INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cancer diagnosed world-wide, with 250000 to 500000 cases diagnosed per year, and it is the third leading cause of cancer-related death in the world[1]. In the United States, the incidence of HCC has nearly tripled over the past 3 decades, with approximately 20000 new cases diagnosed annually, largely owing to the growing incidence of chronic Hepatitis C-related cirrhosis. HCC is associated with a poor prognosis, with 5-year survival rate persistently less than 10%. It is potentially curable by surgery or liver transplantation if detected early. Unfortunately, over 85% of cases are diagnosed at late stages when surgical intervention is no longer a viable option. The only available systemic treatment is a multi-targeted kinase inhibitor, sorafenib. In randomized, placebo-controlled phase III clinical trials, sorafenib modestly improves overall survival (OS) for patients with intermediate to advanced stage HCC[2,3]. An effective second-line agent for those with sorafenib failure or intolerance has yet to be identified. This has led to an ongoing search for molecular pathways and novel compounds for the treatment of advanced HCC.
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase downstream of the phophoinositide-3-kinase (PI3K)-related kinase family. It is a central regulator of various oncogenic processes including cell growth, proliferation, metabolism, and angiogenesis. There is growing evidence to suggest that mTOR deregulation plays a significant role in hepatocellular carcinogenesis. Pre-clinical data indicates that deregulated expression of mTOR pathway effectors is present in 40%-50% of HCCs, and activation of the mTOR pathway is associated with less differentiated tumors, earlier tumor recurrence, and worse survival outcomes[4]. Our review focuses on components and functions of the mTOR pathway and its potential role in the treatment of advanced HCC.
MTOR PATHWAY
Components of mammalian target of rapamycin complexes
The PI3K/AKT/mTOR signaling pathway- also known as the “mTOR pathway”- contains two important components: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Figure 1). They are multiprotein complexes, comprised of both shared and unique components.
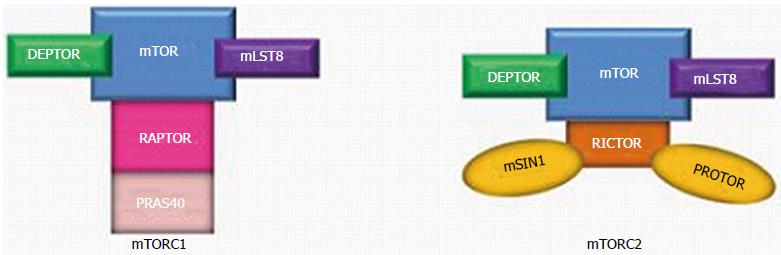
Figure 1 Mammalian target of rapamycin complex 1, Mammalian target of rapamycin complex 2 and their associated proteins.
mTOR: Mammalian target of rapamycin; DEPTOR: DEP domain-containing mTOR-interacting protein; mLST8: Mammalian lethal with SEC13 protein 9; RAPTOR: Regulatory-associated protein of mTOR; PRAS40: 40 kDa Pro-rich AKT substrate; RICTOR: Rapamycin-insensitive companion of mTOR; mSIN1: Mammalian stress-activated map kinase-interacting protein 1; PROTOR: Protein observed with RICTOR.
The mTOR kinase- also known as “mTOR”- is one of three components which is present in both mTORC1 and mTOR2; mammalian lethal with SEC13 protein 9 (mLST8) and DEP domain-containing mTOR-interacting protein (DEPTOR) are two other proteins that are common to both mTORC1 and mTORC2. mLST8 interacts directly with mTOR to enhance its kinase activity, particularly within mTORC2 (its effect within mTORC1 is not clearly understood)[5]. DEPTOR prevents substrate binding to mTORC1 and mTORC2, which leads to inhibition of mTORC1 and mTORC2 activity[6,7].
mTORC1, which is sensitive to the effects of rapamycin, has two unique proteins: regulatory-associated protein of mTOR (RAPTOR) and 40 kDa Pro-rich AKT substrate (PRAS40; also known as AKT1S1). RAPTOR serves as a binding platform where substrates are presented to mTOR for subsequent activation of mTORC1[8]. Conversely, PRAS40, like DEPTOR, is a direct inhibitor of mTORC1 substrate binding which hinders mTORC1 activity[9].
Specific to mTORC2 are rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated map kinase-interacting protein 1 (mSIN1; also known as MAPKAP1) and protein observed with RICTOR (PROTOR) (Figure 1)[10]. There is some evidence that RICTOR contributes to the structural foundation of mTORC2; in the absence of RICTOR, mTORC2 becomes inactive[7]. The functions of mSIN1 and PROTOR remain unclear.
Functions and regulations of mTORC1 and mTORC2
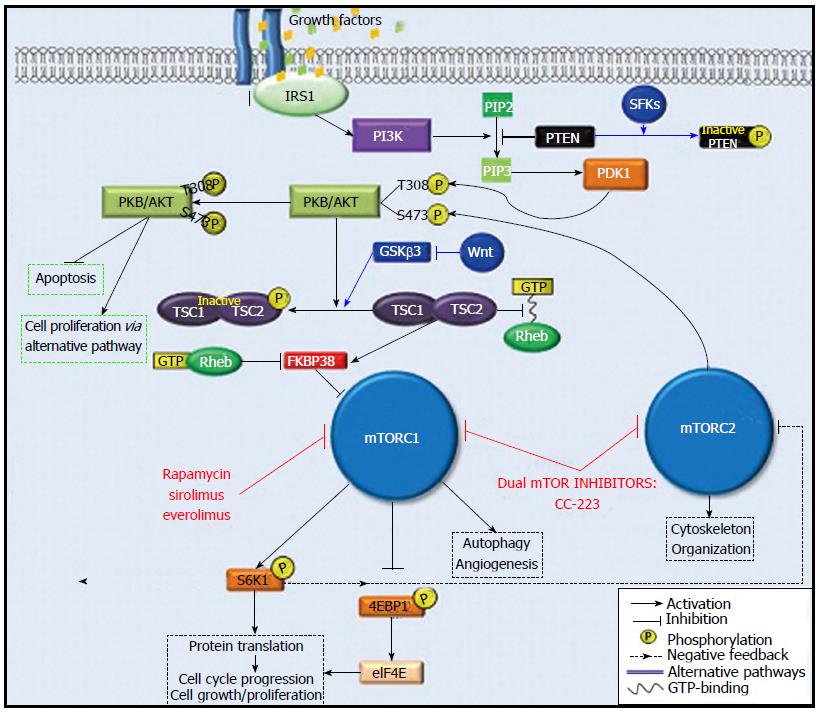
mTORC1: mTORC1 expression is driven by stimulants such as energy status, physiologic stress, and growth factors. Specifically, in the presence of growth factors, insulin receptor substrate 1 (IRS1) activates PI3K. PI3K phosphorylates the second messenger called phosphatidylinositol (4,5)-biphosphate (PIP-2), which becomes phosphatidylinositol (3,4,5)-triphosphate (PIP-3) upon phosphorylation. PIP-3 then promotes the phosphorylation of serine/threonine protein kinase (PKB/AKT) at protein residue Thr308 by 3-phosphoinositide-dependent protein kinase-1 (PDK1). Further downstream signaling through the effector tuberous sclerosis 1-tuberous sclerosis 2 complex (TSC1-TSC2) ultimately leads to the activation of mTORC1.
Activated mTORC1 phosphorylates its two downstream targets, 70S ribosomal protein S6 kinase (S6K1) and the eukaryotic initiation factor 4E binding protein 1 (4E-BP1). S6K1 and 4E-BP1 are major regulators of protein translation; they also drive cell proliferation, angiogenesis, and autophagy[11].
Under normal physiologic conditions, 4E-BP1 binds to the eukaryotic initiation factor 4E (eIF4E) to arrest protein translation. However, when 4E-BP1 is phosphorylated by mTORC1, its binding to eIF4E is interrupted, and this allows protein translation to occur. Concomitantly, phosphorylation of S6K1 by mTORC1 also results in protein translation; however, it also creates a negative feedback loop whereby the phosphorylated S6K1 attenuates PI3K signaling by suppressing IRS1 activity, leading to mTORC1 inhibition. Interestingly, mTORC1 inhibition by rapamycin and its analogues disrupts S6K1-mediated feedback inhibition of PI3K signaling, which allows for increased PKB/AKT phosphorylation (Figure 2). Furthermore, rapamycin-induced inhibition of mTORC1 leads to an accumulation of phosphorylated AKT which can then activate downstream effectors of alternative pathways to inhibit apoptosis and promote cell proliferation[12].
Figure 2 The PI3K/AKT/mTOR pathway.
IRS1: Insulin receptor subsrate 1; PI3K: Phophoinositide-3-kinase; PIP-2: Phosphatidylinositol (4,5)-biphosphate; PIP-3: Phosphatidylinositol (3,4,5)-triphosphate; PTEN: Phosphatase and tensin homologue on chromosome 10 gene; PDK1: Phosphoinositide-dependent protein kinase-1; PKB/AKT: Serine/threonine protein kinase; TSC1-TSC2: Tuberous sclerosis 1-tuberous sclerosis 2 complex; 4EBP1: Eukaryotic initiation factor 4E binding protein 1; eIF4E: Eukaryotic initiation factor 4E; S6K1: 70S ribosomal protein S6 kinase; SFKs: SRC family kinases.
mTORC2: Similar to mTORC1, mTORC2 activity is also promoted by growth factors. The upstream regulatory mechanisms specific to mTORC2 are poorly understood; however, its role in the phosphorylation of PKB/AKT has been well-characterized. The full activation of PKB/AKT requires two steps of phosphorylation: first, at protein residue Thr308 by PDK1, and second, at residue Ser473 by mTORC2[13]. Therefore, mTORC2 indirectly promotes mTORC1 activity through activation of PKB/AKT (Figure 2). Another less understood function of mTORC2 involves the regulation of actin cytoskeleton organization. Unlike the inhibitory effects on mTORC1, the effects of rapamycin and its analogues on mTORC2 are minimal[14].
Constitutive upstream regulators of the mTOR pathway: PTEN and TSC1-TSC2 complex
Phosphatase and tensin homologue on chromosome 10 gene (PTEN) is a multiphosphatase tumor suppressor located on human chromosome 10q23.3. It blocks the downstream activity of PI3K-AKT signaling by degrading PIP-3[11]. Inhibition of PIP-3 by PTEN prevents activation of PKB/AKT which leads to the down-regulation of mTORC1 activity (Figure 2). In the absence of PTEN, activation of mTORC1 is unbridled and hepatocellular carcinogenesis occurs. Watanabe et al[15] showed high incidence of HCC (66%) in PTEN-deficient mice at the end of an 80-wk period. Wang et al[16] demonstrated that decreased PTEN protein expression in HCC tissue samples compared to paired surrounding tissue samples was associated with higher tumor pathologic grade, TNM stage, and more frequent incidence of metastasis.
The TSC1 and TSC2 proteins (also known as hamartin and tuberin, respectively) are regulators of cell proliferation which have been implicated in HCC carcinogenesis. In its active form, the TSC1-TSC2 complex inhibits mTORC1 activation. Specifically, TSC2 acts as a GTPase-activating protein (GAP) which degrades guanosine triphosphate (GTP) and prevents its binding with Rheb, a GTP-binding protein. As a result, Rheb’s ability to inhibit FKBP38, a negative regular mTORC1, is disabled and mTORC1 is inhibited. However, Akt-mediated phosphorylation deactivates the TSC1-TSC2 complex by decreasing its GAP-activity towards Rheb, which permits Rheb-GTP binding[17]. In turn, Rheb carries out its usual function of inhibiting FKBP38, and mTORC1 activation occurs (Figure 2)[17,18]. In fact, loss of either TSC1 or TSC2 promotes autonomous activation of mTORC1. Using liver-specific TSC1 knockout mice, Menon et al[19] demonstrated that chronic activation of mTORC1 in the absence of TSC1 induced hepatocyte damage, independent of hepatic steatosis, which leads to the spontaneous development of HCC.
It is important to note that PTEN and TSC1-TSC2 complex also function as integrating hubs for the regulation of mTOR via alternative signaling pathways. For example, the Src family kinases (SFKs) and the Wnt protein of the Wnt/β-catenin pathway are direct upstream regulators of PTEN and TSC1-TSC2 complex, respectively. Studies of breast cancer cell lines have shown that SFKs phosphorylate PTEN to inhibit its function[20], which then promotes mTORC1 activation. Conversely, the stimulation of Wnt prevents TSC2 phosphorylation through inhibition of GSKβ3, a protein constituent of Wnt/β-catenin pathway, thus inhibiting mTORC1 activation[17].
CLINICAL EXPERIENCE OF MTOR INHIBITION IN HCC
mTORC1 inhibitor in the prevention of HCC recurrence post liver transplantation
Within the past decade, the role of mTOR inhibition in the prevention of HCC recurrence has been examined more thoroughly in the post-liver transplantation patient population. Recurrence is a major cause of morbidity and mortality among these patients, and the recurrence risk is markedly influenced by explant pathology such as poor tumor differentiation and the presence of microvascular invasion[21].
The traditional immunosuppressants used to prevent liver allograft rejection are calcineurin inhibitors (CNIs) such as tacrolimus and cyclosporine. They have been implicated in tumorogenesis both in vitro and in vivo[22,23]. In contrast, mTOR inhibitors are capable of effective immunosuppression (by blocking interleukin-2-mediated acute graft rejection) and concomitant prevention of hepatocellular tumorogenesis (through potent inhibition of angiogenesis). These two reasons make them attractive immunosuppresants for post-liver transplantation patients with a pre-transplant diagnosis of HCC[24].
In retrospective and non-randomized prospective analyses, post-liver transplantation HCC patients treated with sirolimus (a rapamycin analogue which selectively inhibits mTORC1) showed decrease in HCC recurrences[25]. In a study of 70 post-liver transplantation HCC patients treated with sirolimus-based immunosuppression, Toso et al[26] demonstrated an absolute decrease in recurrence rates by 6% (Milan criteria) and 14% (beyond Milan criteria) compared to studies not using sirolimus. A recent meta-analysis in patients with HCC who underwent liver transplantation indicated that sirolimus-treated patients demonstrated longer 5-year relapse-free survival (RFS) and 5-year OS rates (79%-80% and 80%, respectively) compared to CNI-treated patients (54%-60%; 59%-62%, respectively)[27].
Because of this promising data, a prospective, randomized international clinical trial (the “SiLVER trial”) has been developed to assess the role of sirolimus in HCC-free patient survival in liver transplantation recipients with a pre-transplant diagnosis of HCC; the primary endpoint is RFS with a planned 5-year follow-up[28].
mTORC1 inhibitor in advanced HCC
Recently, single-arm phase I/II studies have shown that everolimus (a second-generation mTORC1 inhibitor), has single-agent activity in de novo or recurrent advanced HCC. In a cohort of 36 patients, everolimus hindered disease progression in patients with advanced HCC when used at maximum tolerated dose of 70 mg weekly[29]. In a subsequent phase I/II study by Zhu et al[30], 28 patients with advanced HCC tolerated everolimus at the dose of 10 mg daily. The median progression free survival was 3.8 mo, suggesting a modest antitumor effect of everolimus in advanced HCC[31].
This study led to the global phase III randomized EVOLVE-1 trial, where everolimus was compared to placebo in patients with advanced HCC who discontinued sorafenib due to disease progression or drug intolerance. This trial unfortunately showed no OS benefit for everolimus in the salvage setting of advanced HCC[32]. As discussed in section 2 (b) of this review, mTORC1 and mTORC2 are two complementary components of the mTOR pathway: when mTORC1 is inhibited, mTORC2 is upregulated. This increase in mTORC2 activity generates a surplus of phosphorylated PKB/AKT which, despite mTORC1 inhibition, inhibits apoptosis and promotes cell proliferation via alternative pathways (Figure 2)[33]. This phenomenon may partially explain the unsatisfactory efficacy of everolimus demonstrated in the EVOLVE-1 trial, and suggests a potential mechanism for drug resistance against mTORC1 inhibitors in HCC. Given this theory, dual mTORC1 and mTORC2 inhibition has become an attractive pharmacologic target with therapeutic potential in advanced HCC treatment.
The safety of everolimus in combination with sorafenib has also been evaluated for the treatment of advanced HCC, as it posed the opportunity to target two major pathways involved in HCC pathogenesis. However, phase I studies demonstrated intolerable toxicities with this combination, rendering it infeasible as a therapeutic option[34,35].
POTENTIAL OF DUAL MTOR INHIBITION IN HCC
Pre-clinical studies using second generation mTOR inhibitors (i.e., Pp242, OSI027, AZD8055) in HCC cell lines and xenograft models have demonstrated enhanced antitumor efficacy of dual mTORC1/2 targeting[36-38]. Specifically, CC-223 (CC0482223) is a potent selective inhibitor of both mTORC1 and mTORC2 that impedes tumor resistance by inhibiting AKT phosphorylation. In multiple tumor cell lines, substrates of both mTORC1 and mTORC2 (p-S6RP and pAKT Ser473, respectively) were inhibited by CC-223, whereas rapamycin was a successful inhibitor of its downstream target p-S6RP only.
The therapeutic potential of CC-223 is being tested in a phase I trial of patients with refractory malignancies including HCC. Twenty-seven HCC patients have been enrolled as of June 2013; 93% of them previously received sorafenib. With 45 mg daily dosing of CC-223, 11% of patients exhibited a partial response, and 33% of patients maintained stable disease[39]. Due to this encouraging data, a cohort expansion of CC-223 in HCC patients is ongoing.
FUTURE DIRECTIONS IN MTOR INHIBITION FOR HCC
HCC undergoes constant mutational changes throughout its carcinogenesis and progression; therefore, combination therapy may be of interest. The possibility of non-overlapping pathway inhibition can be considered. For instance, sorafenib and dual mTOR inhibition could be a potentially effective strategy. In addition, epigenetic modification through methylation contributes to therapy resistance in many tumor types and HCC is no exception[40]. Dual mTOR inhibition combined with demethylating agents could also be a valid scientific approach[41].
Dramatic advances in the treatment of HCC have been achieved with improvement in the understanding of the biology of HCC pathogenesis and progression. The mTOR pathway is clearly critical to the progression of HCC. We anticipate that future data on single-agent dual mTOR inhibitors and combination strategies utilizing mTORC dual inhibition with other novel agents will contribute to the advances in HCC treatment.