Published online May 27, 2014. doi: 10.4254/wjh.v6.i5.263
Revised: January 15, 2014
Accepted: April 25, 2014
Published online: May 27, 2014
Processing time: 213 Days and 22.9 Hours
Obesity is a global epidemic contributing to an increasing prevalence of obesity-related systemic disorders, including nonalcoholic fatty liver disease. The rising prevalence of nonalcoholic steatohepatitis (NASH) will in the near future lead to end-stage liver disease in a large cohort of patients with NASH-related cirrhosis and NASH is predicted to be a leading indication for liver transplantation in the coming decade. However, the prevalence of obesity and the progression of hepatic histological damage associated with NASH exhibit significant ethnic disparities. Despite a significantly lower body mass index and lower rates of obesity compared to other ethnic groups, Asians continue to demonstrate a significant prevalence of hypertension, diabetes, metabolic syndrome and NASH. Ethnic disparities in central adiposity and visceral fat distribution have been hypothesized to contribute to these ethnic disparities. The current review focuses on the epidemiology of obesity and NASH among Asian populations.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is rapidly becoming a major contributor of chronic liver disease worldwide. The increasing prevalence of NAFLD among Asians reflects both an increasing awareness and diagnosis and the increasing risk of obesity and obesity-related diseases among this population. Ethnic disparities in the impact of weight gain on the development of obesity-related diseases is especially important for Asian populations, who have greater rates of central obesity and visceral deposition of fat and therefore are at greater risk of obesity-related diseases, such as NAFLD, at a lower body mass index.
- Citation: Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J Hepatol 2014; 6(5): 263-273
- URL: https://www.wjgnet.com/1948-5182/full/v6/i5/263.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i5.263
Non-alcoholic fatty liver disease (NAFLD) spans a spectrum of liver diseases that ranges from simple steatosis of the liver to progressive inflammation and fibrosis, resulting in non-alcoholic steatosis (NASH) and cirrhosis[1]. While the definition of NAFLD relies heavily on the clinical exclusion of significant alcoholic liver disease as well as other concomitant chronic liver diseases that can mimic similar histopathological features, one of the major hallmark features of NAFLD is the consistent association with type 2 diabetes mellitus, hypertension, hyperlipidemia and obesity[1-5]. The rising epidemic of obesity and obesity-related diseases in many post-industrialized countries has been accompanied by a concurrent rise in the prevalence of NAFLD. These emerging trends along with our better understanding of the pathophysiology of NAFLD clearly highlight the important role of obesity and obesity-related diseases in the increasing prevalence of NAFLD.
Several studies have reported the alarming increase in obesity and metabolic syndrome in western countries[6-12]. One recent large population-based study in the United States, utilizing data from the National Health and Nutrition Examination Surveys from 2009-2010 (NHANES), reported obesity rates of 35.5% among men and 35.8% among women[7]. Furthermore, population based studies utilizing United States census based data have demonstrated a concurrent rise in the prevalence of obesity-related diseases, such as hypertension and diabetes mellitus[13-18]. Among the same population, several studies have reported an increasing prevalence of NAFLD, suggesting that the rising rates of NAFLD are a consequence of the rising rates of obesity and metabolic syndrome in these populations. In fact, a recent study by Charlton et al[19] estimates that the rising prevalence of NAFLD in the United States population will soon lead to large cohorts of patients with decompensated cirrhosis from NASH and that NASH will soon become the leading indication for liver transplantation in the United States.
However, the epidemiology of obesity and obesity-related diseases demonstrates significant ethnic disparities. For example, several studies among both western and eastern cohorts demonstrate that Asians as a group consistently have a much lower body mass index (BMI) compared to other ethnic groups[20-23]. The relatively lower BMI is not protective in Asians. The rates of hypertension and diabetes mellitus, while somewhat lower, still continue to demonstrate rising trends among Asians[20]. In addition, cohort studies have demonstrated that despite having significantly lower BMI than other ethnic groups, Asians have a surprisingly high prevalence of NAFLD[24]. While not entirely elucidated, one emerging theory for this discrepancy between BMI and NAFLD prevalence may result from ethnic differences in the distribution of body fat, with more central adiposity and visceral fat deposition reported among individuals of Asian ethnicity[25-29]. Nevertheless, the increasing prevalence of obesity, metabolic syndrome and NAFLD among the Asian population will contribute to a large burden of chronic disease. The current paper reviews the concerning rise in obesity and NAFLD, with a focus on Asian populations.
The global obesity epidemic has been associated with the increasing burden of obesity-related diseases such as coronary artery diseases, hypertension and diabetes mellitus[9-12]. In addition, a link has been established between obesity and NAFLD such that obesity increases the risk of progression of hepatic inflammation and fibrosis leading to NASH-related cirrhosis. However, one emerging theme in the study of obesity is the ethnic disparities in the prevalence of obesity as well as the impact of weight gain on the overall risk of obesity-related diseases.
Several studies have reported ethnic disparities in the prevalence of obesity, with higher obesity rates in minority groups such as blacks and Hispanics[20,30-34]. However, Asians as a group generally have a lower BMI and lower prevalence of obesity compared to other ethnic groups[20-23]. Despite lower obesity prevalence, higher rates of metabolic syndrome have been reported in Asians compared to other ethnic groups at similar BMI levels[20]. These findings demonstrate that BMI thresholds for defining overweight and obesity should not be applied uniformly to all ethnic cohorts.
Current BMI categories set forth by the United States Centers for Disease Control and Prevention (BMI > 25 kg/m2 as overweight and BMI > 30 kg/m2 as obese) were intended to predict an individual’s risk of developing diseases associated with overweight and obese categories[35]. Two large population-based longitudinal studies, the San Antonio Heart Study and the Insulin Resistance Atherosclerosis Study, demonstrated a strong association of BMI with the risk of metabolic syndrome. Obese individuals (BMI > 30 kg/m2) were three to eight times more likely to develop metabolic syndrome compared to individuals with BMI < 25 kg/m2[31,36]. In addition, the association of obesity and metabolic syndrome with development of complications such as cardiovascular disease and diabetes mellitus is well established[37-40]. However, similar to ethnic disparities in the prevalence of obesity, the correlation of BMI with obesity-related diseases is not uniform across all ethnicities. For example, using data from NHANES, Palaniappan et al[30] demonstrated that fasting insulin levels, a marker of insulin sensitivity and risk of diabetes, was 19%-26% higher in blacks and 17-22% higher in Hispanics when compared to non-Hispanic whites with similar BMI. This disparity was also noted among Asians, with one study demonstrating significantly higher rates of metabolic syndrome in Asians compared to other ethnic groups with similar BMI. For example, Palaniappan et al[20] demonstrated that the predicted prevalence of metabolic syndrome in non-Hispanic white women aged 55 years with BMI 25 kg/m2 was 12% compared to 30% in Asians with similar demographics and BMI. Furthermore, compared to white men with BMI 25 kg/m2, comparable prevalence of metabolic syndrome was seen in Asian men with BMI 19.9 kg/m2.
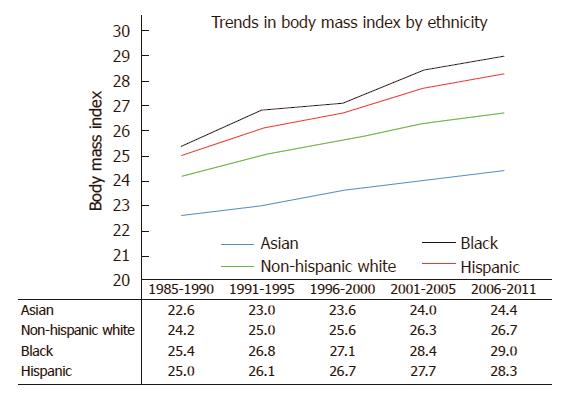
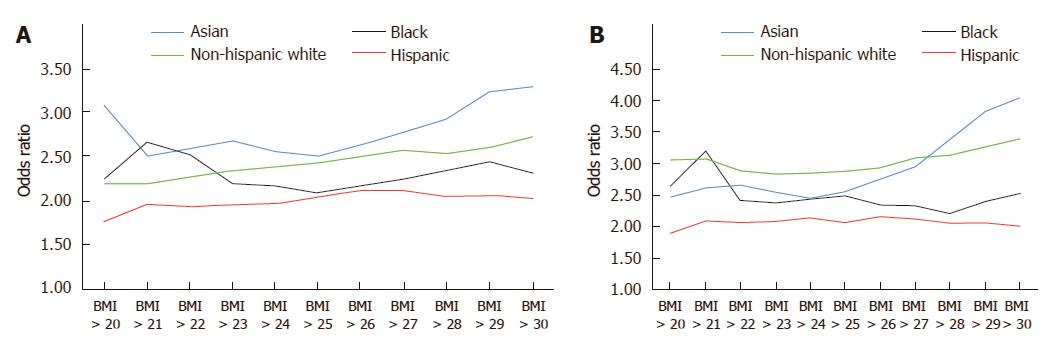
Using data from the California Department of Public Health and the United States Centers for Disease Control and Prevention, our group performed an in-depth analysis of ethnic disparities in obesity and obesity-related diseases with a focus on Asian populations. From 1985 to 2011, Asians as a group had the lowest BMI and lowest obesity prevalence (Asians: 22.6 ± 3.3 kg/m2 in 1985-1990 to 24.4 ± 4.3 kg/m2 in 2006-2011; non-Hispanic whites: 24.2 ± 4.1 kg/m2 in 1985-1990 to 26.7 ± 5.5 kg/m2 in 2006-2011; blacks: 25.4 ± 4.5 kg/m2 in 1985-1990 to 29.0 ± 6.9 kg/m2 in 2006-2011; Hispanics: 25.0 ± 4.1 kg/m2 in 1985-1990 to 28.3 ± 5.8 kg/m2 in 2006-2011) (Figure 1). Despite lower overall BMI, Asians had comparable or even higher rates of hypertension and diabetes mellitus compared to other ethnic groups. To evaluate whether weight gain as measured by BMI affected ethnic groups similarly, we created a multivariate logistic regression model to assess the effect of each one unit increase in BMI on the risk of hypertension or diabetes mellitus (Table 1). In our cohort model, each one unit increase in BMI was associated with 15% increased risk of hypertension in Asians, compared with 11% increase among non-Hispanic whites and 8% increase among blacks and Hispanics. When evaluating the impact of weight gain on the risk of diabetes mellitus, each one unit BMI was associated with 15% increased risk of diabetes mellitus among Asians, compared to 11% increase among non-Hispanic whites, 7% increase among blacks and 8% increase among Hispanics. These data suggest that despite having lower BMI, weight gain as measured by BMI disproportionately affects Asians to a greater degree. Furthermore, similar risks of hypertension and diabetes mellitus among non-Hispanic whites and blacks were seen in Asians at significantly lower BMI. For example, risks of hypertension among Asians with BMI > 22 kg/m2 were similar to non-Hispanic whites with BMI > 27 kg/m2 and blacks with BMI > 28 kg/m2 (Figure 2). While many theories have been proposed to explain these disparities, ethnic differences in body fat distribution may be a major contributing factor. Previous studies evaluating the correlation of BMI with percentage body fat demonstrated that blacks generally have more lean mass and less fat mass compared to whites. In contrast, Asians have more central adiposity and visceral fat distribution, which carries a greater risk of developing cardiovascular and metabolic diseases[25-29].
| Hypertension | Diabetes | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Asian | 1.15 | 1.13-1.18 | < 0.001 | 1.15 | 1.13-1.18 | < 0.001 |
| Non-Hispanic White | 1.11 | 1.10-1.11 | < 0.001 | 1.11 | 1.11-1.12 | < 0.001 |
| Black | 1.08 | 1.07-1.10 | < 0.001 | 1.07 | 1.06-1.09 | < 0.001 |
| Hispanic | 1.08 | 1.07-1.09 | < 0.001 | 1.08 | 1.07-1.09 | < 0.001 |
Acknowledging these disparities, several studies have suggested that current thresholds for defining obesity and overweight in Asians may not accurately reflect the risk of developing obesity-related diseases and BMI thresholds should be lowered for Asian cohorts[41,42]. In 2000, the World Health Organization Western Pacific Regional Office proposed a lower cutoff of BMI > 25 kg/m2 for obesity in Asian populations[43]. Several Asian countries have begun to adopt this modified BMI categorization[44-46]. Additional studies have attempted to incorporate additional anthropometric tools to better stratify the risk of metabolic diseases among Asians. Using a cross sectional population-based survey study of 2947 patients in China, Shao et al[47] demonstrated that waist-height ratio was significantly better at predicting risk of metabolic syndrome than BMI or waist circumference alone. Liu et al[48] performed a similar evaluation among a cross sectional cohort of 772 Chinese patients. BMI, waist circumference and waist-hip ratio were found to have similar predictive power for risk of metabolic diseases, such as hypertension, diabetes mellitus and dyslipidemia. While solely relying on BMI to predict risk of obesity-related diseases such as NAFLD has several limitations, these additional complementary anthropometric tools may improve risk stratification.
While studies in western populations clearly indicate that NASH will be a leading cause of chronic liver disease, less is known about the epidemiology of NAFLD among Asian populations. Recent community-based studies from Asian countries, including Japan, China, Taiwan, and Korea, indicate that the overall NAFLD prevalence reaches as high as 45% with year-specific analyses, demonstrating a continued rise in NAFLD prevalence with time[49-55]. Additional studies from the Asia-Pacific region demonstrated similar trends of NAFLD prevalence in India, Malaysia, Singapore and Indonesia[56-62]. Wong et al[63] performed a large cross-sectional study in Hong Kong to assess the community prevalence of NAFLD using proton nuclear magnetic resonance (p-NMR) spectroscopy. A total of 922 patients randomly selected from the Hong Kong census database without chronic liver disease completed a full clinical assessment. Among this cohort, p-NMR was utilized to measure intrahepatic triglyceride content with a cutoff of 5% used to distinguish patients with and without fatty liver disease. Transient elastography was also utilized to assess for hepatic fibrosis with a cutoff of 9.6 kPa to define advanced fibrosis. Overall, the cohort was 42.2% men and average BMI was 22.8 ± 3.5 kg/m2. A total of 264 patients (26.8%) met the cutoff for diagnosis of fatty liver disease. Average BMI among the fatty liver disease cohort was 25.3 ± 3.4 kg/m2 and among the non-fatty liver disease cohort was 21.8 ± 3.0 kg/m2. Prevalence of advanced fibrosis was 3.7% (n = 8) and 1.3% (n = 7) among fatty liver and non-fatty liver cohorts, respectively. A similar study was performed in Shanghai that included 3175 adults that assessed for prevalence of metabolic syndrome using criteria from the National Cholesterol Education Program - Adult Treatment Panel III and for fatty liver with ultrasonography[64]. Overall, 22.9% and 20.8% of individuals had metabolic syndrome and fatty liver, respectively. The risk for fatty liver was increased among patients with abdominal obesity (waist circumference > 90 cm in men and > 80 cm in women: 32.8-fold increase), diabetes mellitus (31.6-fold increase), dyslipidemia (22.6-fold increase) and hypertension (22.3-fold). Patients that met the diagnostic threshold for metabolic syndrome had a nearly 40 times increased risk for fatty liver. When stratified by BMI, those with fatty liver disease and BMI < 25 kg/m2 had 36.1% prevalence of metabolic syndrome. Furthermore, the presence of fatty liver disease was found to have the best positive predictive value and attributable risk percentage in detecting risk factor clustering for metabolic syndrome.
Variations in fat distribution have been implicated as one potential reason for disparate associations between BMI and NAFLD prevalence among Asian populations. It has been previously reported that the percent body fat as well as fat distribution differs significantly among Asian and non-Asian populations, such that greater central and visceral adiposity is commonly seen in Asians[25-29]. It has also been implied that as a result of this disparate distribution of fat, excessive amounts of visceral adipose tissue may occur in Asians not overweight or obese using BMI cutoffs. Greater central adiposity distribution is associated with higher risks of cardiovascular disease and metabolic syndrome[65-67]. Furthermore, ideal body weight may be different among different ethnicities and different world regions, such that while an individual does not meet BMI threshold for obesity, he may be significantly heavier than ideal body weight, and this translates into increased risk of insulin resistance and metabolic syndrome[68-70]. For example, Chang et al[68] performed a prospective South Korean study of 15,347 men to assess ultrasound-based diagnosis of fatty liver disease. Even among men with BMI 18.5-22.9, mild weight gains of 0.6 to 2.3 kg were associated with 38%-73% increase in the risk for fatty liver disease. This phenomena, termed “metabolically obese”, namely the increased risk of insulin resistance, metabolic syndrome and NAFLD despite normal or lean BMI, has been more commonly seen in Asian populations[68-70].
Another potential theory that may partially contribute to the rising prevalence of NAFLD among Asian populations centers on the role of diet. Carbohydrates in the form of rice are a central component of the Asian diet. However, significant amounts of carbohydrates in the diet can lead to accumulation of triglycerides within the liver, which is mediated by glucose stimulated activation of the liver transcription factor, carbohydrate responsive element-binding protein (ChREBP). This process over time leads to significant hepatic steatosis and eventual progression of disease towards NASH[71-73]. However, the impact of ChREBP on hepatic steatosis among individuals with significant carbohydrate exposure may not necessarily correlate with development of insulin resistance. A recent study by Benhamed et al[74] evaluated ChREBP over expressing mice fed a standard diet, demonstrating that despite having increased expression of genes involved in lipogenesis/fatty acid esterification and resultant hepatic steatosis, the mice remained insulin sensitive. In addition, ChREBP over expressing mice fed a high-fat diet also showed normal insulin levels and improved insulin signaling and glucose tolerance compared with controls, despite having greater hepatic steatosis.
The progression of inflammation and fibrosis in patients with NAFLD is not believed to differ significantly by ethnicity. However, some earlier studies have suggested that NAFLD may be less severe with slower progression among Asian populations[75,76]. This hypothesis is complicated by several potential confounding factors. NAFLD is a relatively more recent phenomenon in Asian countries and the expected progression of disease leading to cirrhosis may occur over the next several decades. Thus, the emergence of fatty liver disease observed in the recent era in Asian populations probably lags behind western populations by several decades and the impact of large cohorts of patients with chronic liver disease and cirrhosis from NASH is expected to flood our health care system in the coming years. Another potential contributing factor is the increasing awareness and subsequent diagnosis of NAFLD among these Asian Pacific regions. Furthermore, the previously reported disparate association between BMI and metabolic syndrome that results from ethnic disparities in central adiposity and visceral fat distribution may alter the natural history of NAFLD among this population.
Despite these potential caveats, it is generally agreed that the progression of disease among patients with simple steatosis is slow compared with other diseases, such as hepatitis C virus (HCV), whereas patients with histological evidence of NASH can progress more rapidly towards advanced fibrosis and cirrhosis[1,2,77]. Long-term longitudinal studies have demonstrated increased mortality among patients with both NAFLD and NASH when compared to controls without underlying liver disease[78-86]. Interestingly, the most common cause of death among patients with NAFLD and NASH was cardiovascular diseases, reflecting the close correlations of NAFLD with metabolic syndrome and cardiovascular disease outcomes. However, simple steatosis is not always benign and progression of disease, while slow, can occur. In a single centered Hong Kong cohort, Wong et al[87] conducted a prospective longitudinal study of 52 patients with biopsy proven NAFLD. Among patients with simple steatosis on histology at baseline (n = 13), 15% had normal histology, 23% still had simple steatosis and 62% had evidence of histological progression towards NASH at 36 mo. While the small sample size may limit the generalization of these findings, this study raises awareness of the dynamic nature of steatosis and that simple steatosis is not necessarily benign and may warrant closer follow up.
However, progression of NAFLD to NASH-related cirrhosis is clearly associated with increased risks of hepatic decompensation and liver-related mortality[88-91]. Hui et al[88] performed a prospective longitudinal cohort study of 23 patients with clinically/pathologically confirmed NASH-related cirrhosis compared with 46 age and gender matched HCV-related cirrhosis patients. Over a median follow up of 60 mo (range 5-177 mo), 9/23 NASH-related cirrhosis patients developed hepatic decompensation (8 with ascites or encephalopathy, 1 with variceal bleeding). The overall survival at 1, 3, and 10 years was 95%, 90% and 84%, respectively. After multivariate regression modeling, there was no significant survival difference between the NASH-related cirrhosis and HCV-related cirrhosis cohorts. A larger United States study compared 152 patients with NASH-related cirrhosis to 150 matched patients with HCV-related cirrhosis[89]. Over 10 years of follow up, NASH patients had significantly lower mortality compared to HCV patients but this mortality difference was primarily seen in patients with Child Pugh Turcotte (CPT) class A cirrhosis. Among patients with CPT class A cirrhosis, NASH patients had significantly lower rates of hepatic decompensation, development of ascites and hepatocellular carcinoma (HCC). Similar findings were reported in a large multi-center international study of 247 patients with advanced fibrosis or cirrhosis secondary to NASH compared to 264 chronic HCV patients with similar stages of fibrosis[91]. Among the NASH cohort, there were 19.4% liver-related complications and 13.4% deaths or liver transplantation over a mean follow up of 85.6 mo. Among the HCV cohort, there were 16.7% liver-related complications and 9.4% deaths or liver transplantations over a mean follow up of 74.9 mo. After adjusting for differences in baseline characteristics, cumulative incidence of liver-related complications was significantly lower in the NASH group compared to the HCV group. However, the incidence of cardiovascular events and overall mortality was not significantly different between NASH and HCV cohorts. The results of these studies indicate that while progression of NAFLD towards NASH cirrhosis is clearly associated with increased risks of hepatic decompensation and mortality, these increased risks may not be as high as that seen among the cohort of chronic HCV cirrhosis patients.
While the risks of HCC from chronic liver disease secondary to hepatitis B and HCV are better defined, the risk of HCC among patients with NASH is less well known. NASH-related HCC occurs primarily in the setting of hepatic cirrhosis[1,92-95]. A large retrospective cohort study from South Korea evaluated 329 patients with fatty liver disease associated HCC and demonstrated an increase in NAFLD-related HCC from 3.8% in 2001-2005 to 12.2% in 2006-2010[96]. A United States based study evaluated 195 NASH-cirrhosis patients from 2003-2007 with serial abdominal computed tomography and serum alpha-fetoprotein every 6 mo with a median follow up of 3.2 years[97]. Among this cohort for NASH-related cirrhosis patients, 12.8% (n = 25) developed HCC with an annual cumulative HCC incidence of 2.6%. Several additional studies, both in western and Asia-Pacific regions, report on the progression of NASH-related cirrhosis towards HCC but this rate of progression is significantly lower than that seen among patients with cirrhosis secondary to chronic HCV. Yasui et al[98] prospectively evaluated 412 NAFLD patients from 1990 to 2006. Among this cohort, 68 patients with NASH-related cirrhosis were compared with 69 age and sex matched HCV-related cirrhosis patient controls to determine HCC risk. Overall, the 5-year cumulative HCC rate was 11.3% for NASH patients and 30.5% for HCV patients. This lower HCC risk among NASH-related cirrhosis patients compared with HCV-related cirrhosis patients was confirmed in additional studies.
While the majority of NASH-related HCC occurs in patients with cirrhosis, several studies have reported HCC development among non-cirrhotic NASH patients, with one Japanese study reporting rates of non-cirrhotic NASH-related HCC ranging from 10%-75% of cases[97-101]. The exact etiology for this non-cirrhotic pathway towards HCC is unclear. However, studies have demonstrated that obesity and diabetes mellitus, both of which are closely associated with NAFLD, are independently associated with increased risk of HCC among patients with chronic liver disease[102-104]. Furthermore, Welzel et al[105] utilized the National Cancer Institute’s Surveillance, Epidemiology and End Results-Medicare database to evaluate the impact of metabolic syndrome on overall HCC risk among the general United States population. Among a cohort of 3649 HCC cases and 195953 comparison cohort, metabolic syndrome (as defined by National Cholesterol Education Program Adult Treatment Panel III criteria) was associated with a significantly increased risk of HCC (OR = 2.13, 95%CI: 1.96-2.13, P < 0.0001).
The implications of these findings on HCC screening among NAFLD patients are a major public health issue. While more studies evaluating the long-term HCC risk among patients with NASH-related cirrhosis are needed, it is reasonable to implement standard HCC screening programs in this cohort as one would for patients with cirrhosis from other chronic liver disease etiologies. However, as with other chronic liver disease etiologies, only a fraction of NASH-related cirrhosis patients will develop HCC and the ability to better define the cohort of patients from those who will not develop HCC will be especially important in the management of this group of patients. More studies are needed to investigate risk factors for HCC development among this cohort that will allow a more targeted approach towards risk stratifications and earlier detection and treatment of HCC. However, the increasingly reported cases of HCC among non-cirrhotic NAFLD patients introduce an unexpected component to the commonly accepted pathogenesis of HCC. Clearly, these patients do not carry the same HCC risk as those patients with non-cirrhotic hepatitis B infection. However, what distinguishes those patients with non-cirrhotic NAFLD that develop HCC from those that do not? What are the important risk factors that should be incorporated into risk stratification models? How should HCC screening programs be implemented among this cohort? More studies are needed to better understand the risk factors associated with HCC development among NASH patients with and without cirrhosis.
The increasing prevalence of patients with NASH who develop cirrhosis and decompensated liver disease will undoubtedly lead to a major increase in the number of patients on the waiting list for liver transplantation. Several studies have already predicted that as a result of the obesity epidemic, the rising rates of NASH will become a leading indication for liver transplantation (Table 2)[19,106-108]. A recent study by Charlton et al[19] retrospectively evaluated liver transplantations in the United States from 2001-2009 utilizing a national liver transplantation database. This study demonstrated a significant increase in the proportion of patients undergoing liver transplantation for NASH from 1.2% in 2001 to 9.7% in 2009, making NASH the third leading indication for liver transplantation. Furthermore, the trajectory of increasing prevalence of NASH among liver transplantation recipients indicates that it will soon become the leading indication for liver transplantation. It has also been suggested that our current estimation of NASH prevalence is an underestimation, as many patients with cirrhosis secondary to cryptogenic cirrhosis may in fact be more accurately categorized as NASH. This hypothesis is supported by evidence demonstrating that cryptogenic cirrhosis patients share many similar characteristics to NASH patients, including risk factors associated with metabolic syndrome, and many patients with cryptogenic cirrhosis can in fact be more accurately categorized as NASH[109-113]. Furthermore, the outcomes associated with cryptogenic cirrhosis are also similar to those seen among patients with NASH[110-113]. Clearly, the rising prevalence of obesity and NASH patients who develop decompensated liver disease will soon become a significant cohort impacting the liver transplantation waiting list.
| Liver disease etiology | Pre-MELD (1992-2002) | Post-MELD (2003-2007) | Post-MELD (2008-2012) |
| Acute liver failure | 2390 (7.9) | 1639 (6.9) | 1285 (5.1) |
| Chronic HCV | 9248 (30.7) | 7970 (33.5) | 7803 (31.2) |
| Chronic HBV | 1419 (4.7) | 802 (3.4) | 604 (2.4) |
| HCC | 466 (1.6) | 1714 (7.2) | 3423 (13.7) |
| ALD | 5027 (16.7) | 3704 (15.6) | 3636 (14.6) |
| ALD + HCV | 2495 (8.3) | 1845 (7.8) | 1529 (6.1) |
| NASH | 8 (0.1) | 796 (3.3) | 2162 (8.7) |
| AIH | 1277 (4.2) | 715 (3.0) | 693 (2.8) |
| Cryptogenic | 3460 (11.5) | 2115 (8.9) | 1634 (6.5) |
| PBC | 1992 (6.6) | 1009 (4.2) | 795 (3.2) |
| PSC | 1648 (5.5) | 1 (4.3) | 922 (3.7) |
| Metabolic | 729 (2.4) | 478 (2.0) | 491 (2.0) |
In Asia-Pacific regions, viral hepatitis and hepatocellular carcinoma are the leading indications for liver transplantation. Furthermore, unlike western countries, living donor liver transplantations play a more significant role in liver transplantation surgeries[114-116]. With the continued rising prevalence of NAFLD and NASH in this region, NASH may soon become a leading contributor of end stage liver disease and need for liver transplantation in the Asia-Pacific regions.
The global obesity epidemic is associated with the increasing prevalence of metabolic syndrome and NAFLD. This phenomenon will contribute to an increasingly large cohort of patients that will develop NASH-related cirrhosis, decompensated liver disease and HCC. The emergence of this cohort is on the horizon and will introduce a significant disease burden in the field of liver transplantation. However, there are significant ethnic disparities in the prevalence and association of obesity with development of NASH. Furthermore, it is not clear if the risk factors associated with development of NASH and progression to cirrhosis and HCC vary by ethnicity. Our current focus on Asian populations clearly indicate that despite having lower average BMI, Asians as a group still maintain significant risks of metabolic syndrome and NAFLD, resulting primarily from the disparately higher central adiposity and visceral fat distribution seen in this cohort. This may further contribute to relatively increased risk of NASH development. More studies are needed to identify factors that influence the ethnicity-dependent rate of hepatic histological damage and the risk of HCC in NASH patients.
P- Reviewers: Ahmed M, Chuang WL, Czaja MJ, Laguna JC, Peltec A, Zelber-Sagi S S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 2. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2288] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 3. | Colicchio P, Tarantino G, del Genio F, Sorrentino P, Saldalamacchia G, Finelli C, Conca P, Contaldo F, Pasanisi F. Non-alcoholic fatty liver disease in young adult severely obese non-diabetic patients in South Italy. Ann Nutr Metab. 2005;49:289-295. [PubMed] |
| 4. | Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg. 2003;138:1240-1244. [PubMed] |
| 5. | Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2774] [Cited by in RCA: 2668] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 7. | Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3909] [Cited by in RCA: 3854] [Article Influence: 296.5] [Reference Citation Analysis (0)] |
| 8. | Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in children and adolescents: United States, 2005-2008. NCHS Data Brief. 2010;1-8. [PubMed] |
| 9. | Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4734] [Cited by in RCA: 4471] [Article Influence: 298.1] [Reference Citation Analysis (0)] |
| 10. | Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39-47. [PubMed] |
| 11. | Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-778. [PubMed] |
| 12. | Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529. [PubMed] |
| 13. | Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 348] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683-689. [PubMed] |
| 15. | Kiernan M, Winkleby MA. Identifying patients for weight-loss treatment: an empirical evaluation of the NHLBI obesity education initiative expert panel treatment recommendations. Arch Intern Med. 2000;160:2169-2176. [PubMed] |
| 16. | Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893-2898. [PubMed] |
| 17. | Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605-619. [PubMed] |
| 18. | Drøyvold WB, Midthjell K, Nilsen TI, Holmen J. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes (Lond). 2005;29:650-655. [PubMed] |
| 19. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 854] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 20. | Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond). 2011;35:393-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Zhang H, Rodriguez-Monguio R. Racial disparities in the risk of developing obesity-related diseases: a cross-sectional study. Ethn Dis. 2012;22:308-316. [PubMed] |
| 22. | Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60:23-28. [PubMed] |
| 23. | Jain A, Mitchell S, Chirumamilla R, Zhang J, Horn IB, Lewin A, Huang ZJ. Prevalence of obesity among young Asian-American children. Child Obes. 2012;8:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 25. | Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 550] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 26. | Aloia JF, Vaswani A, Mikhail M, Flaster ER. Body composition by dual-energy X-ray absorptiometry in black compared with white women. Osteoporos Int. 1999;10:114-119. [PubMed] |
| 27. | Fernández JR, Heo M, Heymsfield SB, Pierson RN, Pi-Sunyer FX, Wang ZM, Wang J, Hayes M, Allison DB, Gallagher D. Is percentage body fat differentially related to body mass index in Hispanic Americans, African Americans, and European Americans? Am J Clin Nutr. 2003;77:71-75. [PubMed] |
| 28. | Rahman M, Temple JR, Breitkopf CR, Berenson AB. Racial differences in body fat distribution among reproductive-aged women. Metabolism. 2009;58:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Du T, Sun X, Yin P, Huo R, Ni C, Yu X. Increasing trends in central obesity among Chinese adults with normal body mass index, 1993-2009. BMC Public Health. 2013;13:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Palaniappan LP, Carnethon MR, Fortmann SP. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002;25:1351-1357. [PubMed] |
| 31. | Palaniappan L, Carnethon MR, Wang Y, Hanley AJ, Fortmann SP, Haffner SM, Wagenknecht L. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27:788-793. [PubMed] |
| 32. | Kershaw KN, Albrecht SS, Carnethon MR. Racial and ethnic residential segregation, the neighborhood socioeconomic environment, and obesity among Blacks and Mexican Americans. Am J Epidemiol. 2013;177:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Jackson CL, Szklo M, Yeh HC, Wang NY, Dray-Spira R, Thorpe R, Brancati FL. Black-white disparities in overweight and obesity trends by educational attainment in the United States, 1997-2008. J Obes. 2013;2013:140743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr. 2013;167:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 35. | Centers for Disease Control and Prevention. Overweight and Obesity. Department of Health and Human Services; Atlanta, GA: 2009. Available from: http: //www.cdc.gov/obesity/. |
| 36. | Han TS, Williams K, Sattar N, Hunt KJ, Lean ME, Haffner SM. Analysis of obesity and hyperinsulinemia in the development of metabolic syndrome: San Antonio Heart Study. Obes Res. 2002;10:923-931. [PubMed] |
| 37. | Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070-1077. [PubMed] |
| 38. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] |
| 39. | Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210-1214. [PubMed] |
| 40. | Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the atherosclerosis risk in communities study: 1987-1998. Diabetes Care. 2002;25:1358-1364. [PubMed] |
| 41. | James WP, Chunming C, Inoue S. Appropriate Asian body mass indices? Obes Rev. 2002;3:139. [PubMed] |
| 42. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [PubMed] |
| 43. | World Health Organization Western Pacific Region. International Association for the Study of Obesity and the International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia Pty Limited 2000; . |
| 44. | Ko GT, Chan JC. Burden of obesity--lessons learnt from Hong Kong Chinese. Obes Rev. 2008;9 Suppl 1:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Huang KC. Obesity and its related diseases in Taiwan. Obes Rev. 2008;9 Suppl 1:32-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Misra A, Khurana L. Obesity-related non-communicable diseases: South Asians vs White Caucasians. Int J Obes (Lond). 2011;35:167-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 288] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 47. | Shao J, Yu L, Shen X, Li D, Wang K. Waist-to-height ratio, an optimal predictor for obesity and metabolic syndrome in Chinese adults. J Nutr Health Aging. 2010;14:782-785. [PubMed] |
| 48. | Liu Y, Tong G, Tong W, Lu L, Qin X. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health. 2011;11:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26 Suppl 1:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Lee K. Relationship between uric acid and hepatic steatosis among Koreans. Diabetes Metab. 2009;35:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138-143. [PubMed] |
| 52. | Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol. 2009;50:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 53. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 54. | Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. J Gastroenterol Hepatol. 2007;22:1482-1489. [PubMed] |
| 55. | Tsai CH, Li TC, Lin CC. Metabolic syndrome as a risk factor for nonalcoholic fatty liver disease. South Med J. 2008;101:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, Dhibar T, Bhattacharya B, Bhattacharya D, Manna B. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 58. | Dassanayake AS, Kasturiratne A, Rajindrajith S, Kalubowila U, Chakrawarthi S, De Silva AP, Makaya M, Mizoue T, Kato N, Wickremasinghe AR. Prevalence and risk factors for non-alcoholic fatty liver disease among adults in an urban Sri Lankan population. J Gastroenterol Hepatol. 2009;24:1284-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Pinidiyapathirage MJ, Dassanayake AS, Rajindrajith S, Kalubowila U, Kato N, Wickremasinghe AR, de Silva HJ. Non-alcoholic fatty liver disease in a rural, physically active, low income population in Sri Lanka. BMC Res Notes. 2011;4:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Magosso E, Ansari MA, Gopalan Y, Abu Bakar MR, Karim Khan NA, Wong JW, Ng BH, Yuen KH, Lutfi Shuaib I, Nesaretnam K. Prevalence of non-alcoholic fatty liver in a hypercholesterolemic population of northwestern peninsular Malaysia. Southeast Asian J Trop Med Public Health. 2010;41:936-942. [PubMed] |
| 61. | Malik A, Cheah PL, Hilmi IN, Chan SP, Goh KL. Non-alcoholic fatty liver disease in Malaysia: a demographic, anthropometric, metabolic and histological study. J Dig Dis. 2007;8:58-64. [PubMed] |
| 62. | Chow WC, Tai ES, Lian SC, Tan CK, Sng I, Ng HS. Significant non-alcoholic fatty liver disease is found in non-diabetic, pre-obese Chinese in Singapore. Singapore Med J. 2007;48:752-757. [PubMed] |
| 63. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 64. | Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, Dai F, Li F, Chen SY. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol. 2005;20:1825-1832. [PubMed] |
| 65. | Lee YS, Kek BL, Poh LK, Saw SM, Loke KY. Association of raised liver transaminases with physical inactivity, increased waist-hip ratio, and other metabolic morbidities in severely obese children. J Pediatr Gastroenterol Nutr. 2008;47:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012;27:1555-1560. [PubMed] [DOI] [Full Text] |
| 67. | Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681-1688. [PubMed] |
| 68. | Chang Y, Ryu S, Sung E, Woo HY, Cho SI, Yoo SH, Ahn HY, Choi NK. Weight gain within the normal weight range predicts ultrasonographically detected fatty liver in healthy Korean men. Gut. 2009;58:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372-379. [PubMed] |
| 70. | Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, Bass N. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 71. | Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159-2170. [PubMed] |
| 72. | Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281-7286. [PubMed] |
| 73. | Ma L, Tsatsos NG, Towle HC. Direct role of ChREBP.Mlx in regulating hepatic glucose-responsive genes. J Biol Chem. 2005;280:12019-12027. [PubMed] |
| 74. | Benhamed F, Denechaud PD, Lemoine M, Robichon C, Moldes M, Bertrand-Michel J, Ratziu V, Serfaty L, Housset C, Capeau J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012;122:2176-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 75. | Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011;26 Suppl 1:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788-793. [PubMed] |
| 77. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 916] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 78. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] |
| 79. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] |
| 80. | Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750-755. [PubMed] |
| 81. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [PubMed] |
| 82. | Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 83. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 84. | Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 85. | Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 86. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 561] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 87. | Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 489] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 88. | Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420-427. [PubMed] |
| 89. | Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682-689. [PubMed] |
| 90. | Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 92. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] |
| 93. | Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291-293. [PubMed] |
| 95. | Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436-1441. [PubMed] |
| 96. | Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, Yoon JH, Lee HS. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non-B non-C hepatocellular carcinoma in a hepatitis B-endemic area. Digestion. 2011;84 Suppl 1:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 959] [Article Influence: 63.9] [Reference Citation Analysis (1)] |
| 98. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433; quiz e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 99. | Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma in nonalcoholic fatty liver cirrhosis and alcoholic cirrhosis: risk factor analysis in liver transplant recipients. Eur J Gastroenterol Hepatol. 2012;24:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1012] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 102. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [PubMed] |
| 103. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 104. | Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 105. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 106. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] |
| 107. | Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133:1814-1820. [PubMed] |
| 108. | Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048-1058. [PubMed] |
| 109. | Charlton M, Kasparova P, Weston S, Lindor K, Maor-Kendler Y, Wiesner RH, Rosen CB, Batts KP. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608-614. [PubMed] |
| 110. | Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, Berg CL. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:346-352. [PubMed] |
| 111. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [PubMed] |
| 112. | Maor-Kendler Y, Batts KP, Burgart LJ, Wiesner RH, Krom RA, Rosen CB, Charlton MR. Comparative allograft histology after liver transplantation for cryptogenic cirrhosis, alcohol, hepatitis C, and cholestatic liver diseases. Transplantation. 2000;70:292-297. [PubMed] |
| 113. | Sutedja DS, Gow PJ, Hubscher SG, Elias E. Revealing the cause of cryptogenic cirrhosis by posttransplant liver biopsy. Transplant Proc. 2004;36:2334-2337. [PubMed] |
| 114. | Nayak NC, Jain D, Vasdev N, Gulwani H, Saigal S, Soin A. Etiologic types of end-stage chronic liver disease in adults: analysis of prevalence and their temporal changes from a study on native liver explants. Eur J Gastroenterol Hepatol. 2012;24:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Egawa H, Tanabe K, Fukushima N, Date H, Sugitani A, Haga H. Current status of organ transplantation in Japan. Am J Transplant. 2012;12:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 116. | Chen CL, Concejero AM, Cheng YF. More than a quarter of a century of liver transplantation in Kaohsiung Chang Gung Memorial Hospital. Clin Transpl. 2011;213-221. [PubMed] |