longest reported survival is 139 mo.
Postoperative complications are mainly related to anastomotic thrombosis or stenosis, congestion of the inferior vena cava (IVC) and incompletely resolved portal hypertension. Complications related to IVC congestion are mild to severe oedema of the lower torso and limbs and renal dysfunction. Mild renal dysfunction, observed in almost all patients, usually resolves spontaneously without the need for haemodialysis; haemodialysis was required in 13 (12.1%) patients. Within the second group of complications, the most commonly observed were ascites and (early or delayed) variceal bleeding. In 21 (19.6%) patients, bleeding occurred post-operatively due to persistent portal hypertension. Varma reported a case in which a venous graft was interposed between a retroperitoneal varix and the PV in order to improve the drainage of the portal venous system[53]. The majority of variceal bleedings were controlled with sclerotherapy, splenic artery embolization or splenectomy with or without gastric devascularization[79].
Thrombosis at the level of the anastomosis was seen in 17 (15.9%) patients. Such complication can sometimes be treated by endovascular stenting.
Hepato-intestinal or MVT represents the very last surgical option in grade IV PVT, allowing replacement of the entire splanchnic venous system of the recipient[81]. Such a radical expedient still represents a major technical and immunological challenge, and carries a high risk of rejection, infection and surgical complications. In recent years, surgical technique and peri-operative management have evolved substantially, achieving one- and three-year survival rates up to 80% and 70%[65,82,83]. Particular attention should be given to the graft procurement and to ensure a low cold ischemia time (≤ 6 h) in order to avoid irreversible intestinal mucosal injury[84,85]. The MVT surgical technique consists of complete replacement of the abdominal viscera after exenteration[86]. Arterial inflow is established with a unique anastomosis between the donor aortic patch encompassing the coeliac trunk and superior mesenteric artery and recipient aorta. Venous anastomosis is routinely performed with a piggy-back technique. A terminal ileostomy is created in order to allow endoscopic monitoring of the bowel graft. The decision to opt for a MVT may be undertaken during the transplant procedure. If adequate portal flow can be restored, the Indiana group led by Vianna proposed that only the liver should be implanted and the multivisceral graft split on the back table; the remaining organs are next directed towards a backup recipient needing an isolated intestinal transplantation. If portal flow cannot be established, then a MVT is required[87].
SVT used to represent a common indication for MVT in adults. The Pittsburgh experience consisting of five hundred transplants included MVT for SVT in 10% of cases[88]. The Indiana group obtained excellent one- and three-year survival rates of 80% and 72%. However, the procedure carried a complication rate of 56% with half the patients requiring a surgical re-exploration[83]. Nowadays, MVT is less frequently offered in cases of SVT due to the introduction of more sophisticated surgical techniques when dealing with extended SVT.
PVT and SVT are no longer a contraindication for LT. However, in order to achieve satisfactory outcomes, the surgical strategy needs to be carefully planned before the transplant procedure. Patients with extended splanchnic thrombosis may need complex vascular interventions; others may even require a multivisceral transplantation.
We thank Reena Ravikumar, Specialty Registrar in General Surgery, London, United Kingdom, for her support with English language revision.
P- Reviewer: Di Costanzo GG, Xia VW S- Editor: Gou SX L- Editor: Webster JR E- Editor: Wu HL
| 1. | Garcia-Pagan JC, Valla DC. Portal vein thrombosis: a predictable milestone in cirrhosis? J Hepatol. 2009;51:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: a review. Am J Med. 1992;92:173-182. [PubMed] |
| 3. | Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873-1881. [PubMed] |
| 4. | Shaw BW, Iwatsuki S, Bron K, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985;161:66-68. [PubMed] |
| 5. | Lerut J, Tzakis AG, Bron K, Gordon RD, Iwatsuki S, Esquivel CO, Makowka L, Todo S, Starzl TE. Complications of venous reconstruction in human orthotopic liver transplantation. Ann Surg. 1987;205:404-414. [PubMed] |
| 6. | Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, Denninger MH, Sauvanet A, Valla D, Durand F. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691-697. [PubMed] |
| 7. | Dumortier J, Czyglik O, Poncet G, Blanchet MC, Boucaud C, Henry L, Boillot O. Eversion thrombectomy for portal vein thrombosis during liver transplantation. Am J Transplant. 2002;2:934-938. [PubMed] |
| 8. | DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 650] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 9. | Raza SA, Jang HJ, Kim TK. Differentiating malignant from benign thrombosis in hepatocellular carcinoma: contrast-enhanced ultrasound. Abdom Imaging. 2014;39:153-161. [PubMed] |
| 10. | Rodríguez-Castro KI, Porte RJ, Nadal E, Germani G, Burra P, Senzolo M. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation. 2012;94:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Stieber AC, Zetti G, Todo S, Tzakis AG, Fung JJ, Marino I, Casavilla A, Selby RR, Starzl TE. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg. 1991;213:199-206. [PubMed] |
| 12. | Molmenti EP, Roodhouse TW, Molmenti H, Jaiswal K, Jung G, Marubashi S, Sanchez EQ, Gogel B, Levy MF, Goldstein RM. Thrombendvenectomy for organized portal vein thrombosis at the time of liver transplantation. Ann Surg. 2002;235:292-296. [PubMed] |
| 13. | Lerut JP, Mazza D, van Leeuw V, Laterre PF, Donataccio M, de Ville de Goyet J, Van Beers B, Bourlier P, Goffette P, Puttemans T. Adult liver transplantation and abnormalities of splanchnic veins: experience in 53 patients. Transpl Int. 1997;10:125-132. [PubMed] |
| 14. | Seu P, Shackleton CR, Shaked A, Imagawa DK, Olthoff KM, Rudich SR, Kinkhabwala M, Busuttil RW. Improved results of liver transplantation in patients with portal vein thrombosis. Arch Surg. 1996;131:840-844; discussion 844-845. [PubMed] |
| 15. | Pan C, Shi Y, Zhang JJ, Deng YL, Zheng H, Zhu ZJ, Shen ZY. Single-center experience of 253 portal vein thrombosis patients undergoing liver transplantation in China. Transplant Proc. 2009;41:3761-3765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Tao YF, Teng F, Wang ZX, Guo WY, Shi XM, Wang GH, Ding GS, Fu ZR. Liver transplant recipients with portal vein thrombosis: a single center retrospective study. Hepatobiliary Pancreat Dis Int. 2009;8:34-39. [PubMed] |
| 17. | Kim SJ, Kim DG, Park JH, Moon IS, Lee MD, Kim JI, Yoon YC, Yoo YK. Clinical analysis of living donor liver transplantation in patients with portal vein thrombosis. Clin Transplant. 2011;25:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Robles R, Fernández JA, Hernández Q, Marin C, Ramirez P, Sánchez Bueno F, Luján JA, Rodriguez JM, Acosta F, Parrilla P. Eversion thromboendovenectomy for organized portal vein thrombosis encountered during liver transplantation. Transplant Proc. 2003;35:1915-1917. [PubMed] |
| 19. | Song S, Kwon CH, Shin M, Kim TS, Lee S, Moon HH, Park JB, Kim SJ, Joh JW, Lee SK. A new technique for complete portal vein and superior mesenteric vein thrombosis in a liver transplant recipient. Exp Clin Transplant. 2014;12:67-70. [PubMed] |
| 20. | Slater RR, Jabbour N, Abbass AA, Patil V, Hundley J, Kazimi M, Kim D, Yoshida A, Abouljoud M. Left renal vein ligation: a technique to mitigate low portal flow from splenic vein siphon during liver transplantation. Am J Transplant. 2011;11:1743-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Akbulut S, Kayaalp C, Yilmaz M, Yilmaz S. Auxiliary reno-portal anastomosis in living donor liver transplantation: a technique for recipients with low portal inflow. Transpl Int. 2012;25:e73-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Aydin C, Ersan V, Baskiran A, Unal B, Kayaalp C, Yilmaz S. Controlling massive hemorrhage from the retropancreatic portal vein as a complication of thromboendovenectomy during liver transplantation with balloon catheter tamponade: how to do it. Surg Today. 2014;44:792-794. [PubMed] |
| 23. | Hiatt JR, Quinones-Baldrich WJ, Ramming KP, Lois JF, Busuttil RW. Bile duct varices. An alternative to portoportal anastomosis in liver transplantation. Transplantation. 1986;42:85. [PubMed] |
| 24. | Lladó L, Fabregat J, Castellote J, Ramos E, Torras J, Jorba R, Garcia-Borobia F, Busquets J, Figueras J, Rafecas A. Management of portal vein thrombosis in liver transplantation: influence on morbidity and mortality. Clin Transplant. 2007;21:716-721. [PubMed] |
| 25. | Wu TH, Chou HS, Pan KT, Lee CS, Wu TJ, Chu SY, Chen MF, Lee WC. Application of cryopreserved vein grafts as a conduit between the coronary vein and liver graft to reconstruct portal flow in adult living liver transplantation. Clin Transplant. 2009;23:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Otte JB, Reynaert M, De Hemptinne B, Geubel A, Carlier M, Jamart J, Lambotte L, Kestens PJ. Arterialization of the portal vein in conjunction with a therapeutic portacaval shunt. Hemodynamic investigations and results in 75 patients. Ann Surg. 1982;196:656-663. [PubMed] |
| 27. | Melandro F, Lai Q, Levi Sandri GB, Guglielmo N, Di Laudo M, Morabito V, Pretagostini R, Berloco PB, Rossi M. A case of portal vein arterialization after a liver transplant. Exp Clin Transplant. 2013;11:287-289. [PubMed] |
| 28. | Ott R, Böhner C, Müller S, Aigner T, Bussenius-Kammerer M, Yedibela S, Kissler H, Hohenberger W, Reck T, Müller V. Outcome of patients with pre-existing portal vein thrombosis undergoing arterialization of the portal vein during liver transplantation. Transpl Int. 2003;16:15-20. [PubMed] |
| 29. | Stange B, Glanemann M, Nüssler NC, Bechstein WO, Neuhaus P, Settmacher U. Indication, technique, and outcome of portal vein arterialization in orthotopic liver transplantation. Transplant Proc. 2001;33:1414-1415. [PubMed] |
| 30. | Erhard J, Lange R, Giebler R, Rauen U, de Groot H, Eigler FW. Arterialization of the portal vein in orthotopic and auxiliary liver transplantation. A report of three cases. Transplantation. 1995;60:877-879. [PubMed] |
| 31. | Aspinall RJ, Seery JP, Taylor-Robinson SD, Habib N. Comments on “arterialization of the portal vein in orthotopic and auxiliary liver transplantation”. Transplantation. 1996;62:1375-1376. [PubMed] |
| 32. | Charco R, Margarit C, López-Talavera JC, Hidalgo E, Castells L, Allende H, Segarra A, Moreíras M, Bilbao I. Outcome and hepatic hemodynamics in liver transplant patients with portal vein arterialization. Am J Transplant. 2001;1:146-151. [PubMed] |
| 33. | Nivatvongs S, Sirijindakul B, Nontasoot B. Portal vein arterialization for liver transplantation with extensive portomesenteric vein thrombosis: a case report. Transplant Proc. 2004;36:2267-2268. [PubMed] |
| 34. | Paloyo S, Nishida S, Fan J, Tekin A, Selvaggi G, Levi D, Tzakis A. Portal vein arterialization using an accessory right hepatic artery in liver transplantation. Liver Transpl. 2013;19:773-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Settmacher U, Stange B, Schaser KD, Puhl G, Glanemann M, Steinmüller T, Heise M, Neuhaus P. Primary permanent arterialization of the portal vein in liver transplantation. Transpl Int. 2003;16:430-433. [PubMed] |
| 36. | Margarit C, Bilbao I, Charco R, Lázaro JL, Hidalgo E, Allende E, Murio E. Auxiliary heterotopic liver transplantation with portal vein arterialization for fulminant hepatic failure. Liver Transpl. 2000;6:805-809. [PubMed] |
| 37. | Müller V, Ott R, Tannapfel A, Hohenberger W, Reck T. Arterialization of the portal vein in liver transplantation: a new microsurgical model in the rat. Transplantation. 2001;71:977-981. [PubMed] |
| 38. | Sheil AG, Stephen MS, Chui AK, Ling J, Bookallil MJ. A liver transplantation technique in a patient with a thrombosed portal vein and a functioning renal-lieno shunt. Clin Transplant. 1997;11:71-73. [PubMed] |
| 39. | Bhangui P, Lim C, Salloum C, Andreani P, Sebbagh M, Hoti E, Ichai P, Saliba F, Adam R, Castaing D. Caval inflow to the graft for liver transplantation in patients with diffuse portal vein thrombosis: a 12-year experience. Ann Surg. 2011;254:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Kato T, Levi DM, DeFaria W, Nishida S, Tzakis AG. Liver transplantation with renoportal anastomosis after distal splenorenal shunt. Arch Surg. 2000;135:1401-1404. [PubMed] |
| 41. | Moon DB, Lee SG, Ahn CS, Kim KH, Hwang S, Ha TY, Song GW, Jung DH, Ryu JH, Kim KW. Technical modification of reno-portal anastomosis in living donor liver transplantation for patients with obliterated portal vein and large spontaneous splenorenal shunts. Hepatogastroenterology. 2008;55:2193-2199. [PubMed] |
| 42. | Miyamoto A, Kato T, Dono K, Umeshita K, Kawabata R, Hayashi S, Kubota M, Kobayashi S, Nagano H, Nakamori S. Living-related liver transplantation with renoportal anastomosis for a patient with large spontaneous splenorenal collateral. Transplantation. 2003;75:1596-1598. [PubMed] |
| 43. | Paskonis M, Jurgaitis J, Mehrabi A, Kashfi A, Fonouni H, Strupas K, Büchler MW, Kraus TW. Surgical strategies for liver transplantation in the case of portal vein thrombosis--current role of cavoportal hemitransposition and renoportal anastomosis. Clin Transplant. 2006;20:551-562. [PubMed] |
| 44. | Pécora RA, Canedo BF, Andraus W, Martino RB, Santos VR, Arantes RM, Pugliese V, D Albuquerque LA. Portal vein thrombosis in liver transplantation. Arq Bras Cir Dig. 2012;25:273-278. [PubMed] |
| 45. | Child CG, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann Surg. 1953;138:600-608. [PubMed] |
| 46. | Silen W, Mawdsley DL, Weirich WL, Harper HA. Studies of hepatic function in dogs with Eck fistula or portacaval transposition. AMA Arch Surg. 1957;74:964-970. [PubMed] |
| 47. | Tarzl TE, Scanlan WA, Thornton FH, Wendel RM, Stearn B, Larzarus RE, Mcallister W, Shoemaker WC. Effect of insulin on glucose metabolism in the dog after portacaval transposition. Am J Physiol. 1965;209:221-226. [PubMed] |
| 48. | Starzl TE, Putnam CW, Porter KA, Halgrimson CG, Corman J, Brown BI, Gotlin RW, Rodgerson DO, Greene HL. Portal diversion for the treatment of glycogen storage disease in humans. Ann Surg. 1973;178:525-539. [PubMed] |
| 49. | Riddell AG, Davies RP, Clark AD. Portacaval transposition in the treatment of glycogen-storage disease. Lancet. 1966;2:1146-1148. [PubMed] |
| 50. | Tzakis AG, Kirkegaard P, Pinna AD, Jovine E, Misiakos EP, Maziotti A, Dodson F, Khan F, Nery J, Rasmussen A. Liver transplantation with cavoportal hemitransposition in the presence of diffuse portal vein thrombosis. Transplantation. 1998;65:619-624. [PubMed] |
| 51. | Azoulay D, Hargreaves GM, Castaing D, Bismuth H. Caval inflow to the graft: a successful way to overcome diffuse portal system thrombosis in liver transplantation. J Am Coll Surg. 2000;190:493-496. [PubMed] |
| 52. | Weeks SM, Alexander JR, Sandhu J, Mauro MA, Fair JH, Jaques PF. Mechanic and pharmacologic treatment of a saddle embolus to the portal vein after liver transplantation and portacaval hemitransposition. AJR Am J Roentgenol. 2000;175:537-539. [PubMed] |
| 53. | Varma CR, Mistry BM, Glockner JF, Solomon H, Garvin PJ. Cavoportal hemitransposition in liver transplantation. Transplantation. 2001;72:960-963. [PubMed] |
| 54. | Norrby J, Mjörnstedt L, Liden H, Friman S, Olausson M. Liver transplantation using cavoportal hemitransposition: a possibility in the presence of extensive portal vein thrombosis. Transplant Proc. 2001;33:2495-2496. [PubMed] |
| 55. | Olausson M, Norrby J, Mjörnstedt L, Liden H, Friman S. Liver transplantation using cavoportal hemitransposition- a life-saving procedure in the presence of extensive portal vein thrombosis. Transplant Proc. 2001;33:1327-1328. [PubMed] |
| 56. | Santaniello W, Ceriello A, Defez M, Maida P, Monti GN, Sicoli F, Calise F. Liver transplant with cavoportal hemitransposition for portal and mesenteric thrombosis: case report. Transplant Proc. 2001;33:1488-1489. [PubMed] |
| 57. | Pinna AD, Nery J, Kato T, Levi D, Nishida S, Tzakis AG. Liver transplant with portocaval hemitransposition: experience at the University of Miami. Transplant Proc. 2001;33:1329-1330. [PubMed] |
| 58. | Bakthavatsalam R, Marsh CL, Perkins JD, Levy AE, Healey PJ, Kuhr CS. Rescue of acute portal vein thrombosis after liver transplantation using a cavoportal shunt at re-transplantation. Am J Transplant. 2001;1:284-287. [PubMed] |
| 59. | Urbani L, Cioni R, Catalano G, Iaria G, Bindi L, Biancofiore G, Vignali C, Mosca F, Filipponi F. Cavoportal hemitransposition: patient selection criteria and outcome. Transplant Proc. 2002;34:3331-3333. [PubMed] |
| 60. | Gerunda GE, Merenda R, Neri D, Angeli P, Barbazza F, Valmasoni M, Feltracco P, Zangrandi F, Gangemi A, Miotto D. Cavoportal hemitransposition: a successful way to overcome the problem of total portosplenomesenteric thrombosis in liver transplantation. Liver Transpl. 2002;8:72-75. [PubMed] |
| 61. | Azoulay D, Adam R, Castaing D, Muresan S, Essomba A, Vibert E, Savier E, Smail A, Veilhan LA, Bismuth H. Liver transplantation with cavoportal or renoportal anastomosis: a solution in cases of diffuse portal thrombosis. Gastroenterol Clin Biol. 2002;26:325-330. [PubMed] |
| 62. | Shrotri M, Sudhindran S, Gibbs P, Watson CJ, Alexander GJ, Gimson AE, Jamieson NV, Delriviere L. Case report of cavoportal hemitransposition for diffuse portal vein thrombosis in liver transplantation. Transplant Proc. 2003;35:397-398. [PubMed] |
| 63. | Kumar N, Atkison P, Fortier MV, Grant DR, Wall WJ. Cavoportal transposition for portal vein thrombosis in a pediatric living-related liver transplantation. Liver Transpl. 2003;9:874-876. [PubMed] |
| 64. | Verran D, Crawford M, Stormon M, Shun A. Liver retransplantation in an infant requiring cavoportal hemi transposition. Pediatr Transplant. 2004;8:416-419. [PubMed] |
| 65. | Ceulemans B, Aerts R, Monbaliu D, Coosemans W, Verslype C, Van Steenbergen W, Yap P, Fevery J, Nevens F, Pirenne J. Liver transplantation using cavoportal transposition: an effective treatment in patients with complete splanchnic venous thrombosis. Transplant Proc. 2005;37:1112-1114. [PubMed] |
| 66. | Bertelli R, Nardo B, Montalti R, Beltempo P, Puviani L, Cavallari A. Liver transplantation in recipients with portal vein thrombosis: experience of a single transplant center. Transplant Proc. 2005;37:1119-1121. [PubMed] |
| 67. | Wang C, Zhang T, Song S, Xiu D, Jiang B. Liver transplant with portocaval hemitransposition: blood supply with only hepatic artery is possible? Transplant Proc. 2005;37:2163-2165. [PubMed] |
| 68. | Ozden I, Suoglu OD, Aydogan A, Bilge O, Yavru A, Sokucu S, Acarli K. Successful living-donor liver transplantation and retransplantation with cavoportal hemitransposition: a case report. Exp Clin Transplant. 2006;4:562-566. [PubMed] |
| 69. | Lipshutz GS, Patel S, Hiatt JR, Yersiz H, Farmer DG, McDiarmid SV, Ghobrial RM, Busuttil RW. Portocaval hemitransposition in pediatric liver transplant recipients: a single-center experience. Liver Transpl. 2006;12:1097-1103. [PubMed] |
| 70. | Egawa H, Tanaka K, Kasahara M, Takada Y, Oike F, Ogawa K, Sakamoto S, Kozaki K, Taira K, Ito T. Single center experience of 39 patients with preoperative portal vein thrombosis among 404 adult living donor liver transplantations. Liver Transpl. 2006;12:1512-1518. [PubMed] |
| 71. | Selvaggi G, Weppler D, Nishida S, Moon J, Levi D, Kato T, Tzakis AG. Ten-year experience in porto-caval hemitransposition for liver transplantation in the presence of portal vein thrombosis. Am J Transplant. 2007;7:454-460. [PubMed] |
| 72. | Li FG, Yan LN, Wang WT. Extensive thrombosis of the portal vein and vena cava after orthotopic liver transplantation with cavoportal hemitransposition: a case report. Transplant Proc. 2008;40:1777-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Yan ML, Zeng Y, Li B, Xu MQ, Wen TF, Wang WT, Yang JY, Yan LN. Postoperative complications after liver transplantation with cavoportal hemitransposition. Hepatobiliary Pancreat Dis Int. 2008;7:322-324. [PubMed] |
| 74. | Gao PJ, Zhu JY, Li GM, Leng XS. Liver transplantation for the patients with end stage liver disease and portal vein thrombosis. Beijing Da Xue Xue Bao. 2009;41:558-560. [PubMed] |
| 75. | Campsen J, Kam I. Combined piggyback technique and cavoportal hemitransposition for liver transplant. Case Rep Med. 2010;2010:595289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Suarez Artacho G, Barrera Pulido L, Alamo Martinez JM, Serrano Diez-Canedo J, Bernal Bellido C, Marín Gomez LM, Padillo Ruiz J, Gómez Bravo MA. Outcomes of liver transplantation in candidates with portal vein thrombosis. Transplant Proc. 2010;42:3156-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Ravaioli M, Zanello M, Grazi GL, Ercolani G, Cescon M, Del Gaudio M, Cucchetti A, Pinna AD. Portal vein thrombosis and liver transplantation: evolution during 10 years of experience at the University of Bologna. Ann Surg. 2011;253:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Shi Z, Yan L, Wen T, Chen Z. Cavoportal hemitransposition in an adult-to-adult, living-donor liver transplantation. Dig Liver Dis. 2011;43:e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Lai Q, Darius T, Monbaliu D, Pinheiro R, Ciccarelli O, Pirenne J, Lerut J. Cavoportal hemitransposition in liver transplant recipients with complete and diffuse splanchnic venous thrombosis. Transplantation. 2012;94 Suppl 10:168. |
| 80. | Chmurowicz T, Zasada-Cedro K, Wojcicki M. Cavoportal hemitransposition for unrecognized spontaneous mesocaval shunt after liver transplantation: a case report. Transpl Int. 2013;26:E46-E49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol. 2012;57:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 82. | Fujishiro J, Pech TC, Finger TF, Praktinjo M, Stoffels B, Standop J, Abu-Elmagd K, Tuerler A, Hirner A, Kalff JC. Influence of immunosuppression on alloresponse, inflammation and contractile function of graft after intestinal transplantation. Am J Transplant. 2010;10:1545-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Vianna RM, Mangus RS, Kubal C, Fridell JA, Beduschi T, Tector AJ. Multivisceral transplantation for diffuse portomesenteric thrombosis. Ann Surg. 2012;255:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 84. | Nickkholgh A, Contin P, Abu-Elmagd K, Golriz M, Gotthardt D, Morath C, Schemmer P, Mehrabi A. Intestinal transplantation: review of operative techniques. Clin Transplant. 2013;27 Suppl 25:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Wunderlich H, Brockmann JG, Voigt R, Rauchfuss F, Pascher A, Brose S, Binner C, Bittner H, Klar E. DTG procurement guidelines in heart beating donors. Transpl Int. 2011;24:733-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Vianna RM, Mangus RS, Tector AJ. Current status of small bowel and multivisceral transplantation. Adv Surg. 2008;42:129-150. [PubMed] |
| 87. | Mangus RS, Tector AJ, Kubal CA, Fridell JA, Vianna RM. Multivisceral transplantation: expanding indications and improving outcomes. J Gastrointest Surg. 2013;17:179-86; discussion p.186-7. [PubMed] |
| 88. | Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, Koritsky DA, Schuster B, Martin L, Cruz RJ. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg. 2009;250:567-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |