Published online Jul 27, 2014. doi: 10.4254/wjh.v6.i7.504
Revised: May 16, 2014
Accepted: June 10, 2014
Published online: July 27, 2014
Processing time: 209 Days and 23.6 Hours
AIM: To study these characteristics and prognostic patterns in a Greek patient population.
METHODS: We analyzed a large cohort of cirrhotic patients referred to the department of Gastroenterology and Hepatology and the outpatient clinics of this tertiary hospital, between 1991 and 2008. We included patients with established cirrhosis, either compensated or decompensated, and further decompensation episodes were registered. A data base was maintained and updated prospectively throughout the study period. We analyzed differences in cirrhosis aetiology, time to and mode of decompensation, hepatocellular carcinoma (HCC) occurrence and ultimately patient survival.
RESULTS: Five hundreds and twenty-two patients with median age 67 (range, 29-91) years and average follow up 9 years-10 mo (range, 1-206 mo) were studied. Commonest aetiology was hepatitis C virus (HCV, 41%) followed by alcohol (31%). The median survival time in compensated cirrhotics was 115 mo (95%CI: 95-133), whereas in decompensated patients was 55 mo (95%CI: 36-75). HCV patients survived longer while HBV patients had over twice the risk of death of HCV patients. The median time to decompensation was 65 mo (95%CI: 51-79), with alcoholics having the highest risk (RR = 2.1 vs HCV patients). Hepatitis B virus (HBV) patients had the highest risk of HCC, alcoholics the lowest. Leading causes of death: liver failure, hepatorenal syndrome, sepsis and HCC progression.
CONCLUSION: Cirrhosis aetiology and decompensation at presentation were predictors of survival. Alcoholics had the highest decompensation risk, HBV cirrhotics the highest risk of HCC and HCV cirrhotics the highest decompensation-free time.
Core tip: Hepatitis C was the most common cause in our cirrhotics and many hepatitis C virus patients were aged and demonstrated a long, mild course. Alcoholic and non alcoholic steatohepatitis cirrhosis is becoming a significant problem. Ascites was the commonest type of decompensation. Survival in compensated cirrhotics was at least double that of decompensated patients. Variceal bleeding was more frequent in alcoholics; nevertheless it was unexpectedly related to better survival than decompensation with ascites or encephalopathy. This was attributed to the improvements in the management of variceal bleeding together with the importance of abstinence from alcohol after the episode was successfully treated. Hepatocellular carcinoma patients with a history of hepatitis B virus had the highest risk of mortality.
- Citation: Samonakis DN, Koulentaki M, Coucoutsi C, Augoustaki A, Baritaki C, Digenakis E, Papiamonis N, Fragaki M, Matrella E, Tzardi M, Kouroumalis EA. Clinical outcomes of compensated and decompensated cirrhosis: A long term study. World J Hepatol 2014; 6(7): 504-512
- URL: https://www.wjgnet.com/1948-5182/full/v6/i7/504.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i7.504
Cirrhosis and its complications represent the end in the spectrum of chronic liver diseases, irrespective of aetiology. The natural history of cirrhosis is classically characterised by an asymptomatic phase termed compensated cirrhosis, followed by the development of complications from portal hypertension and/or liver dysfunction, termed decompensated cirrhosis. The transition has been estimated to occur at a rate of 5%-7% per year. In recent years this process has been proposed as a series of critical steps that if unchecked, culminate in hepatic decompensation[1].
Chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), represents the commonest cause of cirrhosis worldwide[2]. Nevertheless, hepatitis C often has indolent course for a long period of time[3,4] with a median time from infection to cirrhosis of 30 years; several confounding factors have been associated with disease progression[4]. Despite higher risk for decompensation in HCV infection, cirrhosis presents earlier in HBV patients[5]. Longitudinal studies of patients with chronic hepatitis B have shown a 5 year cumulative incidence of developing cirrhosis 8%-20% with a 5 year survival in compensated cirrhosis 85% and 15%-35% in decompensated cirrhosis[6]. Various factors are related with progression in HBV infection, but clearly its course is modified by antiviral therapy and HBV DNA suppression[7,8]. Sustained response to anti-HCV treatment also significantly determines patients’ outcome regarding decompensation, liver failure, death or orthotopic liver transplantation and decreases does not completely eliminate the HCC risk[9].
Hepatocellular carcinoma (HCC) is a major complication of viral cirrhosis, both compensated and decompensated, and a major cause of death[2]. HCC incidence appears to be increasing worldwide[10] and several clinicopathological variables have been identified as predictors for outcome[11,12]. The annual incidence of HBV related HCC ranges from 2%-5%[6]. Two other very common causes of chronic liver disease and subsequent complications are non alcoholic steatohepatitis (NASH) and alcoholic liver disease, which especially in western countries are becoming significant public health issues[13,14].
The purpose of this study was to evaluate-in a region of southern Greece-a cohort of patients with either compensated or decompensated cirrhosis at presentation; to identify the time to and mode of decompensation, investigate the occurrence of HCC and assess the impact of all the aforementioned on patient survival.
The study was performed in a tertiary hospital which is the reference centre for the island of Crete, in the south of Greece. The population of 800000 is largely homogenous. The few non-Greek patients are mostly from Eastern Europe and the Balkans. The study conformed to the principles of declaration of Helsinki and was approved by the ethics committee of the University Hospital of Heraklion. The participants in the study provided verbal consent; for those patients with hepatic encephalopathy, consent was given by relatives. Written informed consent was provided from those undergoing interventional procedures (i.e., liver biopsies, endoscopies and abdominal paracentesis).
We included patients with a diagnosis of cirrhosis who were seen as outpatients in the liver clinic or were hospitalized, mostly for chronic liver disease complications. Starting date of the study was January 1991 and their data were registered in a data base until June 2008. During the long period of this study, many patients that fulfilled the criteria were included and were therefore followed up prospectively. In that sense the study is both prospective and retrospective.
All patients with established cirrhosis were included. Diagnosis was based on liver biopsy (all patients with compensated cirrhosis) and/or clinical evidence of decompensation combined with endoscopic and radiological findings. We excluded from the study (1) paediatric liver disease; (2) patients with primary biliary cirrhosis (PBC); (3) autoimmune hepatitis (AIH) cirrhosis; and (4) 12 patients who did not wish to participate in the study. PBC patients have a discrete clinical course and our experience has been reported elsewhere[15]. AIH patients are few in Crete (less than 20 patients have been diagnosed during the study period) and all but one run a good course under treatment. Thus AIH as a separate group for cirrhosis aetiology was excluded due to small numbers.
The diagnosis of decompensated cirrhosis was based on the presence of any of the following: ascites, variceal bleeding or encephalopathy. The classification as compensated cirrhosis precluded any past history of the above criteria. The diagnosis of liver failure was made when one or more of the following were observed in decompensated cirrhotics: Hepato-renal syndrome type 2/type 1, progressively worsening liver biochemistry with prolongation of international normalized ratio and/or deepening jaundice (frequently due to sepsis), severely worsening encephalopathy, or liver failure in the context of massive infiltration from tumour.
All patients with HBV related cirrhosis had received standard antivirals, initially lamivudine/adefovir and later either entecavir or tenofovir. Antiviral treatment started at the time of initial diagnosis of chronic HBV infection and continued after the diagnosis of cirrhosis until death or end of follow up.
No patient with decompensated hepatitis C related cirrhosis received antiviral treatment with the standard regimen. The number of compensated HCV cirrhotics in this population on treatment with interferon and ribavirin was too small to draw conclusions. HCV decompensated cirrhotics received only supportive treatment as indicated (diuretics, antibiotics, varices ligation, repeated paracentesis and terlipressin). Approximately half of HCV cirrhotics had no antiviral treatment prior to their cirrhosis diagnosis due to either old age, side effects or unavailability of treatment. In any case antivirals were discontinued on diagnosis of cirrhosis according to the guidelines at a certain time period. A 30% of alcoholics discontinued alcohol consumption.
A careful evaluation was performed to document any episode of decompensation at scheduled outpatient Hepatology clinic visits or at hospitalization for any reason. For patients who had not attended the outpatient clinic for three months after their previous visit, information regarding the outcome was obtained by telephone interviews with patients or relatives.
Liver biopsies were taken using ultrasound guidance and were initially performed with Menghini needles, later substituted by Tru-cut needles; few biopsies were done intraoperatively or transjugularly. All patients with bleeding were scoped within 24 h to diagnose and treat portal hypertensive bleeding. Ascites and encephalopathy were diagnosed according to standard criteria; all patients with ascites on presentation and according to clinical suspicion underwent abdominal paracentesis to check for spontaneous bacterial peritonitis ever since this was internationally accepted practice. Screening for HCC was performed every 6 mo with ultrasound (US) and a-fetoprotein, and during the last 3 years of the study contrast-enhanced US was used. HCC was diagnosed either histologically or according to European association of the study of the liver/American association of the study if the liver criteria ever since these were published[16,17].
Viral hepatitis markers were detected by Abbott Elisa immunoassays and viral load was measured quantitatively using polymerase chain reaction test wherever appropriate since the method was available. Alcohol misuse (defined as exceeding 40 g of ethanol daily in male-20 g daily in female patients) was identified after interviewing the patient during hospitalisation or in the outpatient alcoholic clinic, as well from information provided by social services. The study included patients with a diagnosis of alcoholic cirrhosis who were either active drinkers or were abstainers at evaluation. Three distinct end points were considered: decompensation, death (or liver transplantation) and HCC. Few patients received a transplant due to the late development of liver transplantation services in the country. NASH related cirrhosis and cryptogenic cirrhosis were evaluated as a single group.
Univariate comparisons of patient characteristics between the aetiological groups were undertaken using the chi-squared test and one-way ANOVA according to the type of characteristic. Bonferroni post-hoc comparisons were made when the ANOVA comparison was found to be statistically significant.
The median follow-up time was calculated using the reverse Kaplan Meier estimator[18]. Kaplan-Meier estimates of survival curves were constructed for both overall survival and decompensation-free survival. Median survival times were compared using the log-rank test. Both univariate and multivariate Cox’s proportional hazards models were used to estimate hazard ratios (relative risks). A significance level of 5% was chosen for all hypothesis tests. SPSS version 17 was used throughout.
A total of 522 patients were included in the study. The majority of these patients had compensated cirrhosis on presentation (n = 360, 69%). One hundred and eighty five patients developed decompensation during follow up (35.4% of the entire cohort and 51.3% of the initially compensated cirrhotics) and there were 231 deaths (44%) over the follow-up period. Median follow-up was 9 years 10 mo, and ranged from 1 mo to just over 17 years. There were 183 patients with a minimum follow up of 5 years in the entire cohort.
Seventy eight patients (15%) were lost to follow up. The distribution of cirrhosis causes in those lost to follow up was found to be similar to those remaining in the study (n = 444). Leading causes of death were: liver failure which resulted in 55 deaths (23.8%) hepatorenal syndrome (n = 50, 21.6%), sepsis (n = 25, 10.8%), massive portal hypertensive bleeding (n = 15, 6.5%). These were followed by HCC progression, extrahepatic cancer, cardiovascular events, and other causes. In 21 patients (9%) who died the cause was not verified.
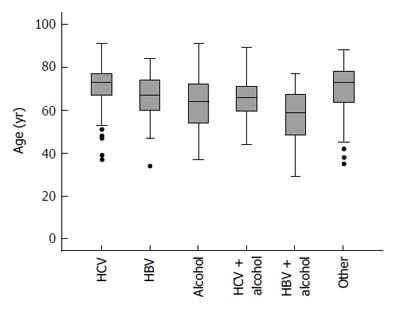
Characteristics of the patient cohort are presented in Table 1. Mean patient age was 67 (range 29 to 91) years. The most common cause of cirrhosis was hepatitis C (41%, 215 patients), followed by alcoholic liver disease (31%, 162 patients). The age distribution within each etiologic group is summarized in Figure 1.
| n (%) | |
| Number of patients | 522 |
| Male | 342 (66) |
| Female | 180 (34) |
| Cirrhosis aetiology | |
| HCV | 180 (34) |
| HCV/ALD | 35 (7) |
| HBV | 67 (13) |
| HBV/ALD | 15 (3) |
| ALD | 162 (31) |
| NASH/other | 63 (12) |
| HCC | 88 (17) |
| Patients alive | 213 (41) |
| Patients died | 231 (44) |
| Lost to follow up | 78 (15) |
| Initially compensated | 358 (69) |
| Initially decompensated | 164 (31) |
| Decompensated during Follow Up | 185 (35) |
| Initial episode of decompensation | |
| Ascites | 256 (73) |
| Variceal bleed | 37 (11) |
| Encephalopathy | 10 (3) |
| More than 1 | 22 (6) |
| Not known | 24 (7) |
The mean age of the alcoholic liver disease (ALD) patients was 62 (SD ± 12) years, of HBV patients 67 (SD ± 10) years, of HBV + ALD patients 56 (SD ± 15) years, of HCV patients 71 (SD ± 9) years, HCV + ALD patients 65 (SD ± 11) years, of NASH/cryptogenic patients 70 (SD ± 13) years. Average patient age differed to a statistically significant extent between groups (P < 0.0001). Post-hoc pairwise comparisons indicated that HCV and NASH/cryptogenic patients were older on average than ALD or HBV/ALD patients (P < 0.0001 in all cases). HBV/ALD patients were also younger, on average, than patients with HBV alone (P = 0.035). There have been only 5 patients with HDV co-infection, three of them with HBV/HDV/HCV with a history of intravenous drug use. Six patients had co infection HBV/HCV. Due to the small number no separate analysis for the viral co infections was performed.
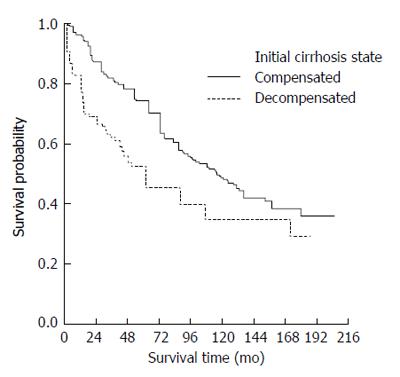
The median survival time of those presenting with compensated cirrhosis was 115 (95%CI: 95-135) mo whereas decompensated patients had a median survival of 55 (95%CI: 36-75) mo. Kaplan-Meier survival curves also indicated a worse overall prognosis for patients presenting with decompensated cirrhosis (Figure 2) (P < 0.0001).
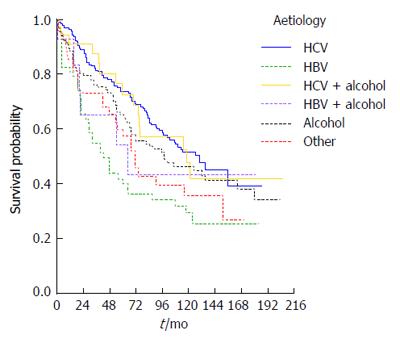
Survival was also strongly influenced by cirrhosis aetiology: Kaplan-Meier survival curves are presented according to etiologic group in Figure 3, in which HBV patients (90% e-antigen negative) appear to have the worst overall survival (P = 0.004). Using univariate Cox regression analysis, HBV patients were found to have just over twice the risk of death of HCV patients (RR = 2.1, P < 0.0001) whilst the NASH/cryptogenic group had a RR of 1.6 (P = 0.042) compared to the HCV group.
At presentation, both cirrhosis aetiology and decompensation remained significant predictors of survival (P values 0.007 and < 0.0001 respectively) after adjusting for age (P = 0.633) and sex (P = 0.505) in a multivariable model. The RR was 2.6 for patients that were decompensated at diagnosis (95%CI: 1.9-3.6) compared to compensated patients. Patients with HBV had RR = 1.8 (95%CI: 1.2-2.7, P = 0.005) compared to HCV patients but none of the other groups had a statistically significantly elevated risk compared to HCV patients (all P values > 0.1).
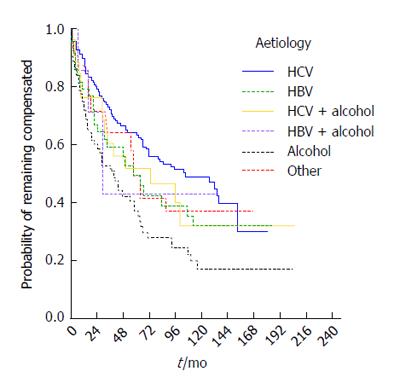
The median time to decompensation was 65 mo (95%CI: 51-79 mo) and varied according to aetiology. Kaplan-Meier curves are presented by etiologic group in Figure 4 (P = 0.003). The highest median decompensation-free time was seen in the HCV patient group (median 105, 95%CI: 60-150 mo), the lowest in the NASH/cryptogenic (median 58, 95%CI: 48-68) and HBV groups (median 57, 95%CI: 35-79 mo). Aetiology remained a statistically significant predictor of risk of decompensation in a multivariable Cox model (P = 0.026), adjusting for age (P = 0.611) and sex (P = 0.878). Patients with alcoholic aetiology had the highest risk of decompensation compared to those with HCV (RR = 2.1, 95%CI: 1.3-3.2).
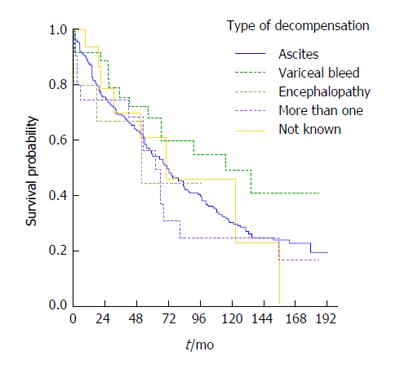
The most frequent type of decompensation was presentation of ascites (73%, 256 patients) while 6% (22 patients) had more than one complication on the same date, 15 having both ascites and encephalopathy. The latter group of patient had high mortality (64%, 14 patients out of 22, died during follow-up). Variceal bleeding was diagnosed in 37 patients. The leading aetiology in patients with variceal bleeding was ALD (51%, 19 patients), followed by NASH/crypto (27%, 10 patients) and then HCV (19%, 7 patients).
From the Kaplan-Meier curve it appears that patients who decompensated with variceal bleeding had the best overall survival, followed by those decompensating with ascites whilst the worst outcomes were evident in the group presenting with more than one complication (Figure 5). The corresponding log rank test indicated, however that the differences in survival were not statistically significant (P = 0.354).
HCC was diagnosed in 10 patients at the time of first presentation, whilst 78 patients developed HCC over the follow-up period. The mean time to the development of HCC in the entire cohort was 164 mo (95%CI: 156-172 mo). The incidence of HCC during the follow-up period was associated with cirrhosis aetiology (P = 0.003), even after adjusting for age and sex in a multivariable model (P = 0.027); the only pairwise statistically significant comparison was ALD compared to HBV, with ALD patients having an HCC risk of 0.3 times that of those with HBV aetiology (95%CI: 0.15-0.60, P = 0.001). In addition, female cirrhotics had an HCC risk 0.38 times that of men (95%CI: 0.20-0.71). Age was not statistically significant (P = 0.205). Cirrhosis aetiology was a borderline statistically significant predictor of survival after the diagnosis of HCC (P = 0.064) after adjusting for age (P = 0.494) and sex (P = 0.159).
In this homogenous cohort of patients with extended follow up from a single centre we studied the clinical course of cirrhosis and analyzed it according to the aetiology. The most common aetiology in this southern region of Greece was hepatitis C, in keeping with previous publications from the island and mainland[19,20]. In our cohort alcoholic was the second most common cause of cirrhosis and this cause has displayed an increasing trend over recent years.
Hepatitis C patients were, on average, older followed by the NASH/cryptogenic group, with HBV cirrhotics tending to be diagnosed at a younger age in this study. The older age in the HCV cohort could be expected as HCV infection is asymptomatic in the majority of patients with slow progression over decades, until cirrhosis is established[5]. Alcohol abuse in HCV infected patients is a well recognised negative prognostic factor and explains the younger average age of this group compared to the HCV group. Even in the younger HBV cirrhotics, however, alcohol abuse significantly lowered the age of cirrhosis diagnosis. In our study, age appeared to influence the survival after decompensation while comorbidities like cardiovascular diseases or diabetes had significant effect in those with NASH related cirrhosis.
NASH is an increasingly recognised cause of cirrhosis, delineating a significant percentage of cryptogenic cases[13]. Although the adult obesity epidemic has not been yet evident in Greece, most of the NASH cirrhotics were identified recently and many of these cases were linked to diabetes mellitus. Their relatively small number in our study is obviously due to the usually long period until cirrhosis develops. This picture is expected to change over the next decade. In our department in a survey of 2000 liver biopsies, NAFLD/NASH comprised 22.5% of the biopsies between the years 2003-2006, as compared to 5% between the years 1990-1995[21].
The majority of patients were diagnosed with compensated cirrhosis, but a considerable number decompensated during follow up. Patients who presented with compensated cirrhosis had a significantly better survival than those presenting with decompensated cirrhosis. A recent interesting study from the United Kingdom showed that survival of cirrhosis is significantly higher in patients diagnosed and followed in an ambulatory setting than those with first diagnosis in the occasion of a hospital admission[22]. In this study aetiology affected prognosis in young patients to a greater extent than in older ones.
In our cohort, those with HCV aetiology remained compensated for a longer period of time on average, with alcoholics having the highest risk for decompensation. These data are similar to those of other studies of Greek cirrhotic patients (as reported by Giannousis et al[20]) as well as to those from a cohort of 4537 cirrhotics from a general practice data base in the United Kingdom. In the later study, alcoholic aetiology had higher rate of decompensation compared to others during the first year after diagnosis; nevertheless this difference was not evident following the first year[23].
Ascites was the most common type of presentation in decompensated cirrhosis while patients with multiple presentations (i.e., combination of ascites, variceal bleeding, and encephalopathy) had the worst prognosis. In a study on acute-on-chronic liver failure (ACLF) by Moreau et al[24], ascites was a risk factor for development ACLF because it is an independent predictor of kidney failure following bacterial infections. Benvegnù et al[2] reported (using a large cohort of viral cirrhosis, mainly HCV related, cirrhosis patients) that the most frequent complication was HCC, followed by ascites which is also the experience published by Sangiovanni et al[25], in an elegant natural history study of 214 HCV patients. A recent paper[26] showed that the HCC incidence was significantly higher among HCV patients with varices compared to those without.
In the present study, alcoholics had significantly more episodes of variceal bleeding. Unexpectedly patients who decompensated with variceal bleeding displayed a better survival compared to other presentations of decompensation. This may be attributed to the large number of alcoholics in this group of decompensated patients, in whom abstinence may have effectively influenced the prognosis. Moreover the established approach in variceal bleeding which includes a combination of pharmacologic and early endoscopic therapy may also be responsible for improved survival displayed in these patients[27]. Primary and secondary prophylaxis might also account for the decreased incidence of variceal bleeding observed in the recent years as compared to episodes seen in the first years of the study.
Our cohort’s average survival was almost similar in compensated cirrhotics (10 years) and slightly better in decompensated (4.5 years) to the survival reported in the seminal natural history paper by D’Amico et al[28]. The somewhat better survival in our decompensated group could be due to our study being more recent (with documented improvements in the medical and endoscopic management of these patients), and also due to the development of alcohol services in our department and the course of HCV patients with the longest survival. Fattovich et al[3] in a previous classical study also reported a long survival in a cohort of 384 HCV cirrhotics[25,29]. It should be stressed that survival in HCV cirrhosis was better compared to HBV cirrhotics despite the fact that HCV cirrhotics received only symptomatic and supportive treatment while practically the vast majority HBV patients received antiviral treatment.
The lowest survival rates were found in the HBV group. This might be related to the increased incidence of HCC in this group and to the fact that more than 90% of our HBV patients had HBeAg negative chronic hepatitis. Indeed the incidence of cirrhosis and its subsequent complications are much more frequent in HBeAg negative than in HBeAg positive HBV infected patients, both in Europe and in Asia[7]. Moreover, we have included patients from the first era of the antiviral therapy when treatment for HBV aetiology was not as effective as treatments now available. This, together with the development of lamivudine resistance in a percentage of the HBV patients (data under preparation) as well as with the correlation with HCC all contributed to the uneven outcome of these patients. The poor outcomes for the combined aetiology, HBV plus alcohol group, is no surprise as alcohol can worsen the natural course of viral hepatitis at any time[4,7].
Alcoholic cirrhotics despite their higher decompensation risk had a relatively high overall survival rates and this can again be explained by the fact that a proportion of these patients successfully discontinued or reduced their alcohol intake. Thirty percent of the whole cohort of patients with alcoholic aetiology became abstinent, mostly by attending the alcohol services at the hospital. Similar to our findings, the study by Toshikuni et al[30] reported that survival of HCV cirrhotics was similar to survival of alcoholic cirrhotics, with the same risk for decompensation and mortality. A study in Danish patients[31] showed that alcoholic cirrhotics had high prevalence of complications at the time of diagnosis and these were predictors of 1-year mortality. In this series ascites was also the most frequent type of decompensation, while there was also high risk of variceal bleeding or encephalopathy. As in our series, more than one complication was associated with worse prognosis.
HCC development was observed mostly in HCV and HBV cirrhosis, and NASH had the smallest incidence. The risk was highest in HBV cirrhosis and lowest in those with alcoholic aetiology. Similarly, Fattovich et al[11] reported that in the absence of HBV or HCV infection, HCC incidence is lower in alcoholic cirrhotics and these data were confirmed by a retrospective study from Japan. However recent data confirm that heavy alcohol consumption significantly increases the risk of HCC in HBV-related cirrhotics[32].
Survival after HCC development was marginally related to the aetiology in our group of patients in keeping with the data by Trevisani et al[12]. However, the development of HCC was a catastrophic event in the natural course of the disease[2,25]. The poor survival of the HCC group was also influenced by the fact that many of these patients were referred from district hospitals after the diagnosis of large tumours, not amenable to radical treatments (resection or transplantation). This, together with a heterogeneous approach to HCC screening amongst the referring hospitals obviously affected both the actual incidence and the outcome. Treatment of these patients has been reported in the randomized trial with sc octreotide[33] or im long acting somatostatin analogues[34]. The remaining few patients underwent chemoembolization and have been reported elsewhere (Samonakis et al[34] submitted). Only 3 patients were transplanted due to the recent development of transplant services in the country, where even today there is only one liver transplant centre with a rather limited activity.
The causes of death in this cohort of cirrhotic patients were mostly related to complications of liver disease and/or HCC rather than the presence of comorbidities. This in keeping with most published experience in natural history studies[35]. An exception to this was the NASH-cryptogenic group where death from cardiovascular complications was frequent (data not shown). It is increasingly recognised that cardiovascular diseases may seriously contribute to the mortality of cirrhosis, contrary to previously thoughts that liver cirrhosis is protective for coronary artery disease[36].
This study has several limitations. Due to the original design it has a retrospective and a prospective arm. Moreover some patients were lost to follow up after a successful management of an acute episode, so data on survival or cause of death are missing for this population. We could not provide an analysis in relation to the model end-stage liver disease (MELD) score as it was introduced after 2002. A recent publication[37] showed that aetiology of cirrhosis has an impact on 1-year survival predicted by the MELD score. The study findings are further limited by a long accrual period. Standard survival analysis methods, such as those applied in the present study, are valid under the assumption that the probabilities of death are stable with respect to absolute time.
In conclusion, in this cohort of patients with a long follow up we found that cirrhosis aetiology and decompensation were predictors of survival at presentation. Alcoholics had the highest risk of decompensation and HBV cirrhotics were at the highest risk of developing HCC. On average HCV cirrhotics had the highest decompensation-free time. The improvement in the management of cirrhosis complications, recent advances in the treatment of viral hepatitis and the development of specialized services for alcoholic liver disease could affect the development of complications and ultimately patient survival.
We are grateful to Dr. Moschandreas for her advice regarding data analysis and for English language corrections. We are also grateful to Dr. Economaki, Dr. Leontidis, Dr. Chatzicostas for their contribution in data selection and information for patient update.
Cirrhosis and its complications represent the end in the spectrum of chronic liver diseases, irrespective of aetiology. Its natural history is typically characterised by an asymptomatic phase termed compensated cirrhosis, followed by the development of complications from portal hypertension and/or liver dysfunction, termed decompensated cirrhosis. Cirrhotics have diverse presentation and prognosis according to stage, aetiology and geographic region.
The research objective was to study disease progression in the authors’ cohort of compensated and decompensated cirrhotics; to identify the time to and mode of decompensation, to assess the occurrence of hepatocellular carcinoma and the ultimate impact of cirrhosis on patient survival.
Previous studies from various parts of the world have presented local experience. This is the largest study in their country, both with respect to the study population and the follow up period. It reflects various critical issues on the epidemiology, natural history and survival of this large cohort of cirrhotics.
The study provides insight on the natural course of common causes of liver cirrhosis, denotes the increasing problem of alcoholic liver disease, whereas provides useful information on the importance of aetiology in prognosis.
Cirrhosis arises as a result of different mechanisms of liver injury and represents a common denominator to various aetiologies; it represents an increasing cause of liver morbidity and mortality. Chronic infection with hepatitis B and C virus, represent the commonest cause of cirrhosis. Hepatocellular carcinoma is a primary neoplasm that frequently develops on a cirrhotic liver.
The article of Samonakis et al entitled “Natural history study of compensated and decompensated cirrhosis: a long term single centre study” is a retrospective study aimed to reporting the characteristics and evolution of a wide cohort of cirrhotic patients in Greece. The article is of interest in clinical practice, the methodology is appropriate and the size of the population is big enough to support the conclusions that authors have drawn in this comprehensive work.
P- Reviewer: Castiella A, Koch TR, Romero MR, Squadrito G S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 392] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 2. | Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 957] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 4. | Massard J, Ratziu V, Thabut D, Moussalli J, Lebray P, Benhamou Y, Poynard T. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 2006;44:S19-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 7. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 967] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 8. | Manno M, Cammà C, Schepis F, Bassi F, Gelmini R, Giannini F, Miselli F, Grottola A, Ferretti I, Vecchi C. Natural history of chronic HBV carriers in northern Italy: morbidity and mortality after 30 years. Gastroenterology. 2004;127:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Manesis EK, Papatheodoridis GV, Touloumi G, Karafoulidou A, Ketikoglou J, Kitis GE, Antoniou A, Kanatakis S, Koutsounas SJ, Vafiadis I. Natural course of treated and untreated chronic HCV infection: results of the nationwide Hepnet.Greece cohort study. Aliment Pharmacol Ther. 2009;29:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1793] [Article Influence: 85.4] [Reference Citation Analysis (2)] |
| 12. | Trevisani F, Magini G, Santi V, Morselli-Labate AM, Cantarini MC, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Farinati F. Impact of etiology of cirrhosis on the survival of patients diagnosed with hepatocellular carcinoma during surveillance. Am J Gastroenterol. 2007;102:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S11-S16. [PubMed] |
| 14. | Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004;19:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Koulentaki M, Moscandrea J, Dimoulios P, Chatzicostas C, Kouroumalis EA. Survival of anti-mitochondrial antibody-positive and -negative primary biliary cirrhosis patients on ursodeoxycholic acid treatment. Dig Dis Sci. 2004;49:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421-430. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3243] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 18. | Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 2147] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 19. | Kouroumalis EA, Skordilis PG, Moschandrea J, Alexandrakis G, Charoulakis N, Tzardi M, Manousos ON. Natural history of advanced hepatocellular carcinoma in Crete. Association with hepatitis C virus. Eur J Gastroenterol Hepatol. 1997;9:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Giannousis IP, Papatheodoridis GV, Deutsch MJ, Manolakopoulos SG, Manesis EK, Koskinas JS, Archimandritis AJ. The burden and recent epidemiological changes of the main chronic liver diseases in a Greek referral tertiary centre. Eur J Gastroenterol Hepatol. 2010;22:172-179. [PubMed] |
| 21. | Avgerinos A, Koulentaki M, Tzardi M, Samonakis D, Matrella E, Kouroumalis EA. Increased incidence of steatohepatitis during the last sixteen years in Crete. EASL special conference on NASH/NAFLD. 2009;. |
| 22. | Ratib S, Fleming KM, Crooks CJ, Aithal GP, West J. 1 and 5 year survival estimates for people with cirrhosis of the liver in England, 1998-2009: a large population study. J Hepatol. 2014;60:282-289. [PubMed] |
| 23. | Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2169] [Article Influence: 180.8] [Reference Citation Analysis (5)] |
| 25. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 26. | Gomez EV, Rodriguez YS, Bertot LC, Gonzalez AT, Perez YM, Soler EA, Garcia AY, Blanco LP. The natural history of compensated HCV-related cirrhosis: a prospective long-term study. J Hepatol. 2013;58:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Samonakis DN, Triantos CK, Thalheimer U, Patch DW, Burroughs AK. Management of portal hypertension. Postgrad Med J. 2004;80:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2133] [Article Influence: 112.3] [Reference Citation Analysis (3)] |
| 29. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 30. | Toshikuni N, Izumi A, Nishino K, Inada N, Sakanoue R, Yamato R, Suehiro M, Kawanaka M, Yamada G. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol. 2009;24:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675-1682. [PubMed] |
| 32. | Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, Lo GH, Chen YS, Yen YC, Hu JT. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol. 2013;58:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Kouroumalis E, Skordilis P, Thermos K, Vasilaki A, Moschandrea J, Manousos ON. Treatment of hepatocellular carcinoma with octreotide: a randomised controlled study. Gut. 1998;42:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Samonakis DN, Moschandreas J, Arnaoutis T, Skordilis P, Leontidis C, Vafiades I, Kouroumalis E. Treatment of hepatocellular carcinoma with long acting somatostatin analogues. Oncol Rep. 2002;9:903-907. [PubMed] |
| 35. | Asrani SK, Kamath PS. Natural history of cirrhosis. Curr Gastroenterol Rep. 2013;15:308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Kalaitzakis E, Rosengren A, Skommevik T, Björnsson E. Coronary artery disease in patients with liver cirrhosis. Dig Dis Sci. 2010;55:467-475. [PubMed] |
| 37. | Angermayr B, Luca A, König F, Bertolini G, Ploner M, Gridelli B, Ulbrich G, Reiberger T, Bosch J, Peck-Radosavljevic M. Aetiology of cirrhosis of the liver has an impact on survival predicted by the Model of End-stage Liver Disease score. Eur J Clin Invest. 2009;39:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |