Published online Jun 27, 2014. doi: 10.4254/wjh.v6.i6.394
Revised: March 8, 2014
Accepted: May 14, 2014
Published online: June 27, 2014
Processing time: 167 Days and 18.5 Hours
Systemic lupus erythematosus (SLE) encompass a broad spectrum of liver diseases. We propose here to classify them as follows: (1) immunological comorbilities (overlap syndromes); (2) non-immunological comorbilities associated to SLE; and (3) a putative liver damage induced by SLE itself, referred to as “lupus hepatitis”. In the first group, liver injury can be ascribed to overlapping hepatopathies triggered by autoimmune mechanisms other than SLE occurring with higher incidence in the context of lupus (e.g., autoimmune hepatitis, primary biliary cirrhosis). The second group includes non-autoimmune liver diseases, such as esteatosis, hepatitis C, hypercoagulation state-related liver lesions, hyperplasic parenchymal and vascular lesions, porphyria cutanea tarda, and drug-induced hepatotoxicity. Finally, the data in the literature to support the existence of a hepatic disease produced by SLE itself, or the occurrence of a SLE-associated prone condition that increases susceptibility to acquire other liver diseases, is critically discussed. The pathological mechanisms underlying each of these liver disorders are also reviewed. Despite the high heterogeneity in the literature regarding the prevalence of SLE-associated liver diseases and, in most cases, lack of histopathological evidence or clinical studies large enough to support their existence, it is becoming increasingly apparent that liver is an important target of SLE. Consequently, biochemical liver tests should be routinely carried out in SLE patients to discard liver disorders, particularly in those patients chronically exposed to potentially hepatotoxic drugs. Diagnosing liver disease in SLE patients is always challenging, and the systematization of the current information carried out in this review is expected to be of help both to attain a better understanding of pathogenesis and to build an appropriate work-up for diagnosis.
Core tip: The existence of liver disease associated with lupus itself, or increased susceptibility to concomitant liver diseases, either autoimmune or non-autoimmune ones, is still somewhat controversial, and difficult to diagnose. Data in the literature are scarce, and often based on case reports or clinical studies with limited patient size or histological evidence. The pros and cons to support the existence of such pathological entities, and the still preliminary studies on the mechanisms involved, are critically discussed here. We concluded that liver is often a target of systemic lupus erythematosus, and biochemical liver tests should be systematically carried out in these patients.
- Citation: Bessone F, Poles N, Roma MG. Challenge of liver disease in systemic lupus erythematosus: Clues for diagnosis and hints for pathogenesis. World J Hepatol 2014; 6(6): 394-409
- URL: https://www.wjgnet.com/1948-5182/full/v6/i6/394.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i6.394
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with variable clinical presentation, usually characterized by several immunological signs and symptoms[1-3]. It primarily affects women under 50 years of age, and is diagnosed on the basis of presence of at least 4 out of 11 criteria identified by the American College of Rheumatology (ACR), either sequentially or simultaneously, namely malar rash, discoid rash, photosensitivity, oral ulcers, nonerosive arthritis, pleuritis or pericarditis, renal disorders (proteinuria or cellular casts), neurologic disorder (seizures or psychosis), hematologic disorder (hemolytic anemia, leukopenia or thrombocytopenia) and immunologic disorders (anti-DNA, anti-Sm or antiphospholipid antibodies)[4-6].
The most common symptoms are fever, weight loss, and a general lack of wellbeing and athralgia, while the most frequent signs are skin rashes. Biochemical exams typically present anemia, and increased rates of erythrosedimentation. Treatment includes nonsteroidal anti-inflammatory drugs (NSAIDs), corticoids, and immunomodulators. Death is generally caused by progressive renal insufficiency, severe impairment of the central nervous system, or multi-organic failure after systemic infection[4].
Even though, as above mentioned, alterations of skin, joints and kidney, as well as of the cardiovascular, hematological and central nervous systems, are part of the criteria indicating morbidity, the liver can also be affected[1-5]. Although a true liver disease triggered by SLE itself is a controversial issue, 25% to 50% of patients may present alterations in the liver function tests (LFTs)[7]. The for and against data in the literature to support the existence of the multiple associations of SLE with liver disease will be discussed in detail in this review. Our literature inclusion criteria limited the citation of clinical cohort studies to those written in English language and published in peer reviewed journals; only very exceptional studies in other languages were included, when dealing with topics with extremely scarce information. The quotation studies in abstract form, when equivalent full papers were unavailable, was also very exceptional, and limited to peer reviewed, highly prestigious meetings.
Subclinical liver disease is common in SLE, and 25%-50% of patients with lupus may develop abnormal liver function at some point[8,9]. The more common laboratory abnormalities associated with the different kinds of liver disease related to lupus are summarized in Table 1. In addition, an overview of the main biochemical and histological findings reported in the literature is depicted in Table 2.
| Ref. | Study type | Patientswith SLE | NO. of patients with biochemicalalterations and alteration types | Liver histological findings |
| Mackay et al[11] | Retrospective | 19 | (n = 19) ↑ AST, ALT | Minimal changes, portal fibrosis, steatosis, inflammation (n = 11) Normal (n = 6) Chronic hepatitis (n = 2) |
| Chwalińska-Sadowska et al[12] | Retrospective | 18 | NA | Minimal changes (n = 13) Normal (n =5) |
| Runyon et al[13] | Retrospective | 238 | (n = 124) ↑ AST, ALT, total bilirubin, ALP, GGT, LDH (≥ 2 × ULN) | (n = 33) Steatosis (n = 12) Others: cirrhosis, chronic hepatitis, granulomatosis, chronic hepatitis, steatosis, cholestasis, centrilobular necrosis |
| Gibson et al[14] | Retrospective | 81 | (n = 64) ↑ AST, ALT, ALP | (n = 7) Portal inflammation (n = 5) Steatosis (n = 1) Chronic hepatitis (n = 1) |
| Miller et al[15] | Prospective | 260 | (n = 84) ↑ AST, ALT, ALP | Minimal changes (n = 14) |
| Matsumoto et al[17] | Retrospective | 73 | NA | Hepatic arteritis (n = 11) Steatosis (n = 53) RNH (n = 5) Viral hepatitis (n = 2) SLE-PBC overlap syndrome (n = 1) SLE-AIH overlap syndrome (n = 1) |
| Luangjaru et al[9] | Retrospective | 225 | (n = 80) ↑ AST, ALT ( ≤ 4 × ULN) | NA |
| Chowdhary et al[7] | Retrospective | 192 | (n = 40) ↑ AST, ALT | HCV (n = 3) Steatosis (n =5) SLE-AIH overlap syndrome (n = 4) SLE-PBC overlap syndrome (n = 3) Cryptogenic cirrhosis (n = 1) |
| Piga et al[3] | Retrospective | 242 | (n = 59) ↑ AST, ALT (≥ 2 × ULN) | NA |
| Her et al[138] | Retrospective | 141 | (n = 46) ↑ Total bilirubin, AST, ALT, LDH, ALP (≥ 2 × ULN) | NA |
| Huang et al[90] | Retrospective | 1533 | (n = 134) ↑ AST, ALT (≥ 2 × ULN during 2 yr) | Chronic Hepatitis (n = 6) Minimal changes (n = 4) Normal (n = 3) |
| Zheng et al[2] | Retrospective | 504 | (n = 47) ↑ Total bilirubin (13%), ALT (98%), ALP (42%), GGT (49%) | (n = 10) Portal blood cell infiltration (n = 8) Hydropic degeneration (n = 8) Steatosis (n = 2) Mild cholestasis (n = 2) Focal necrosis (n= 1) Nodular cirrhosis (n = 1) |
| Takahashi et al[18] | Prospective | 206 | (n = 123) ↑ AST, ALT (99%) ↑ ALP and GGT (81%) | (n = 25) Lupus hepatitis (n = 16): Unspecific reactive hepatitis (88%) Active hepatitis (12%) SLE-AIH overlap syndrome (n = 6): Interface hepatitis (100%) Cirrhosis (33%) SLE-PBC overlap syndrome (n = 3) |
| Hepatic alteration | Laboratory abnormalities |
| Hepatic steatosis | GGT, ALT/AST |
| Viral hepatitis | ALT, AST, HCV, cryoglobulinemia |
| Toxic hepatitis | ALP, GGT, AST/ALT, bilirubin |
| Nodular regenerative hyperplasia | ALT, AST, thrombocytopenia |
| Primary biliary cirrhosis | ALP, GGT, AMA |
| Autoimmune hepatitis | ANA, ASMA, gammaglobulin |
| Hepatic venous thrombosis | Antiphospolipidic antibodies |
| Lupus hepatitis | Anti-ribosomal P autoantibodies |
Hepatomegalia is detected in 12%-55% of SLE patients, depending on the analyzed series[10]. In an original article by Mackay et al[11], the authors observed hepatomegalia and/or alterations in LFTs in 19 SLE patients, normal liver biopsies in 6 cases, and minimal histological changes in another 11 ones (fatty liver, portal fibrosis, and mild to moderate portal infiltrate). Histological changes compatible with chronic hepatitis with progression to cirrhosis were confirmed in the remaining 2 patients. Similar findings were obtained by Polish researchers in a study of 18 SLE patients; whereas 5 of them showed normal liver histologies, the other 13 ones showed only minimal hepatocellular changes[12]. These results do not agree with those observed by Runyon et al[13] who, in a retrospective review of 238 patients with SLE, observed hepatomegalia in 39% of patients, splenomegaly in 6% and jaundice in 24%. Twenty one percent of patients were defined as carriers of liver disease based on abnormal liver histologies or, in some cases, elevation of liver enzymes 2 times over the upper limit of normal (ULN).
In the same study, liver histology of 33 patients showed steatosis (36%), cirrhosis and chronic active hepatitis (12%), hepatic granulomatosis, centrilobular necrosis (9%), and chronic hepatitis and microabscesses (6%). These findings were very challenging for the common view at the beginning of 80 s, and prompted other researchers to replicate these results. However, only one year after this report, Gibson et al[14] failed to reproduce such a high rate of severe liver disease associated with SLE. They reported 55% of patients with increase in transaminase levels among 81 patients with SLE, and identified SLE as the only explanation for this abnormality in 29% of the cases. Histological analysis of 7 of these patients revealed portal inflammation in 5, fatty liver in 1, and active chronic hepatitis in the remaining one. They also reported a 23% increase in the levels of alanine aminotransferase (ALT)/aspartate aminotransferase (AST) and alkaline phosphatase (ALP) (≤ 2 times ULN), with a notable predominance among patients that presented active clinical signs of SLE. All of these abnormalities normalized with steroid treatment.
A prospective analysis by Miller et al[15] recruited 260 patients with SLE that were followed up for a 12-mo period. In the follow-up examinations, liver enzymes levels were high in 23% of them. Clinical liver disease was observed in only 2% of the cases, while causes for liver compromise unrelated to SLE were verified in only 15% of the cases. No specific cause for liver disease other than SLE could be identified in 8% of the patients. The histological analysis carried out on 14 patients found only minimal and non-specific changes. It is noteworthy that the increase in transaminase levels in 12 out of 15 patients appeared concomitantly with lupus activity.
A much lower frequency of liver abnormalities was reported by Fox et al[16] in a retrospective cohort of 200 patients, where an increase of liver enzymes was documented in only 2.5% of the cases. These biochemical changes were associated with liver clinic manifestations only in few cases, and had no relationship with plasmatic ribosomal-P antibodies.
Very interesting findings were published by Matsumoto et al[17], who analyzed liver histology of 73 patients with SLE. They identified fatty liver as the major feature in 72% of the cases, while nodular regenerative hyperplasia, viral hepatitis, primary biliary cirrhosis (PBC), and autoimmune hepatitis (AIH) were identified as the main cause of liver disease only in few cases (6.8%, 4.1%, 2.7%, and 2.7%, respectively).
Finally, Takahashi et al[18] reported recently that liver dysfunction was apparent in 123 (59.7%) out of 206 patients. They identified different causes of liver dysfunction as follows: induced by drug (30.9%), caused by SLE itself (28.5%), fatty liver (17.9%), AIH (4.9%), PBC (2.4%), cholangitis (1.6%), alcohol (1.6%), and viral hepatitis (0.8%). The liver dysfunction tends to be mild, except when caused by AIH.
From the studies reported above, it is readily apparent that the published data linking liver diseases with SLE during the last four decades are highly heterogeneous, and that a high number of cases lack adequate histological documentation.
The frequent association between SLE and LFT alterations may be accounted for by three possibilities, namely: (1) the existence of some kind of liver parenchymal injury associated with SLE alone, often referred to as “lupus hepatitis”; (2) the occurrence of an overlap syndrome by which SLE shows additional features of another autoimmune liver disease; and (3) the concurrency of comorbility of SLE with a non-autoimmune hepatopathy, e.g., drug-induced liver damage, viral hepatitis or thrombotic liver disease, among others.
Although it is still a controversial issue, there is compiling evidence in the literature that lupus itself is not associated with a specific, severe and progressive liver injury. However, several authors have pointed a role for SLE in triggering an often subclinical hepatopathy, referred to as “lupus hepatitis”. They described this disease as an asymptomatic hypertransaminasemia frequently associated with exacerbations of the lupus disease, which returns to normal values after corticosteroid therapy[2,14,15].
May be a part the confusion begun in the early 50’s, when AIH was wrongly referred to as “lupoid hepatitis”[11]. Subsequent studies added more confusion when no serology was available to rule out overlapping chronic viral diseases [hepatitis C virus (HCV), hepatitis B virus (HBV), cytomegalovirus, etc.] in SLE patients with hypertransaminasemia.
In the early 80’s, Runyon et al[13] reactivated the debate publishing a very controversial study describing both a “canalicular cholestasis” profile and SLE-related cirrhosis as diseases triggered by lupus itself. As mentioned before, the sample analyzed in this study consisted of 33 lupus patients presenting different types of liver damage that were documented by liver biopsy, namely steatosis, chronic hepatitis, hemochromatosis, granulomatose hepatitis, cholestasis and cirrhosis. Serological and virological markers to rule out hepatitis C did not exist at this time.
As was also stressed above, another condition that is needed to rule out among SLE patients with hypertransaminasemia is an overlap with AIH, which represents a separate disease from lupus, both because of its distinct pathogenic mechanism (specific organ) and its distinctive biochemical, serological, and histological characteristics that allow for a clear differentiation.
Hypergammaglobulinemia, autoantibodies [antinuclear antibody (ANA), antismooth muscle antibody (ASMA), anti-liver-kidney microsome antibodies], a histological profile characterized by piecemeal necrosis (interface hepatitis), and a rich plama cells infiltrate are highly distinctive aspects of AIH. On the other hand, if a lupus patient presents evidence of progressive non-autoimmune chronic hepatitis characterized by persistent severe inflammatory damage, we need to consider first other probable diagnosis of chronic liver injury, such as hepatitis B or C, or other autoimmune diseases overlapping with lupus. The discrimination is further complicated by the fact that liver histopathological features in patients with lupus hepatitis are miscellaneous and non-specific, similar to those in other liver diseases. It is therefore important, before diagnosing lupus hepatitis, to rigorously rule out other liver diseases, including drug-induced liver injury, alcohol liver disease, viral hepatitis (hepatitis A, B, C, D, E, Epstein-Barr virus or cytomegalovirus), and other autoimmune-associated liver diseases [AIH, PBC, primary sclerosing cholangitis (PSC)].
A recent study by Zheng et al[2] based on this strict discrimination criteria reported a 9.3% lupus hepatitis incidence among 504 SLE patients evaluated. However, the prevalence reported in the literature is rather variable, with both lower[4,8,17,19] and higher[14,18,20] rate values.
Zheng et al[2] also reported that the prevalence of lupus hepatitis in patients with active SLE was higher than those with inactive SLE (11.8% vs 3.2%). The patients with lupus hepatitis mostly showed mild to moderate elevations of serum transaminase levels, though 6 patients had jaundice as the predominant feature. ALP and Gamma glutamil transferase elevations were far less frequent. Only 12.8% had liver injury-related clinical manifestations. Lupus hepatitis responds well to moderate to high doses of corticosteroids[3].
In patients suspected to have lupus hepatitis, it has been often reported a correlation between hepatic enzymes abnormalities and autoantibodies to ribosomal P proteins (anti-ribosomal P), a highly specific marker for SLE[19,21,22]. Indeed, several reports suggest that SLE-related hepatitis may be associated with, or even caused by this autoantibody.
Anti-ribosomal P occurs in 12%-16% of patients with lupus[21-24], although this proportion increased to 30% when more sensitive methods were employed [enzyme-linked immunosorbent assay (ELISA) based upon the combination of different ribosomal-P antigens], with Caucasian ethnicity having lower values[25]. The proportion of serum anti-ribosomal P occurrence raised to 44% among SLE patients with liver dysfunction and, from them, 70% had SLE-associated hepatitis, a far higher value as compared with SLE patients suffering from other hepatic alterations, such as fatty liver (29%), drug-induced hepatitis (17%), or SLE-AIH overlap syndrome (20%)[26]. Furthermore, Koren et al[27] reported the development of chronic active hepatitis in a patient with SLE followed several months later by the appearance of high serum levels of anti-ribosomal P antibodies, and suggested a possible causal relationship. As for the mechanism explaining this causal relationship, anti-ribosomal P positive sera from SLE patients were found to react strongly “in vitro” with a polypeptide antigenically related to a 38 kD ribosomal P0 protein present on the plasma membrane of hepatoma cells[28], thus further strengthening the possibility that anti-ribosomal P antibodies could be directly detrimental in lupus patients by inducing hepatocellular lysis, and further transaminase release. Finally, anti-ribosomal P antibodies up-regulate the expression of proinflammatory cytokines by peripheral monocytes in SLE, which may be a contributing factor for hepatitis development[29].
Given that auto-antibodies directed against eukaryotic P proteins are highly specific to SLE, they can be used as diagnostic markers of the disease. However, there is no standard methodology for its detection and titration in clinical practice. The plasma titers of this antibody often fluctuate in relation to lupus activity, and were formerly associated with neuropsychiatric kidney and liver failure[22,26].
Several isolated cases have been reported of association of anti-ribosomal P antibody occurrence with hepatitis, and also with kidney failure[27,30]. However, it was Arnett et al[19] the first to report this association in a cohort study in 1995. They found lupus-related hepatitis in 3% of 131 lupus patients in a retrospective study that analyzed the hepatic manifestations of SLE. The clinical outcome for these patients was variable, from a minimum, subclinical increase of transaminases to acute hepatitis and overt liver failure. Unfortunately, histological studies were not carried out in this study to correlate the degree of liver injury associated with lupus hepatitis and the levels of anti-ribosomal P antibodies.
Although these lines of evidence link anti-ribosomal P antibodies to liver damage in SLE patients, the association is still highly controversial. For example, lack of a clear association between lupus hepatitis and anti-ribosomal P levels was reported in a recently published retrospective study of 73 patients with SLE, where 12 of them (16%) were reported to have lupus hepatitis. In this group, 6 patients had a concurrent liver involvement with the diagnosis of SLE, and it occurred later during an exacerbation of the disease in the remaining 5 patients[19]. Clinical manifestations were as follows: hepatomegaly (n = 4), jaundice (n = 4), abdominal pain (n = 3), ascitis (n = 2), portal hypertension (n = 1), and hepatic failure with encephalopathy (n = 1). Despite elevated liver enzymes were noted in 11 cases and cholestasis in 8 ones, the presence of anti-ribosomal P antibodies was observed only in one case, and therefore an association between lupus hepatitis and any kind of specific antibody could not be documented. Liver biopsy in 5 patients showed chronic active hepatitis in 3 cases, chronic hepatic granulomas in 1 case, and nonspecific inflammation in another one. Although the authors showed clear evidence of immunosuppressive therapy response in most patients, liver biopsy was performed in less than half of them, and their description was not detailed enough to clearly differentiate lupus hepatitis from AIH.
In part, disagreements on the association between anti-ribosomal P antibody and lupus hepatitis can be explained by different features of the studied populations (e.g., ethnicity), environmental factors affecting autoantigen expression, and distinct degrees of sensitivity/specificity of the methods used to detect anti-ribosomal P antibodies. Usually, associations between anti-ribosomal P antibody levels and hepatitis were investigated by using not well-standardized, or even “in-house” immunological methods[19,26]. Unfortunately, large cohort studies where lupus hepatitis or other SLE hepatic manifestations have been reliably documented, and where well-standardized, high sensitivity/specificity immunological methods are employed to detect anti-ribosomal P antibodies (e.g., those using a mixture the ribosomal P antigens P0, P1, and P2), are lacking, and we eagerly await them to confirm or deny the existence of this association.
To complicate the picture further, Calich et al[31] reported recently the presence of anti-ribosomal P antibodies in patients having AIH not associated with lupus (9.7%; 9/93), and suggested that this antibody predicts worse prognosis of the disease, with follow-up data showing higher prevalence of cirrhosis in anti-ribosomal P antibody-positive AIH patients (100%, 7/7). This finding suggests that anti-ribosomal P antibodies can be involved in the pathogenesis of other hepatic autoimmune diseases, apart from lupus hepatitis. The debate is still open, and it is apparent that we need more data to support the role and impact of anti-ribosomal P antibodies in both SLE and AIH pathogenesis.
The existence of overlap syndromes linking SLE with other autoimmune liver diseases is matter of controversies since, again, the data in the literature are scarce.
According to the so called “theory of the mosaic of autoimmunity”[32], each of these associations may represent a particular variant of a major underlying autoimmune disease, which can shows up under the form of multiple autoimmune liver diseases coexisting in the same patient. Other good examples of such variants are more typical hepatic overlap syndromes, such as AIH-PBC and AIH-PSC[33].
Although AIH or PBC are rare among SLE patients taken as a whole[34], the co-existence of SLE with either of these liver diseases is not uncommon among the subgroup of SLE patients with liver enzyme abnormalities. Chowdhary et al[7] reported a strong association between SLE and autoimmune liver disease. They found that 8 out of 40 SLE patients (20%) were AIH carriers, while 6 (15%) showed evidence of PBC.
In another study by Efe et al[35], 36 SLE patients out of 147 (25%) had liver enzyme abnormalities, and 7 of them (4.7%) had SLE associated with another autoimmune liver disease. The rate rose to 19.4% when the subset of SLE patients having HLTs altered was considered and, from them, 72.3% fulfilled the criteria for AIH proposed by the International Autoimmune Hepatitis Group. The therapy with ursodeoxycholic acid, prednisone, immunosuppressive thiopurine analogs, or a combination of them, was successful in these patients.
There have been very few reported cases of AIH associated with SLE. It is therefore apparent that AIH and SLE overlap syndrome is a rare condition, although its exact incidence is unclear.
Oka et al[36] reported 5 (3%) patients with AIH in an analysis of 162 cases of SLE meeting the ACR criteria. Similar findings were documented by Tamai et al[37], who found 10% of AIH in a series of 21 SLE cases.
There is evidence in the literature suggesting that SLE and AIH are different diseases, even when clinical, biochemical and serological characteristics may show overlapping features, such as the presence of polyarthralgia, hypergammaglobulinemia, and positive ANA, ASMA and anti-ribonucleoprotein[38]. In these cases, liver histology is the decisive tool to define diagnosis. The presence of cirrhosis or periportal hepatitis associated with lymphocytes and plasma cell infiltration, as well as rosette formation of liver cells, tips the scales towards AIH. On the other hand, the presence of mainly lobular and occasionally portal inflammation with a paucity of lymphoid infiltrates is more compatible with SLE. Finally, a mixed histological pattern is expected in SLE-AIH syndrome, displaying chronic hepatitis with severe inflammatory activity characterized by focal necrosis of hepatic cells, erosion of the lobular limiting plate, periportal hepatitis, infiltration by lymphocytes and plasma cells, presence of fibrosis in the portal areas and, eventually, cirrhosis[39,40]. In this context, positivity for anti-Sm antibodies, which are highly specific though relatively insensitive to SLE, helps to confirm SLE-AIH overlapping. In addition, presence of antibodies to double-stranded (ds) DNA, another hallmark of SLE, were found to be associated with poorer immediate response to corticosteroid treatment in AIH[41].
PBC is also an autoimmune liver disease, and overlapping with PBC is likely to some extent. However, the co-existence of PBC and SLE is the subject of few reports in the literature, mostly based upon single case reports[42,43]. A large-scale study reported that, among 1032 PBC patients, 27 (0.03%) had also SLE[44]. Interestingly, anti-dsDNA and anti-ribosomal-P antibodies, two serological markers of SLE, were detected in 22% and 5%, respectively, of “pure” PBC patients[45].
SLE-PBC association has been documented mainly in patients with arthritis, polyserositis, and high titers of anti-native DNA and anti-mitochondrial antibodies (AMAs), two pathognomonic signs of SLE and PBC, respectively. Again, PBC can appear in a pre-existing lupus as an expression of an immunological disorder that has not been totally clarified. Osteopontin, a soluble ligand with pleomorphic immunologic activities that plays an important role in inflammation and immunity, may be a link. Osteopontin was reported to be highly expressed in the murphy roths large/lpr mouse[46], a well recognized models of SLE, and it is involved as a chemoattractant cytokine in the recruitment of macrophages and T lymphocytes in the liver granulomas in PBC[47]. Interestingly, Han et al[48], in a large cohort of 1141 SLE patients, confirmed the association between osteopontin and SLE.
Finally, AIH-PBC overlap syndrome has been reported to occur in 2.8% of SLE patients, suggesting the association of not only two but even three autoimmune diseases (SLE-AIH-PBC overlap syndrome)[49]. Furthermore, anti-dsDNA antibodies, which are known to be strongly associated with SLE, were detected in 60%[50] or 56%[51] of patients with AIH-PBC overlap syndrome.
Evidence for SLE-PSC overlap syndrome is limited at best, and only based upon few case reports[52-55]. Whether this clinical association indicates that some immune disorders are common to the two autoimmune diseases or whether they were casual associations remains to be ascertained.
SLE patients often present comorbidity with a number of non-autoimmune liver diseases. In many cases, the prevalence of the concomitant hepatopathy is higher when associated with SLE than alone, indicating either increased susceptibility to the concomitant disease triggered by SLE or vice versa.
Autoimmunity and viral infections are closely associated fields, and viruses have been proposed as a likely etiological, contributing or even triggering factor of systemic autoimmune diseases[56]. This holds true also for SLE, since some hypotheses have identified viruses as potential agents that trigger SLE, with a close relationship to the pathogenic mechanism of damage[57].
Very little association has been found between SLE and patients infected with HCV. Most reports linking the two diseases refer to the presence in these patients of skin lesions, anti-DNA antibodies, hypocomplementemia and cryoglobulinemia[57].
In a study of 134 patients carrying SLE, the presence of anti-HCV antibodies (ELISA) was observed in 18 patients (13%), while the prevalence among voluntary blood donors in a large number of countries ranges from 0.5% to 2%, only. Active infection by HCV was confirmed in 15 (11%) of the patients with positive ELISA HCV[57]. Similar results were obtained in other study where HCV was detected in 4 out of 40 SLE patients (10%), whereas prevalence among voluntary blood donors was only of 0.13%[58]. Steroid therapy in these patients did not seem to alter the HCV course[59]. Whether this reflects a true higher HCV prevalence associated to SLE or it is a mere consequence of the multiple admissions and blood transfusions that these patients are subjected remains to be defined. Large-scale studies avoiding these potential bias are awaited.
It should be on the other hand acknowledged that HCV chronic infection is associated with different biochemical and histological manifestations of autoimmunity that, in certain cases, can mimic SLE[60]. Different types of non-organ-specific autoantibodies can be detected in chronic hepatitis C (e.g., anti-soluble liver antigen, ANA, AMT, rheumatoid factor) and, less frequently, it is associated with low anti-DNA titers; for example, about 20% with hepatitis C patients are ANA positive[61]. In addition, chronic hepatitis C can occur with cryoglobulinemia, which can lead to a wrong SLE diagnosis, due to the simultaneous occurrence of ANA, dermathological and renal lesions and plaquetopenia; this is why, in patients suspected to have SLE, HCV infection must be excluded using routine anti-HCV serology and, HCV-RNA tests. Several factors lead to the production of autoantibodies in HCV patients, including leakage of intracellular components due to the persistent destruction of infected cells[61], the molecular mimicry between HCV and auto-antigens[62], and the functional abnormalities of infected B lymphocytes, with production of excessive autoantibodies and cryoglobulins[63].
Fukuyama et al[64] reported for the first time in the literature the development of an SLE profile after interferon α-2 therapy. There are over 10 currently published cases that link the use of interferon to treat hepatitis C with the appearance of SLE associated with different levels of severity, including one patient with a serious lupus cardiomyopathy that threatened his/her life.
Although chronic infection with HCV can induce clinical and serological changes that can be confused with an autoimmune disease (arthritis, nephropathy, and cytopenias), the appearance of malar rash, discoid lesion, photosensitivity, neurological damage, high titers of ANA or anti-DNA antibodies, and anti-Sm antibody occurrence usually constitute sufficient evidence to diagnose SLE[7].
The clinician must consider three situations in the context of a HCV antibody in a patient with SLE, namely: (1) it may be a false positive HCV ELISA test due to the high levels of autoantibodies that are frequently presented in SLE patients; (2) could be true association between SLE and hepatitis C; and (3) HCV can trigger the occurrence of low levels of ANA and/or anti-DNA, associated with cryoglobulinemia, without typical skin changes[19].
One common complication of SLE patients is the so called “lupus nephritis”, and HCV may play a role. Few cases of lupus nephritis coexisting with HCV infection have been described[65,66]. Although speculative, it is likely that the altered immune response in SLE facilitates HCV infection, and vice versa, that different autoantibodies associated with HCV infection facilitate the development of lupus nephritis due to formation of immune complex deposits in the kidneys. The increase in serum B-lymphocyte activating factor levels in chronic HCV patients with infection and SLE may be a contributing factor, by reinforcing B-cell activation and autoantibody production[67].
SLE patients have a high potential to develop thromboembolic disorders that can impact on hepatic circulation[68]. The frequent presence of anti-phospholipid antibodies among these patients can include thrombotic manifestations in different territories of the splachnic vasculature, both in arterial and venous areas (thrombosis of the hepatic artery, portal thrombosis, and Budd-Chiari syndrome)[69]. Portal hypertension profiles and esophageal varices have also been reported in several cases as secondary events linked to thrombosis of the portal vein, triggered by the presence of anti-cardiolipin antibodies[10].
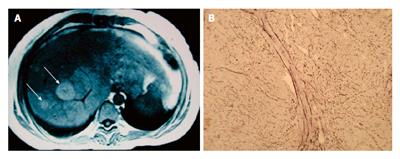
Regenerative nodular hyperplasia (RNH), which follows hepatic vein thrombosis and hepatic circulation disorders, has also been reported in association with SLE (Figure 1)[70]. The pathogenesis of RNH complicating SLE is believed to be related to vasculitis of intrahepatic arteries, leading to secondary portal venous obliteration and thrombosis of the adjacent portal veins[1]. Alternatively, occlusion of intrahepatic small vessels may result from coagulopathy in patients with associated anti-phospholipid syndrome[9]. It has been suggested that anti-phospholipid antibodies play a pathogenic veno-occlusive role in the pathogenesis of RNH[71].
One of the most attractive theories regarding RNH origin involves the storage of immune complexes in small caliber intrahepatic vessels, and the further appearance of obliterative venopathy[70]. The liver histology pattern is characterized by the presence of multiple hepatic nodes that do not have their own walls and that, in the absence of fibrosis, are circumscribed by thin bands formed by the flattening of hepatocyte columns emulating thin fibrous membranes. This condition is another component of a long list of diseases linked to non-cirrhotic portal hypertension. It is often associated with hematological diseases and various conditions that typically present systemic impairment (rheumatoid arthritis, CREST syndrome, Felty’s syndrome)[72]. Another theory suggests that the association between RNH and anti-phospholipid antibodies is due to the cellular regeneration process that begins in the liver to maintain its functional capacity after the ischemic injury induced by these antibodies in the hepatic microcirculation[68].
RNH should be suspected in any patient with both SLE and portal hypertension in the absence of cirrhosis. The diagnosis can be established after a liver biopsy. Due to the large size of the regenerative nodes, there is a chance for the needle to be positioned in an area with no histological damage, which accounts for sampling error. When RNH is to be diagnosed, laparoscopic wedge biopsy is a safe and efficient way to obtain enough tissue to preserve the hepatic architecture required for analysis, avoiding in turn the morbidity associated with an unnecessary open resection[73].
Hepatic imaging of RNH shows several additional findings, including focal nodular hyperplasia (FNH), hepatocellular adenoma, regenerative nodules, and liver metastatic disease. Computed tomography can show normal liver, numerous small nodules, or larger coalesced nodules spanning several centimeters. On nuclear medicine imaging, these lesions may take up sulfur colloid, but will remain iso- or hypodense in both arterial and portal venous phases; this helps to distinguish RNH from FNH[74]. The use of magnetic resonance imaging (MRI) to enhance diagnostic accuracy is still controversial. RNH lesions appear hyperintense on T1-weighted imaging and iso- or hypointense on T2 images (Figure 1). However, the sensitivity and specificity are variable, according to a recent report[75].
RNH may be differentiated from large regenerative nodules (LRN) by either tomography or MRI. LRN can have a distinct presentation, and very often results in enhancing liver nodules, whereas RNH usually does not[76].
The spontaneous rupture of the liver has also been reported in patients with SLE as a serious consequence related to the occurrence of a large area of infarction, due to a thrombotic phenomena of the hepatic artery[77].
Focal disturbance of the hepatic blood supply associated with lupus might also facilitates the hyperplastic development of benign lesions in the liver, such as FNH and hemangiomas[78]. In a recent study analyzing a cohort of 35 SLE patients, FNH was observed at higher rates (5.7%) than in the normal population (0.6%-3.0%), and the same holds true for hemangiomas (54.2% vs 0.4%-20% in the general adult population)[79]. Whereas FNH is thought to be part of an abnormal adaptive regenerative response of the liver parenchyma to local hemodynamic disturbances[80], hemangioma formation may be also favored by an increase of angiogenic factors whose circulating levels are increased in SLE patients, such as estrogens[81], vascular endothelial growth factor, and interleukin-18[82,83]. Confirmation of an increased incidence of these kinds of hepatic benign lesions in SLE patients awaits large-scale studies.
The association of SLE with porphyria cutanea tarda (PCT), the most frequent type of porphyria, is rare, and data defining whether this concomitance is pure coincidence or true association are still lacking[84-86].
Common features in both diseases may be a confusing factor. SLE is similar to PCT regarding photosensitivity, but the presence of blisters involving crusts and miliae in sun-exposed areas of PCT patients, which is characteristic of PCT but rare in SLE (< 5% of the cases)[87], can help to differentiate both diseases.
Co-existence of PCT is usually associated with antimalarial drugs for treating lupus (e.g., chloroquine, hydroxychloroquine), and the regular use of these drugs in SLE patients should be considered a risk for PCT. This usually represents a diagnostic problem, given the frequent association of PCT with a long list of drugs apart from antimalarial agents, which makes the diagnosis of the cause even more complicated[88,89]. The risk associated with antimalarial drugs is dose-dependent; this is why several authors have contraindicated the daily intake of these drugs for SLE due to the risk of massive porphyrinuria, which is often associated with fever, nausea and hepatocelular injury, leading eventually to hepatic necrosis[78-81].
Patients with SLE seem to have a relatively high rate of drug-induced hepatotoxicity (Table 3). For example, Huang et al[90] reported 35 cases of drug-induced hepatotoxicity among 1533 SLE patients reviewed. In another study by Takahashi et al[18], liver damage could be ascribed to drug-induced liver injury in 31% from a total of 123 SLE patients with overt liver dysfunction.
| Drug | Liver injury and clinical significance |
| Corticosteroids | Hepatomegalia |
| Fatty liver | |
| NSAIDs | Asymptomatic ALT increase |
| Hepatocellular, cholestatic, or mixed injury | |
| ASA | Acute and chronic hepatocellular injury |
| (resolve with withdrawal) | |
| Methotrexate | Asymptomatic ALT increase at high doses |
| Esteatosis, fibrosis, or cirrhosis | |
| Anti-malarial drugs1 | Rare hepatotoxic effects |
| Porphyria cutanea tarda | |
| Azatioprine | Cholestasis, peliosis, SOS, RNH |
| Thioguanine | SOS, RNH, portal hypertension |
| Ciclophosphamide | Rare case reports at conventional doses |
| SOS at high doses (resolve with dose reduction) | |
| Mycophenolate mofetil | Asymptomatic ALT increase |
| (resolve with dose reduction) | |
| Rituximab | No liver reactions have been reported |
| Belimumab | No liver reactions have been reported |
At the moment, it is impossible to know with certainty whether this high incidence is due to the chronic use, at relatively high doses, of different drugs commonly prescribed to treat this disease, or whether there is any kind of particular susceptibility that makes these patients prone to drug-induced hepatotoxicity. Of note, SLE patients have been shown to have elevated levels of systemic oxidative stress, which well correlated with liver enzyme elevations[91]. This relationship can be tentatively explained by drug-induced oxidative stress in the liver of these patients, with consequent liver injury. The elevated pro-oxidant liver status associated with a pro-inflammatory conditions like SLE may also make the organ prone to develop hepatotoxicity by drugs exerting detrimental effects via oxidative mechanisms. Indeed, several drugs used in autoimmune disease may themselves be converted into free radicals “in vivo”, thus aggravating oxidative damage[92,93]. Controlled, comparative studies on differential susceptibility to the same drug in patients with SLE and other autoimmune disease (e.g., rheumatoid arthritis) are lacking, but they would be useful to establish whether SLE is indeed a peculiar prone condition for drug-induced liver injury.
Around 80% of SLE patients are treated with analgesic and NSAIDs, prescribed for febrile syndrome, athralgia/arthritis, serositis and/or cephale[94]. Hepatitis , fulminant hepatic failure, cholestasis, and mixed damage were reported to be caused by these compounds[95-98].
Lupus patients usually present a higher rate of NSAID-related complications than SLE-negative subjects. The most common complications are increased transaminase levels, skin rashes triggered by sun, increased retention of body fluids with arterial hypertension, gastric ulcers, and aseptic meningitis. NSAIDs should not be indicated over the counter in SLE, and prescription must always be accompanied by recommendations related to strict clinical and laboratory vigilance[94].
For many years, aspirin was the most common drug associated with SLE-related liver damage. Increments of ALT, AST and ALP have been reported in up to 25% of the SLE patients consuming high doses of aspirin (> 2 g/d)[94].
In the early 70’s, the first publications appeared identifying aspirin as responsible for the hepatic damage in SLE patients[99,100]. It was not however until 1981 that Zimmerman, in a review focused on this issue, showed with certainty that aspirin generates both acute and chronic dose-dependent liver damage[101].
The onset of aspirin-induced liver disease is marked by the appearance of anorexia, nausea and non-specific pain in the upper abdomen. The patient usually does not present jaundice, and ALT and AST values are usually not more than 10 times ULN values. It is very common that AST levels are higher than ALT, and that these alterations are associated with normal ALP levels[102].
Although hepatotoxicity can occur with low levels of plasma salicylate, the mechanism is often dose-dependent, and the biochemical abnormalities revert when the drug is discontinued. In 3% of the cases, the lesion can be severe enough to lead to fatal hepatic failure. Chronic liver damage observed in the hepatic histology as a chronic active hepatitis pattern is much less common, and also returns to normality when the drug is withdrawn[103].
There is also controversial evidence that rheumatic patients usually have underlying conditions that increase the risk of aspirin-induced hepatic failure. However, SLE-related hypoalbuminemia and juvenile rheumatoid arthritis are well documented risk factors as well[104-106].
Thiopurine analogues, such as azathriopine (AZA) and 5-mercaptopurine, are immunosuppressive drugs often employed in autoimmune diseases, including their use to gain or maintain remission in SLE. Hepatotoxicity induced by thiopurine analogues occurs very often with increase in serum transaminase levels. It is associated generally with not severe liver injury, which responds to dose reduction in most patients. RNH is also a very rare but potentially severe complication of thiopurine-based therapies. It is often asymptomatic, and neither biochemical nor molecular markers are indicative of RNH. The suspicion should arise when there are clinical symptoms of portal hypertension, increments of transaminase levels, or thrombocytopenia. A liver biopsy is essential in this case to confirm diagnosis[107].
A recent review by Musumba[108] reports that inflammatory bowel disease patients treated with AZA have a cumulative incidence of RNH at 5 and 10 years of 0.6% and 1.3%, respectively, whereas those treated with high TG doses (> 40 mg/d) have an incidence of RNH of up to 62%; this rate is even higher in patients with elevated liver enzymes and/or thrombocytopenia, as compared with those lacking these abnormalities (76% vs 33%).
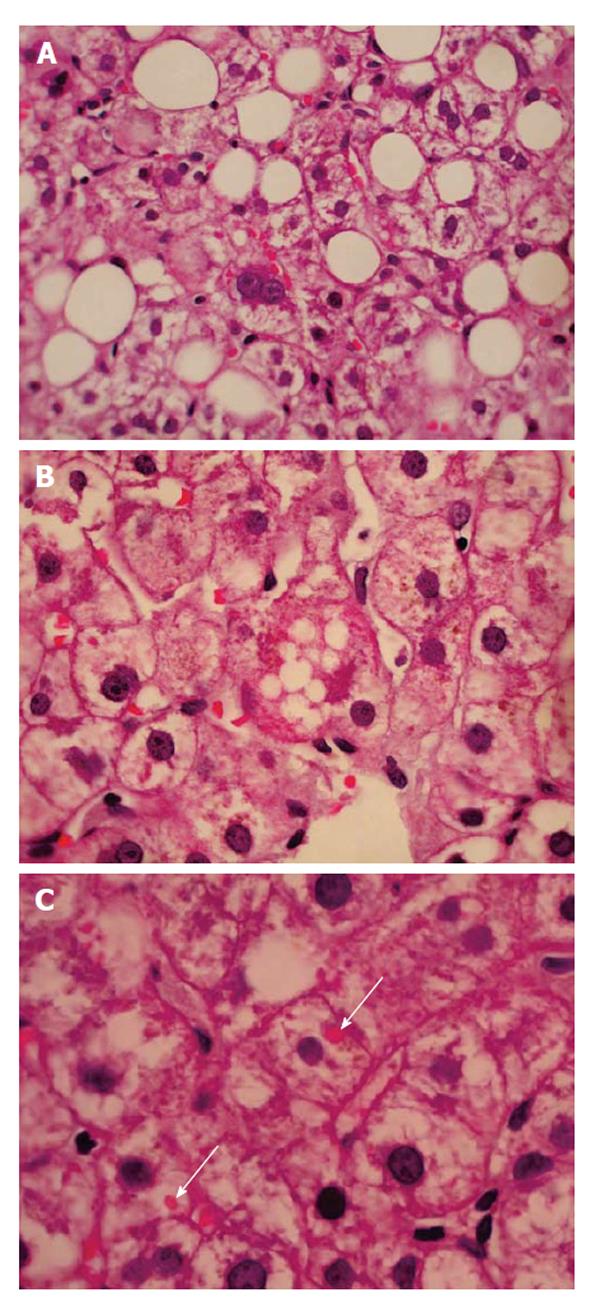
Methotrexate (MTX) is currently the first-line therapy for early and chronic rheumatic and psoriasic arthritis, but it is also indicated to symptomatic patients with SLE[109]. The recognition of risk of chronic liver damage with MTX has prompted the need for intensive biochemical monitoring from several decades ago onwards. The frequency of hepatotoxicity varies widely according to differences in sampling, definitions of damage, dose regimens, and presence of other risk factors[110]. Although one study showed transaminase elevations higher than twice the upper limit of normal in 13% of patients[111], another report assessing 6000 patients receiving MTX, transaminase elevation was described in only 0.6% of patients[112]. Despite this wide difference, most studies concluded that prolonged use of low-dose MTX monotherapy (10 mg/wk for 2-15 years) has favorable long-term safety, and that the development of significant liver fibrosis and cirrhosis is very low[113]; rather, steatosis was the main finding when biopsies were carried out for surveillance dictated by cumulative MTX dose (Figure 2)[114]. Due to this disparity, Society’s guidelines differ on how patients on MTX should be monitored to prevent MTX-induced liver fibrosis[115,116].
Although liver biopsy is still suggested in these patients in case of persistent elevation of transaminase after drug discontinuation, and for ruling out other potential cause of chronic liver disease, there is robust evidence that Fibroscan Elastography may become in a near future the gold standard for fibrosis investigation in patients treated with MTX[117,118]. Most studies concluded that MTX therapy is safe, and that Fibroscan is useful for monitoring liver fibrosis in patients treated with this drug. Conclusions drawn from several studies indicate that severe liver fibrosis is a rare event in patients treated with MTX, and that it is probably unrelated to the dose. A recent work also studied the accuracy and feasibility of Fibroscan and Fibrotest to detect MTX-induced liver fibrosis in 24 psoriasis patients[119]. The results obtained using Fibroscan and Fibrotest were compared with those obtained by liver histology. In this cohort, Fibrotest accurately predicted the presence of liver fibrosis, while Fibroscan accurately predicted the absence of liver fibrosis in MTX users. These findings suggest that a combination of approaches should prospectively be evaluated in monitoring and detecting significant MTX-induced liver fibrosis.
An association between MTX-induced toxicity and genetic polimorfism was suggested. Fisher et al[120] conducted a meta-analysis of published studies including 1400 patients for association of the C677T polymorphism of the gene encoding methylene tetrahydrofolate reductase (MTHFR), and over 660 patients for the A1298C variant. They observed that the former but not the latter MTHFR gene variant was significantly related to MTX toxicity, including hepatotoxicity (OR = 1.71; CI: 1.32-2.21, P < 0.001). Despite results for MTHFR A1298C are not conclusive, C677T polymorphism appears to be a promising risk factor for the development of low-dose-MTX-induced hepatotoxicity. Only few studies reported variants in genes that are predictive for MTX-induced hepatotoxicity[121].
Recent results showed that the administration of metformin in rats receiving MTX normalized altered liver function tests and improved liver histopathological findings. Therefore, this result suggests that this drug confers hepatoprotection against MTX-induced hepatotoxicity[122].
Minor abnormalities of liver enzymes are relatively common when using anti-tumor necrosis factor (TNF) agents, such as infliximab, etanercept, and adalimumab, as anti-inflammatory and immunosuppressive compounds for the treatment of autoimmune diseases[123,124]. Severe hepatic reactions are much less common, and include jaundice, hepatitis, cholestasis, and acute liver failure[125-127]. AIH is a rare, but increasingly recognized adverse event linked to treatments with anti-TNF agents[122]. In addition, lupus-like syndrome and anti-TNF-α-induced SLE were the most common disorders listed in a registry of autoimmune diseases associated with anti-TNF-α agents[128,129]. Finally, rituximab is listed as able to reactivate HBV, even in patients with HBsAg negative and anti-HBsAg positive. This concept was recently reinforced by Seto et al[130], who reported a HBV reactivation rate of 24% in HBs Ag-negative, anti-HBc-positive patients undergoing rituximab-based chemotherapy for hematologic malignancies, with most of reactivations occurring during the first 6 mo of therapy. The Food and Drug Administration recently announced the requirement of a Boxed Warning for the anti-cancer immunosuppressive drugs Rituxan (rituximab). The Boxed Warning is specific for the risk of HBV reactivation in patients who were previously infected with the virus. Use of these drugs in patients with previous HBV infection can result in severe liver damage if the virus is reactivated[131].
Minocycline, a drug used in the treatment of rheumatoid arthritis and acne, can induce a lupus-like syndrome[132]. In addition, statins, which inhibit hydroxymethylglutaryl-coenzyme A reductase, are widely used nowadays in SLE patients due to their immunomodulator and antiatherogenic effect. Several reports have suggested that this drugs may also induce acute hepatitis and a lupus-like syndrome[133]. Finally, cyclophosphamide, an immunosuppressive and potent alkylating agent that improves the outcome of major organ disease when administered at high doses to SLE patients unresponsive to conventional therapy[134], was reported to induce hepatotoxicity associated with liver inflammation in isolated cases[135,136]. There is a report of one case in the literature showing that this effect may occur even when the drug is administered at low doses[137].
Liver abnormalities is very common among patients with SLE, especially if they are assessed from the biochemical point of view. It is generally asymptomatic, and frequently associated with steatosis, reactive unspecific changes and drug-related hepatotoxicity. Severe and progressive liver injury may occur, and even more often in the context of a coexisting primary liver disease or during pharmacotherapy.
SLE by itself is not usually associated with aggressive liver disease, but with an often asymptomatic entity referred to as “lupus hepatitis”, which is characterized by a mild increase in serum transaminase levels. However, there are overlapping profiles with other autoimmune disease, such as AIH and PBC, related to chronic and aggressive damage, sometimes accompanied by changes in immunological liver tests that help to establish an accurate diagnosis. These overlap syndromes are thought to be variants of an underlying general autoimmune disease, which shows up in a variable arrangement of autoimmune disorders. An etiological role for anti-ribosomal P antibodies in triggering both lupus hepatitis and AIH has been proposed, but it remains uncertain and controversial.
SLE patients often present comorbility with non-autoimmune liver diseases. They includes HCV, thrombotic events in the splachnic vasculature, PCT, and drug-induced hepatotoxicity, among others.
Hepatic circulation disorders may lead to adaptive parenchymal regenerative processes (e.g., RNH, FNH) or formation of hemangiomas. RNH must be ruled out in all lupus patients who present evidence of portal non-cirrhotic hypertension associated with hepatic pseudonodular images.
Drug-induced liver toxicity is also a common event in SLE, and may be ascribed to the chronic use, at high doses, of medicines used to control the autoimmune disorder (e.g., thiopurine analogues, anti-TNF-α agents, statins, minocycline, cyclophosphamide) or to mitigate SLE symptoms (e.g., NSAIDs, MTX). SLE is an oxidative-stress-prone condition, and the pro-oxidant effects of many of these drugs may be a causal factor.
Due to the relatively frequent multifaceted manifestations of liver diseases in SLE, with an often difficult differential diagnosis each others, an assessment of immunological, serological and virological markers should be systematically carried out in patients with elevated levels of liver enzymes. Testing for AMA, ASMA, and HCV may be particularly helpful. In addition, an analysis of the patient’s medical history so as to have an accurate record of the drugs taken by the patient should be carefully done. Finally, histology is in some cases the only reliable method of diagnosis, and should be carried out accordingly. We hope the information provided by this review helps to systematize the knowledge of the field, so as to make the challenge of identifying liver diseases associated with SLE more approachable to the clinician.
P- Reviewers: Atta AM, Efe C, Vazquez-Del Mercado M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Schlenker C, Halterman T, Kowdley KV. Rheumatologic disease and the liver. Clin Liver Dis. 2011;15:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Zheng RH, Wang JH, Wang SB, Chen J, Guan WM, Chen MH. Clinical and immunopathological features of patients with lupus hepatitis. Chin Med J (Engl). 2013;126:260-266. [PubMed] |
| 3. | Piga M, Vacca A, Porru G, Cauli A, Mathieu A. Liver involvement in systemic lupus erythematosus: incidence, clinical course and outcome of lupus hepatitis. Clin Exp Rheumatol. 2010;28:504-510. [PubMed] |
| 4. | Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of systemic lupus erythematosus. J Clin Gastroenterol. 2011;45:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Schiavon LL, Carvalho-Filho RJ, Narciso-Schiavon JL, Lanzoni VP, Ferraz ML, Silva AE. Late-onset systemic lupus erythematosus-associated liver disease. Rheumatol Int. 2012;32:2917-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7731] [Cited by in RCA: 8651] [Article Influence: 309.0] [Reference Citation Analysis (0)] |
| 7. | Chowdhary VR, Crowson CS, Poterucha JJ, Moder KG. Liver involvement in systemic lupus erythematosus: case review of 40 patients. J Rheumatol. 2008;35:2159-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | van Hoek B. The spectrum of liver disease in systemic lupus erythematosus. Neth J Med. 1996;48:244-253. [PubMed] |
| 9. | Luangjaru S, Kullavanijaya P. Gastrointestinal and hepatobiliary manifestations in systemic lupus erythematosus. J Med Assoc Thai. 2005;88:71-75. |
| 10. | Abraham S, Begum S, Isenberg D. Hepatic manifestations of autoimmune rheumatic diseases. Ann Rheum Dis. 2004;63:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Mackay IR, Taft LI, Cowling DC. Lupoid hepatitis and the hepatic lesions of systemic lupus erythematosus. Lancet. 1959;1:65-69. [PubMed] |
| 12. | Chwalińska-Sadowska H, Milewski B, Nazarewicz T. Clinical and immunomorphological evaluation of pathological changes in the liver in collagenoses. Mater Med Pol. 1976;8:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 88] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Runyon BA, LaBrecque DR, Anuras S. The spectrum of liver disease in systemic lupus erythematosus. Report of 33 histologically-proved cases and review of the literature. Am J Med. 1980;69:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 138] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Gibson T, Myers AR. Subclinical liver disease in systemic lupus erythematosus. J Rheumatol. 1981;8:752-759. [PubMed] |
| 15. | Miller MH, Urowitz MB, Gladman DD, Blendis LM. The liver in systemic lupus erythematosus. Q J Med. 1984;53:401-409. [PubMed] |
| 16. | Fox RA, Reichlin MW, Reichilin M, Isenberg DA. Liver function test abnormalities in systemic lupus erythematosus [abstract]. Br J Rheumatol. 1997;36:S10. |
| 17. | Matsumoto T, Kobayashi S, Shimizu H, Nakajima M, Watanabe S, Kitami N, Sato N, Abe H, Aoki Y, Hoshi T. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Takahashi A, Abe K, Saito R, Iwadate H, Okai K, Katsushima F, Monoe K, Kanno Y, Saito H, Kobayashi H. Liver dysfunction in patients with systemic lupus erythematosus. Intern Med. 2013;52:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Arnett FC, Reichlin M. Lupus hepatitis: an under-recognized disease feature associated with autoantibodies to ribosomal P. Am J Med. 1995;99:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Khalifa M, Benjazia E, Rezgui A, Ghannouchi N, Alaoua A, Braham A, Létaief A, Bahri F. [Lupus hepatitis: a case series of 12 patients]. Rev Med Interne. 2011;32:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Carmona-Fernandes D, Santos MJ, Canhão H, Fonseca JE. Anti-ribosomal P protein IgG autoantibodies in patients with systemic lupus erythematosus: diagnostic performance and clinical profile. BMC Med. 2013;11:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 409] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Teh LS, Isenberg DA. Antiribosomal P protein antibodies in systemic lupus erythematosus. A reappraisal. Arthritis Rheum. 1994;37:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Schneebaum AB, Singleton JD, West SG, Blodgett JK, Allen LG, Cheronis JC, Kotzin BL. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991;90:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 181] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Mahler M, Kessenbrock K, Szmyrka M, Takasaki Y, Garcia-De La Torre I, Shoenfeld Y, Hiepe F, Shun-le C, von Mühlen CA, Locht H. International multicenter evaluation of autoantibodies to ribosomal P proteins. Clin Vaccine Immunol. 2006;13:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Ohira H, Takiguchi J, Rai T, Abe K, Yokokawa J, Sato Y, Takeda I, Kanno T. High frequency of anti-ribosomal P antibody in patients with systemic lupus erythematosus-associated hepatitis. Hepatol Res. 2004;28:137-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Koren E, Schnitz W, Reichlin M. Concomitant development of chronic active hepatitis and antibodies to ribosomal P proteins in a patient with systemic lupus erythematosus. Arthritis Rheum. 1993;36:1325-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Koren E, Reichlin MW, Koscec M, Fugate RD, Reichlin M. Autoantibodies to the ribosomal P proteins react with a plasma membrane-related target on human cells. J Clin Invest. 1992;89:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Nagai T, Arinuma Y, Yanagida T, Yamamoto K, Hirohata S. Anti-ribosomal P protein antibody in human systemic lupus erythematosus up-regulates the expression of proinflammatory cytokines by human peripheral blood monocytes. Arthritis Rheum. 2005;52:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Hulsey M, Goldstein R, Scully L, Surbeck W, Reichlin M. Anti-ribosomal P antibodies in systemic lupus erythematosus: a case-control study correlating hepatic and renal disease. Clin Immunol Immunopathol. 1995;74:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Calich AL, Bonfa E. The anti-ribosomal P antibodies and prognosis in autoimmune hepatitis. Liver Int. 2014;34:324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | de Carvalho JF, Pereira RM, Shoenfeld Y. The mosaic of autoimmunity: the role of environmental factors. Front Biosci (Elite Ed). 2009;1:501-509. [PubMed] |
| 33. | Beuers U. Hepatic overlap syndromes. J Hepatol. 2005;42 Suppl:S93-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Irving KS, Sen D, Tahir H, Pilkington C, Isenberg DA. A comparison of autoimmune liver disease in juvenile and adult populations with systemic lupus erythematosus-a retrospective review of cases. Rheumatology (Oxford). 2007;46:1171-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Efe C, Purnak T, Ozaslan E, Ozbalkan Z, Karaaslan Y, Altiparmak E, Muratori P, Wahlin S. Autoimmune liver disease in patients with systemic lupus erythematosus: a retrospective analysis of 147 cases. Scand J Gastroenterol. 2011;46:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Oka H. The survey of autoimmune hepatitis in Japan. Tokyo: Japanese Ministry of Health and Welfare 1988; 235-241. |
| 37. | Tamai Y, Ito K, Kin F, Fukase M. American rheumatism association (ARA) preliminary criteria for the classification of systemic lupus erythematosus and autoimmune hepatitis. Rheumachi. 1974;14:88-94. |
| 38. | Leggett BA. The liver in systemic lupus erythematosus. J Gastroenterol Hepatol. 1993;8:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Usta Y, Gurakan F, Akcoren Z, Ozen S. An overlap syndrome involving autoimmune hepatitis and systemic lupus erythematosus in childhood. World J Gastroenterol. 2007;13:2764-2767. [PubMed] |
| 40. | Efe C, Ozaslan E, Nasiroglu N, Tunca H, Purnak T, Altiparmak E. The development of autoimmune hepatitis and primary biliary cirrhosis overlap syndrome during the course of connective tissue diseases: report of three cases and review of the literature. Dig Dis Sci. 2010;55:2417-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Czaja AJ, Morshed SA, Parveen S, Nishioka M. Antibodies to single-stranded and double-stranded DNA in antinuclear antibody-positive type 1-autoimmune hepatitis. Hepatology. 1997;26:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | González LA, Orrego M, Ramírez LA, Vásquez G. Primary biliary cirrhosis/autoimmune hepatitis overlap syndrome developing in a patient with systemic lupus erythematosus: a case report and review of the literature. Lupus. 2011;20:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Nachbar F, Korting HC, Hoffmann RM, Kollmann M, Meurer M. Unusual coexistence of systemic lupus erythematosus and primary biliary cirrhosis. Dermatology. 1994;188:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 45. | Agmon-Levin N, Shapira Y, Selmi C, Barzilai O, Ram M, Szyper-Kravitz M, Sella S, Katz BS, Youinou P, Renaudineau Y. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J Autoimmun. 2010;34:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Miyazaki T, Ono M, Qu WM, Zhang MC, Mori S, Nakatsuru S, Nakamura Y, Sawasaki T, Endo Y, Nose M. Implication of allelic polymorphism of osteopontin in the development of lupus nephritis in MRL/lpr mice. Eur J Immunol. 2005;35:1510-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Harada K, Ozaki S, Sudo Y, Tsuneyama K, Ohta H, Nakanuma Y. Osteopontin is involved in the formation of epithelioid granuloma and bile duct injury in primary biliary cirrhosis. Pathol Int. 2003;53:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Han S, Guthridge JM, Harley IT, Sestak AL, Kim-Howard X, Kaufman KM, Namjou B, Deshmukh H, Bruner G, Espinoza LR. Osteopontin and systemic lupus erythematosus association: a probable gene-gender interaction. PLoS One. 2008;3:e0001757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Efe C, Wahlin S, Ozaslan E, Berlot AH, Purnak T, Muratori L, Quarneti C, Yüksel O, Thiéfin G, Muratori P. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur J Gastroenterol Hepatol. 2012;24:531-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Muratori P, Granito A, Pappas G, Pendino GM, Quarneti C, Cicola R, Menichella R, Ferri S, Cassani F, Bianchi FB. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am J Gastroenterol. 2009;104:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Efe C, Purnak T, Ozaslan E, Wahlin S. The serological profile of the autoimmune hepatitis/primary biliary cirrhosis overlap syndrome. Am J Gastroenterol. 2010;105:226; author reply 226-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Oh DC, Ng TM, Ho J, Leong KP. Systemic lupus erythematosus with concurrent protein-losing enteropathy and primary sclerosing cholangitis: a unique association. Lupus. 2006;15:102-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Kadokawa Y, Omagari K, Matsuo I, Otsu Y, Yamamoto U, Nishino T, Ohba K, Miyazaki M, Harada T, Taguchi T. Primary sclerosing cholangitis associated with lupus nephritis: a rare association. Dig Dis Sci. 2003;48:911-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Audan A, Bruley Des Varannes S, Georgelin T, Sagan C, Cloarec D, Serraz H, Le Bodic L. [Primary sclerosing cholangitis and systemic lupus erythematosus]. Gastroenterol Clin Biol. 1995;19:123-126. [PubMed] |
| 55. | Lamy P, Valla D, Bourgeois P, Rueff B, Benhamou JP. [Primary sclerosing cholangitis and systemic lupus erythematosus]. Gastroenterol Clin Biol. 1988;12:962-964. [PubMed] |
| 56. | Bargellesi A, Vigi V, Conconi F. [Further research on the intracellular “pool” of alpha-globin in beta-thalassemia]. Boll Soc Ital Biol Sper. 1968;44:1666-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Ramos-Casals M, Font J, García-Carrasco M, Cervera R, Jiménez S, Trejo O, de la Red G, Sánchez-Tapias JM, Ingelmo M. Hepatitis C virus infection mimicking systemic lupus erythematosus: study of hepatitis C virus infection in a series of 134 Spanish patients with systemic lupus erythematosus. Arthritis Rheum. 2000;43:2801-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Ahmed MM, Berney SM, Wolf RE, Hearth-Holmes M, Hayat S, Mubashir E, Vanderheyde H, Chang WL, King JW. Prevalence of active hepatitis C virus infection in patients with systemic lupus erythematosus. Am J Med Sci. 2006;331:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Perlemuter G, Cacoub P, Sbaï A, Hausfater P, Thibault V, Le TH, Wechsler B, Buffet C, Piette JC. Hepatitis C virus infection in systemic lupus erythematosus: a case-control study. J Rheumatol. 2003;30:1473-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 60. | McMurray RW, Elbourne K. Hepatitis C virus infection and autoimmunity. Semin Arthritis Rheum. 1997;26:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Manns MP, Obermayer-Straub P. Viral induction of autoimmunity: mechanisms and examples in hepatology. J Viral Hepat. 1997;4 Suppl 2:42-47. [PubMed] |
| 62. | Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260-263. [PubMed] |
| 63. | Sansonno D, Cornacchiulo V, Iacobelli AR, Gatti P, Distasi M, Dammacco F. Hepatitis C virus infection and clonal B-cell expansion. Clin Exp Rheumatol. 1996;14 Suppl 14:S45-S50. [PubMed] |
| 64. | Fukuyama S, Kajiwara E, Suzuki N, Miyazaki N, Sadoshima S, Onoyama K. Systemic lupus erythematosus after alpha-interferon therapy for chronic hepatitis C: a case report and review of the literature. Am J Gastroenterol. 2000;95:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Danesh FR, Lynch P, Kanwar YS. Lupus membranous glomerulonephritis mimicking hepatitis C-associated nephropathy. Am J Kidney Dis. 2002;39:E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Albero MD, Rivera F, Merino E, Gil MT, Jimenez LA, Aranda I, Olivares J. Hepatitis C virus infection complicating lupus nephritis. Nephrol Dial Transplant. 1996;11:1342-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Toubi E, Gordon S, Kessel A, Rosner I, Rozenbaum M, Shoenfeld Y, Zuckerman E. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Vaiphei K, Bhatia A, Sinha SK. Liver pathology in collagen vascular disorders highlighting the vascular changes within portal tracts. Indian J Pathol Microbiol. 2011;54:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Takahaski C, Kumagai S, Tsubata R, Sorachi K, Ozaki S, Imura H, Nakao K. Portal hypertension associated with anticardiolipin antibodies in a case of systemic lupus erythematosus. Lupus. 1995;4:232-235. [PubMed] |
| 70. | Hubscher O, Elsner B. Nodular transformation of the liver in a patient with systemic lupus erythematosus. J Rheumatol. 1989;16:410-412. [PubMed] |
| 71. | Morlà RM, Ramos-Casals M, García-Carrasco M, Cervera R, Font J, Bruguera M, Rojas-Rodríguez J, Ingelmo M. Nodular regenerative hyperplasia of the liver and antiphospholipid antibodies: report of two cases and review of the literature. Lupus. 1999;8:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J Hepatol. 2012;56 Suppl 1:S25-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 73. | Foster JM, Litwin A, Gibbs JF, Intengen M, Kuvshinoff BW. Diagnosing regenerative nodular hyperplasia, the “great masquerader” of liver tumors. J Gastrointest Surg. 2006;10:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | Zech CJ, Seiderer J, Reinisch W, Ochsenkuhn T, Schima W, Diebold J, Wrba F, Reiser MF, Schoenberg SO. Thioguanin-induced nodular regenerative hyperplasia of the liver-ROC analysis of different MR techniques. Eur Radiol. 2007;17:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Laharie D, Vergniol J, Bioulac-Sage P, Diris B, Poli J, Foucher J, Couzigou P, Drouillard J, de Lédinghen V. Usefulness of noninvasive tests in nodular regenerative hyperplasia of the liver. Eur J Gastroenterol Hepatol. 2010;22:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Ames JT, Federle MP, Chopra K. Distinguishing clinical and imaging features of nodular regenerative hyperplasia and large regenerative nodules of the liver. Clin Radiol. 2009;64:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Haslock I. Spontaneous rupture of the liver in systemic lupus erythematosus. Ann Rheum Dis. 1974;33:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Bralet MP, Terris B, Vilgrain V, Brégeaud L, Molas G, Corbic M, Belghiti J, Fléjou JF, Degott C. Epithelioid hemangioendothelioma, multiple focal nodular hyperplasias, and cavernous hemangiomas of the liver. Arch Pathol Lab Med. 1999;123:846-849. [PubMed] |
| 79. | Berzigotti A, Frigato M, Manfredini E, Pierpaoli L, Mulè R, Tiani C, Zappoli P, Magalotti D, Malavolta N, Zoli M. Liver hemangioma and vascular liver diseases in patients with systemic lupus erythematosus. World J Gastroenterol. 2011;17:4503-4508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 365] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 81. | Folomeev M, Dougados M, Beaune J, Kouyoumdjian JC, Nahoul K, Amor B, Alekberova Z. Plasma sex hormones and aromatase activity in tissues of patients with systemic lupus erythematosus. Lupus. 1992;1:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Robak E, Woźniacka A, Sysa-Jedrzejowska A, Stepień H, Robak T. Serum levels of angiogenic cytokines in systemic lupus erythematosus and their correlation with disease activity. Eur Cytokine Netw. 2001;12:445-452. [PubMed] |
| 83. | Robak E, Woźniacka A, Sysa-Jedrzejowska A, Stepień H, Robak T. Circulating angiogenesis inhibitor endostatin and positive endothelial growth regulators in patients with systemic lupus erythematosus. Lupus. 2002;11:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | van Tuyll van Serooskerken AM, Habets JM, Badeloe S, Poblete-Gutiérrez P, Frank J. Porphyria cutanea tarda in pre-existent lupus erythematosus--is there an association. Int J Dermatol. 2007;46 Suppl 3:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 85. | Gibson GE, McEvoy MT. Coexistence of lupus erythematosus and porphyria cutanea tarda in fifteen patients. J Am Acad Dermatol. 1998;38:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Fritsch S, Wojcik AS, Schade L, Machota Junior MM, Brenner FM, Paiva Edos S. Increased photosensitivity Case report of porphyria cutanea tarda associated with systemic lupus erythematosus. Rev Bras Reumatol. 2012;52:968-970. [PubMed] |
| 87. | Haendchen L, Jordão JM, Haider O, Araújo F, Skare TL. Porphyria cutanea tarda and systemic lupus erythematosus. An Bras Dermatol. 2011;86:173-175. |
| 88. | Cram DL, Epstein JH, Tuffanelli DL. Lupus erythematosus and porphyria. Coexistence in seven patients. Arch Dermatol. 1973;108:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Bissell DM, Schmid R. Hepatic porphyrias. Disease of the Liver - 6th Ed. Philadelphia, Toronto: JB Lippincott 1987; 1075-1092. |
| 90. | Huang D, Aghdassi E, Su J, Mosko J, Hirschfield GM, Gladman DD, Urowitz MB, Fortin PR. Prevalence and risk factors for liver biochemical abnormalities in Canadian patients with systemic lupus erythematosus. J Rheumatol. 2012;39:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Lozovoy MA, Simão AN, Panis C, Rotter MA, Reiche EM, Morimoto HK, Lavado E, Cecchini R, Dichi I. Oxidative stress is associated with liver damage, inflammatory status, and corticosteroid therapy in patients with systemic lupus erythematosus. Lupus. 2011;20:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | McKinnon RA, Nebert DW. Possible role of cytochromes P450 in lupus erythematosus and related disorders. Lupus. 1994;3:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Kovacic P, Jacintho JD. Systemic lupus erythematosus and other autoimmune diseases from endogenous and exogenous agents: unifying theme of oxidative stress. Mini Rev Med Chem. 2003;3:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Horizon AA, Wallace DJ. Risk: benefit ratio of nonsteroidal anti-inflammatory drugs in systemic lupus erythematosus. Expert Opin Drug Saf. 2004;3:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Bessone F, Colombato L, Fassio E, Reggiardo MV, Vorobioff J, Tanno H. The spectrum of nimesulide-induced-hepatotoxicity. An overview. Anti-Inflamm Anti-Allergy Agents Med Chem. 2010;9:355-365. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Bessone F, Colombato L, Pasamonti ME, Godoy A, Vorobioff J, Tanno H. Nimesulide: clinical and histological evidences of severe hepatotoxicity. J Hepatol. 2001;34:46. |
| 97. | Bessone F, Tanno H. [Hepatotoxicity induced by non-steroidal anti-inflammatory drugs]. Gastroenterol Hepatol. 2000;23:200-205. [PubMed] |
| 98. | Banks AT, Zimmerman HJ, Ishak KG, Harter JG. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology. 1995;22:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Seaman WE, Ishak KG, Plotz PH. Aspirin-induced hepatotoxicity in patients with systemic lupus erythematosus. Ann Intern Med. 1974;80:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 98] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Travers RL, Hughes GR. Salicylate hepatotoxicity in systemic lupus erythematosus: a common occurrence. Br Med J. 1978;2:1532-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Zimmerman HJ. Effects of aspirin and acetaminophen on the liver. Arch Intern Med. 1981;141:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 73] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage. World J Gastroenterol. 2010;16:5651-5661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 186] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 103. | Russell AS, Sturge RA, Smith MA. Serum transaminases during salicylate therapy. Br Med J. 1971;2:428-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Rainsford KD. Side-effects and toxicology of the salicylates. Aspirin and Related Drugs. Boca Raton, Florida: CRC Press 2004; 367-554. |
| 105. | Teoh NC, Farrell GC. Hepatotoxicity associated with non-steroidal anti-inflammatory drugs. Clin Liver Dis. 2003;7:401-413. [PubMed] |
| 106. | De Santis M, Crotti C, Selmi C. Liver abnormalities in connective tissue diseases. Best Pract Res Clin Gastroenterol. 2013;27:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Cohen-Ezra O, Avni Y, Morgenstern S, Ben-Ari Z. [Nodular regenerative hyperplasia as a complication of thiopurine treatment in a patient with inflammatory bowel disease]. Harefuah. 2012;151:675-68, 721. [PubMed] |
| 108. | Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013;38:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | West SG. Methotrexate hepatotoxicity. Rheum Dis Clin North Am. 1997;23:883-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Phillips CA, Cera PJ, Mangan TF, Newman ED. Clinical liver disease in patients with rheumatoid arthritis taking methotrexate. J Rheumatol. 1992;19:229-233. [PubMed] |
| 111. | Erickson AR, Reddy V, Vogelgesang SA, West SG. Usefulness of the American College of Rheumatology recommendations for liver biopsy in methotrexate-treated rheumatoid arthritis patients. Arthritis Rheum. 1995;38:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 113. | Beyeler C, Reichen J, Thomann SR, Lauterburg BH, Gerber NJ. Quantitative liver function in patients with rheumatoid arthritis treated with low-dose methotrexate: a longitudinal study. Br J Rheumatol. 1997;36:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 114. | Grismer LE, Gill SA, Harris MD. Liver biopsy in psoriatic arthritis to detect methotrexate hepatotoxicity. J Clin Rheumatol. 2001;7:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 115. | Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, Bombardier C, Carmona L, van der Heijde D, Bijlsma JW. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 116. | Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb AB, Koo JY, Lebwohl M, Lim HW. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 117. | Barbero-Villares A, Mendoza J, Trapero-Marugan M, Gonzalez-Alvaro I, Daudén E, Gisbert JP, Moreno-Otero R. Evaluation of liver fibrosis by transient elastography in methotrexate treated patients. Med Clin (Barc). 2011;137:637-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Barbero-Villares A, Mendoza Jiménez-Ridruejo J, Taxonera C, López-Sanromán A, Pajares R, Bermejo F, Pérez-Calle JL, Mendoza JL, Algaba A, Moreno-Otero R. Evaluation of liver fibrosis by transient elastography (Fibroscan®) in patients with inflammatory bowel disease treated with methotrexate: a multicentric trial. Scand J Gastroenterol. 2012;47:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 119. | Berends MA, Snoek J, de Jong EM, Van Krieken JH, de Knegt RJ, van Oijen MG, van de Kerkhof PC, Drenth JP. Biochemical and biophysical assessment of MTX-induced liver fibrosis in psoriasis patients: Fibrotest predicts the presence and Fibroscan predicts the absence of significant liver fibrosis. Liver Int. 2007;27:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Fisher MC, Cronstein BN. Metaanalysis of methylenetetrahydrofolate reductase (MTHFR) polymorphisms affecting methotrexate toxicity. J Rheumatol. 2009;36:539-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Dávila-Fajardo CL, Swen JJ, Cabeza Barrera J, Guchelaar HJ. Genetic risk factors for drug-induced liver injury in rheumatoid arthritis patients using low-dose methotrexate. Pharmacogenomics. 2013;14:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Hadi NR, Al-Amran FG, Swadi A. Metformin ameliorates methotrexate-induced hepatotoxicity. J Pharmacol Pharmacother. 2012;3:248-253. [PubMed] |
| 123. | Sokolove J, Strand V, Greenberg JD, Curtis JR, Kavanaugh A, Kremer JM, Anofrei A, Reed G, Calabrese L, Hooper M. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1612-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 124. | Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, Serrano J, Rochon J, Fontana RJ, Bonacini M. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11:558-564.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 125. | Leak AM, Rincon-Aznar B. Hepatotoxicity associated with etanercept in psoriatic arthritis. J Rheumatol. 2008;35:2286-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 126. | Menghini VV, Arora AS. Infliximab-associated reversible cholestatic liver disease. Mayo Clin Proc. 2001;76:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 127. | Tobon GJ, Cañas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 128. | Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology (Oxford). 2009;48:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 129. | Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 516] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 130. | Seto W-K, Chan TSY, Hwang Y-Y, Choi O, Wong D, Fung J, Lie A K-W, Lai C-L, Kwong Y-L, Yuen M-F. Interim analysis of hepatitis B reactivation in patients with prior HBV exposure undergoing hematopoietic stem cell transplant: a prospective study. Hepatology. 2013;58:650A. [DOI] [Full Text] |
| 131. | Robinson H, Walker-Bone K. Anti-TNF-alpha therapy for rheumatoid arthritis among patients with chronic hepatitis B infection. Rheumatology (Oxford). 2009;48:448-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 132. | Lenert P, Icardi M, Dahmoush L. ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: case-based review. Clin Rheumatol. 2013;32:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 133. | Soubrier M, Mathieu S, Hermet M, Makarawiez C, Bruckert E. Do all lupus patients need statins. Joint Bone Spine. 2013;80:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Takada K, Illei GG, Boumpas DT. Cyclophosphamide for the treatment of systemic lupus erythematosus. Lupus. 2001;10:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 135. | Bacon AM, Rosenberg SA. Cyclophosphamide hepatotoxicity in a patient with systemic lupus erythematosus. Ann Intern Med. 1982;97:62-63. [PubMed] |
| 136. | Goldberg JW, Lidsky MD. Cyclophosphamide-associated hepatotoxicity. South Med J. 1985;78:222-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Subramaniam SR, Cader RA, Mohd R, Yen KW, Ghafor HA. Low-dose cyclophosphamide-induced acute hepatotoxicity. Am J Case Rep. 2013;14:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 138. | Her M, Lee Y, Jung E, Kim T, Kim D. Liver enzyme abnormalities in systemic lupus erythematosus: a focus on toxic hepatitis. Rheumatol Int. 2011;31:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |