Published online Jun 27, 2014. doi: 10.4254/wjh.v6.i6.443
Revised: April 18, 2014
Accepted: May 16, 2014
Published online: June 27, 2014
Processing time: 142 Days and 19.3 Hours
AIM: To determine if there is a reasonable prospect of success of a re-use liver transplantation.
METHODS: We systematically searched for reports of liver graft re-use using electronic searches of PubMed and Web of Knowledge. We performed hand searches of references lists of articles reporting re-use of grafts.
RESULTS: A systematic review of the literature reveals 28 liver transplantations using previously transplanted grafts. First and second recipients ranged in age from 4 to 72 years and 29 to 62 years respectively. Liver disease in the first recipient was varied including 5 (18%) patients with fulminant liver failure who died subsequently of cerebral edema. The second transplantation was performed after a median interval of 5 d (one day-13 years). Viral hepatitis was present in 3 (11%) of the initial recipients and in 8 (29%) of final recipients. Hepatocellular carcinoma was present in 6 (21%) of the final recipients. Early survival after the final transplantation was 93%, whereas long-term survival was 78% with a mean follow-up of 23.3 (3-120) mo.
CONCLUSION: Outcomes of transplantation using previously transplanted grafts in this select population are similar to those seen with conventional grafts.
Core tip: Reuse of a previously transplanted liver graft may be considered if the first recipient suffers neurological death at some time after liver transplantation.
- Citation: Tanaka H, McAlister VC, Levstik MA, Ghent CN, Marotta PJ, Quan D, Wall WJ. Reuse of liver grafts following the brain death of the initial recipient. World J Hepatol 2014; 6(6): 443-447
- URL: https://www.wjgnet.com/1948-5182/full/v6/i6/443.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i6.443
The growing disparity between the demand for and supply of organs for transplantation has restricted the availability of grafts for patients whose indications for transplantation fall outside of conventional guidelines and it has led to new strategies to increase donor utility. On rare occasions, a donor situation is such that it is not acceptable for routine transplantation but a novel rationale is present for an expectation of success so that the graft may be offered to candidates who would otherwise be excluded from transplantation. We encountered this situation for a patient with hepatitis C virus (HCV) and hepatocellular carcinoma (HCC) that had advanced beyond criteria for transplantation when another liver recipient unexpectedly suffered neurological death from intra-cerebellar bleeding, 13 d after transplantation. Organ donation for allocation to patients on the conventional liver transplantation waiting list had been declined by the organ procurement organization. To determine if there was a reasonable prospect of success of a re-use transplantation, we undertook a systematic survey of the literature.
We systematically searched for reports of liver graft re-use using electronic searches of PubMed (1966 to January 2013) and Web of Knowledge (1981 to January 2013). The following key words were used: “liver transplantation”; “reuse”; “graft” or “liver graft”. The search was limited to the English literatures and humans. We performed hand searches of references lists of articles reporting re-use of grafts. We collected the data to determine the age range of each donor and recipient, their liver disease and cause of death (if applicable), the interval between the initial and final liver transplantation and the outcome of the final transplantation.
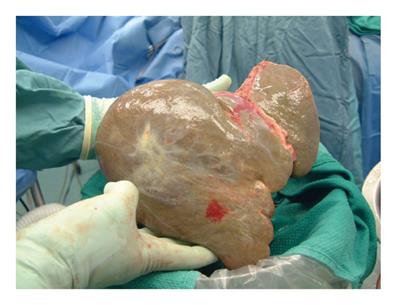
Systematic review of the literature revealed 14 papers describing 27 liver recipients of previously transplanted grafts with an early survival rate of over 90% for both the patients and the re-used grafts[1-14]. No review of this aspect of liver transplantation was located. We proceeded with the re-use liver transplantation in London, ON. The initial recipient was a 55-year-old man with end-stage liver disease secondary to hepatitis C. His blood type was O and hepatitis B core antibody was positive. The initial donor for this recipient was a 69-year-old man with blood type O, who developed brain death from intracranial hemorrhage. Unfortunately, he suffered a huge intracerebellar bleed on the 4th day after transplantation and was declared brain dead on day 13. The second recipient was 54-year-old man with hepatitis C cirrhosis and a history of ruptured HCC two years earlier. He had been put on capecitabine 1000 mg/m2 and multiple liver lesions were embolized by angiogram 7 mo earlier. Even though his HCC appeared to be stable and there was no evidence of extrahepatic disease, long-term survival without liver replacement was considered unlikely. The opportunity was discussed with the patient and his family including its known risks and uncertainties. The blood type of the second recipient was B and hepatitis B serology was negative. At retrieval surgery 14 d after the initial transplant, the liver graft was found to be larger than before with a stiff texture (Figure 1). The liver graft was perfused with Histidine Tryptophan Ketogluterate (HTK) solution via the portal vein. Arterial perfusion was done on the back-table confirming good flow of the perfusate. In the final recipient wide resection of tissue surrounding the liver was performed including areas of diaphragm, peritoneum, omentum, extrahepatic nodes and lymphatic tissue. Occlusive thrombus was removed from the native portal vein. Cold ischemic and warm ischemic times were 9 h and 1.5 h, respectively. His postoperative course was straightforward except for temporary renal impairment. His transaminases went up more than 4000 IU/L, but graft function improved significantly thereafter. His induction immunosuppressive therapy was basiliximab and steroid, and he was maintained on sirolimus and steroid thereafter. Prophylaxis for hepatitis B started according to our protocol. He was put on capecitabine again on day 4. He was discharged 15 d after transplantation. Evidence of recurrent hepatitis C virus was diagnosed 8 mo later. Although the graft continued to function well, he expired 16 mo after transplantation due to recurrence of HCC.
Data from the 28 reuse transplantations are given in Table 1. Initial donors and recipients ranged in age from 4 to 72 years and 29 to 62 years respectively. Liver disease in the first recipient was varied with the notable exception of higher than expected incidence of fulminant liver failure in 5 (18%) patients. These patients became donors when brain death from cerebral edema was diagnosed after liver transplantation. The commonest cause of death of the initial recipient was cerebrovascular accident 4 d (median, one day-13 years) after transplantation. Brain anoxia was the cause of death in one patient but is not recorded in the remaining patients. The second transplantation was performed 5 d (median, one day-13 years) after the initial transplantation. Viral hepatitis was present in 3 (11%) of the initial recipients and in 8 (29%) of final recipients. HCC was present in 6 (21%) of the final recipients. One reused graft failed to function and a second graft failed from hepatic artery thrombosis giving an initial patient and graft survival of 93%. Long-term survival is 78% with a mean follow-up of 23.3 (3-120) mo.
| Location | Donor | Interval (d) initial | Recipient | Outcome | ||||
| (ref. NO.) | Age (yr) | Liver disease | Cause of death | to final transplant | Age (yr) | Liver disease | Early after second transplantation | Long-term |
| London, Canada (current report) | 55 | HCV | CVA | 14 | 54 | HCV/HCC | No complications | Died of recurrent HCC at 16 mo |
| Madrid, Spain[1,2] | 57 | PBC | CVA | 1 | 29 | CR post LTx for PSC and CCC | No complication | Died of recurrent CCC at 48 mo |
| Madrid, Spain[2] | 54 | PSC | CVA | 2 | 32 | CR post LTx for HCV | Sepsis | Died at 4 mo |
| Madrid, Spain[2] | 51 | CR post LTx (cause N/A) | CVA | 2 | 56 | HCV/HCC | AR | Alive at 25 mo |
| Creteil, France[3] | 24 | CR post LTx for cryptogenic cirrhosis | CVA | 5 | 52 | Alcoholic | AR | Alive at 6 mo |
| Essen, Germany[4] | N/A | Cryptogenic cirrhosis | CVA | 1 | 46 | Recurrent HBV post LTx | AR | Alive at 5 mo |
| Barcelona, Spain[5] | 55 | Alcoholic | CVA | 5 | 58 | HCV | No complication | Alive at 14 mo |
| Brussels, Belgium[6] | 47 | ALF (acetaminophen) | Cerebral edema | 2 | 53 | HCV/HCC | AR | Alive at 22 mo |
| Lille Cedex, France[7] | 21 | ALF (acetaminophen) | Cerebral edema | 2 | 61 | HCV | No complication | Alive at 11 mo |
| UNOS #1[8] | 6 | N/A | N/A | 1 | N/A | N/A | N/A | Alive at 111 mo |
| UNOS #2[8] | 60 | Cryptogenic cirrhosis | CVA | 8 | 44 | N/A | N/A | Alive at 62 mo |
| UNOS #3[8] | 21 | N/A | N/A | 1 | N/A | N/A | N/A | Alive at 3.5 mo |
| UNOS #4[8] | 49 | N/A | N/A | 4.9 yr | N/A | N/A | failed at 0.1 mo (cause N/A) | - |
| UNOS #5[8] | 48 | N/A | N/A | 5 | N/A | N/A | N/A | Failed at 11 mo |
| UNOS #6[8] | 56 | HCV | Anoxic brain injury | 2 | N/A | HCV, alcoholic | No complication | Alive at 25.4 mo |
| UNOS #7[8] | 49 | N/A | N/A | 6 | N/A | N/A | N/A | Alive at 4.8 mo |
| UNOS #8[8] | 35 | ALF (acetaminophen) | Cerebral edema | 3 | 56 | PSC | No complication | Alive at 12 mo |
| UNOS #9[8] | 46 | N/A | N/A | 2 | N/A | N/A | N/A | Alive at 11 mo |
| UNOS #10[8] | 25 | N/A | N/A | 2.8 yr | N/A | N/A | N/A | Alive at 5.9 mo |
| UNOS #11[8] | 44 | N/A | N/A | 17 | N/A | N/A | N/A | Alive at 3.0 mo |
| Barcelona, Spain[9] | 55 | Alcoholic | CVA | 5 | 58 | HCV | No complication | Alive at 120 mo |
| Barcelona, Spain[9] | 58 | Alcoholic | CVA | 14 | 55 | Budd Chiari synd | No complication | Alive at 13 mo |
| Barcelona, Spain[9] | 58 | Alcoholic | CVA | 10 | 47 | Ischemic cholangitis | AR | Alive at 7 mo |
| Creteil, France[10] | 72 | Alcoholic | CVA | 13 yr | 61 | Cryptogenic cirrhosis, | No complication | Alive at 12 mo |
| Montreal, Canada[11] | 26 | ALF (acetaminophen overdose) | Cerebral edema | 2 | 62 | Hemochromatosis HCC | No complication | Alive at 30 mo |
| Berlin, Germany[12] | 53 | Cryptogenic cirrhosis | Cerebral edema | 24 | 43 | Alcoholic, HCC | Biliary obstruction by stones | Alive at 6 mo |
| Stuttgart, Germany[13] | 38 | Budd Chiari synd | CVA | 5 yr | 51 | Polycystic liver disease | No complication | Alive at 18 mo |
| Malatya, Turkey[14] | 4 | ALF (hepatitis A) | Cerebral edema | 5 | 31 | Cryptogenic cirrhosis, HCC | HAT at one month, died at 1.3 mo | - |
The outcomes described in this report of liver transplantation using previously transplanted grafts is comparable to transplantation from conventional donors. There may be a publication bias where poor outcomes have been excluded from reportage. The inclusion of patients from mandatory databases and the large number of centers reporting from several jurisdictions may mitigate this risk of publication bias.
There are several causes that lead to severe brain damage in liver transplant recipients[15,16], and some of those circumstances make re-use of liver grafts possible. In the series described here, cerebrovascular accident and cerebral edema are the commonest causes of death of the donor of the previously transplanted graft. Brain death from cerebral edema is a particular concern in candidates with fulminant liver failure as recovery from coma may unpredictably occur after a considerable interval from successful liver replacement. Knowledge that these grafts may be available for re-use should a recovery not occur, may permit the teams to give candidates with fulminant failure the benefit of the doubt.
Moreno González et al[2] considered several factors to be important for successful reuse of liver grafts: all reused grafts should be obtained from young and stable initial donors, excellent graft function in the first recipient, early reuse (within 48 h), short preservation times, biopsy showing minimal preservation injury, negative donor-recipient crossmatch, ABO compatibility, absence of viral, bacterial, and fungal infection. While it is wise to be prudent, the current report suggests that criteria for donation after transplantation may be similar to conventional donation after neurological death. The age of donor here ranged from 4 to 72 years. The interval between transplantations was up to several years. Biopsy before re-use was not routinely reported but should be considered. All of the teams reported efforts to shorten cold and warm ischemic times. Extension of criteria to include donation after cardiac death has not been reported.
There is limited experience of re-use of HCV infected grafts with only two reports in this series. Both of the final recipients experienced recurrence of HCV. One died from recurrent HCC at 16 mo (our case) but the other is well at 25.4 mo after transplantation[8]. Biopsy of HCV infected grafts should be performed before re-use using the same protocols as for initial transplantation.
Clinical indications for the re-use of the liver grafts is varied in the current series but the incidence of HCC, chronic rejection and recurrent hepatitis suggest that candidates may have been offered this unconventional form of transplantation because access to the conventional list was limited. There has been no established guideline so far for the recipients’ indication of reuse liver transplantation. A marginal recipient whose general condition is deteriorating or whose stage of malignancy is almost beyond the criteria for liver transplant and suitable donor is not available may take advantage of the reuse liver transplant. If so, the results presented here confirm that the courage shown by the patients was properly rewarded. Even though the results in this select group of transplantations are good, the world wide experience is so limited that we do not advocate for previously transplanted grafts to be included in the conventional donor pool. This report will hopefully guide medical teams faced with unusual circumstances where a liver recipient unexpectedly dies after transplantation in a manner that permits organ donation.
Nowadays transplant programs are increasingly accepting marginal donors such as old donors, donors with fatty liver, or other conditions such that delayed graft function or poor outcome might be anticipated after the transplant compared to the transplants from non-marginal donors. The local Ethical committee should be ideally called before accepting the reuse liver, and this paper will help the committee understand the feasibility of the rare form of transplants.
The growing disparity between the demand for and supply of organs for transplantation has restricted the availability of grafts for patients whose indications for transplantation fall outside of conventional guidelines and it has led to new strategies to increase donor utility.
Reuse of a previously transplanted liver graft may be considered if the first recipient suffers neurological death at some time after liver transplantation.
This report will hopefully guide medical teams faced with unusual circumstances where a liver recipient unexpectedly dies after transplantation in a manner that permits organ donation.
This is a very novel article focused on the Reuse of liver grafts following the brain death of the initial recipient. Subject to certain restrictions, there maybe some bias. However, liver transplantation secondary use, which provide a new method to solve the liver source,and it deserves further study.
P- Reviewers: Fabris L, Zheng X S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Moreno EG, García GI, González-Pinto I, Gómez SR, Loinaz SC. Successful reuse of a liver graft. Br J Surg. 1991;78:813-814. [PubMed] |
| 2. | Moreno González E, Gómez R, Gonzalez Pinto I, Loinaz C, Garcia I, Maffettone V, Corral M, Marcello M, Gonzalez A, Jimenez C. Reuse of liver grafts after early death of the first recipient. World J Surg. 1996;20:309-312; discussion 312-313. [PubMed] |
| 3. | Tantawi B, Cherqui D, Duvoux C, Dhumeaux D, Fagniez PL. Reuse of a liver graft five days after initial transplantation. Transplantation. 1996;62:868-869. [PubMed] |
| 4. | Friedrich J, Malago M, Lange R, Kemnitz J, Danninger F, Erhard J. Successful regrafting of a transplanted liver. Transpl Int. 1997;10:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Figueras J, Pares D, González C, Ramos E, Rafecas A, Fabregat J, Torras J, Jaurrieta E. Reuse of a transplanted liver. Transpl Int. 1997;10:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Rubay R, Wittebolle X, Ciccarelli O, Roggen F, Talpe S, Laterre PF, Reding R, Lerut J. Re-use of a liver allograft; an exceptional opportunity to enlarge the organ donor pool. Transpl Int. 2003;16:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Pruvot FR, Roumilhac D, Dharancy S, Lambotte P, Auboiron A, Gambiez L, Jegaden O, Declerck N. Re-use of a liver graft and multi-organ procurement from a liver transplant patient. Transpl Int. 2004;17:49-53. [PubMed] |
| 8. | Ortiz J, Reich DJ, Manzarbeitia C, Humar A. Successful re-use of liver allografts: three case reports and a review of the UNOS database. Am J Transplant. 2005;5:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Castellote J, Lladó L, Xiol X, Julià D, Ballester R, Ramos E, Fabregat J, Rafecas A. Successful reuse of liver grafts after death of the first recipient. Clin Transplant. 2006;20:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Tayar C, Karoui M, Laurent A, Hadjhamida MB, Nhieu JT, Duvoux C, Cherqui D. Successful reuse of liver graft 13 years after initial transplantation. Transplantation. 2006;82:1547-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Nafidi O, Letourneau R, Willems BE, Lapointe RW. Reuse of liver graft from a brain dead recipient. Clin Transplant. 2007;21:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Olschewski P, Fikatas P, Pratschke J, Neumann U, Neuhaus P, Puhl G. Endoscopic management of biliary obstruction after successful reuse of a liver graft. Ann Transplant. 2009;14:51-54. [PubMed] |
| 13. | Rentsch M, Meyer J, Andrassy J, Fischer-Fröhlich CL, Rust C, Mueller S, Angele M, Löhe F, Jauch KW, Graeb C. Late reuse of liver allografts from brain-dead graft recipients: the Munich experience and a review of the literature. Liver Transpl. 2010;16:701-704. [PubMed] |
| 14. | Karabulut K, Eris C, Piskin T, Kayaalp C, Yilmaz S. Reuse of a pediatric liver graft: a case report. Case Rep Transplant. 2012;2012:350817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Moreno E, Gómez SR, Gonzalez I, Loinaz C, Garcia I, Perez A, Palomo C, Alvarado A, Maffettone V, Perez-Cerda F. Neurologic complications in liver transplantation. Acta Neurol Scand. 1993;87:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Menegaux F, Keeffe EB, Andrews BT, Egawa H, Monge H, Concepcion W, So SK, Esquivel CO. Neurological complications of liver transplantation in adult versus pediatric patients. Transplantation. 1994;58:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |