INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor. Surgical resection and liver transplantation are the only potentially curative options, but they are contraindicated in most patients[1,2]. Other available imaging guided tools for the treatment of HCC are radiofrequency ablation, ethanol injection, transarterial chemoembolization and radioembolization. These options are not curative, they do increase survival and they are used to downstage patients in order to be suitable to liver transplantation. Moreover, recent studies have underlined the potential of antiangiogenetic treatment in patients with untreatable, unresectable HCCs[3,4]. Imaging follow-up plays a crucial role in evaluating treatment effectiveness and therefore in taking important decisions in the management of these patients. In this article, we will review the imaging appearance of HCC after treatment.
EVALUATION OF TUMOR RESPONSE WITH IMAGING
Currently there are no recommendations regarding the timing for radiological follow-up to assess treatment response and schedules shaped by randomized controlled trials (RCTs) are very heterogeneous[5]. At our institution, treatment efficacy is evaluated using dynamic computed tomography (CT) or magnetic resonance (MR) examinations at one, 3 and 6 mo after treatment, and every 6 mo afterwards. The first evaluation performed at one month is crucial to assess response to treatment and the presence of postprocedural complications. Subsequent follow-up is essential to detect tumor recurrence or the occurrence of new foci of HCC.
Liver resection with radiofrequency tissue coagulation device
Radiofrequency (RF)-assisted liver resection technique uses RF energy to obtain parenchymal dissection by creating a zone of coagulative necrosis along the transection plane[6]. This reduces the risk of intraoperative blood loss when compared with conventional liver resection[6]. Liver resection is indicated in patients with preserved liver function and single HCC, ideally in a subcapsular location[1]. Resected area must be larger than the original tumor and with a margin greater than 5 mm[7]. On CT, resection margin typically appears as an hypoattenuating, non enhancing halo[8,9] (Figure 1). On MR, resection margin is commonly hyperintense on T2-weighted images and hypointense on T1-weighted images and does not enhance after gadolinium injection[8,9]. Residual viable tissue, when present, is usually located along the resection site and shows arterial enhancement and venous wash-out[8,9]. A fluid collection within the resected area is commonly found at initial follow-up and disappears with time[9]. Uncommon complications include biloma, hepatic abscess, pleural effusion and adjacent organ injury (i.e., small bowel perforation).
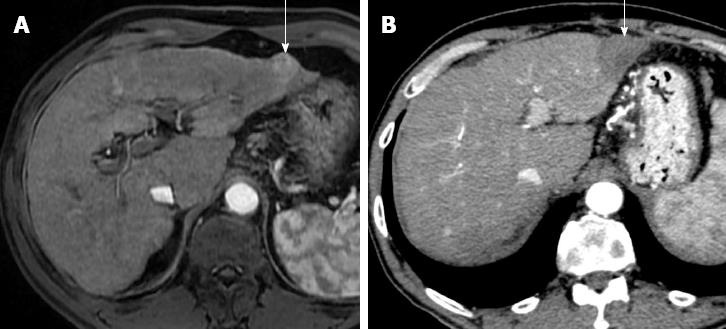
Figure 1 Successful hepatocellular carcinoma resection with radiofrequency tissue coagulation device.
A: Pretreatment arterial phase T1-weighted gradient-echo magnetic resonance shows hypervascular hepatocellular carcinoma (HCC) (arrow); B: On arterial phase computed tomography obtained 4 mo after treatment no hypervascular focus is evident (arrow). Note that resected area is larger to preexisting HCC.
Percutaneous ablation
Percutaneous ablation induces coagulative necrosis by modifying tumor temperature using RF, microwave, laser, cryotherapy or by direct intratumoral injection of chemical substances (ethanol or acetic acid). This procedure is recommended for patients with preserved liver function and a maximum of 3 small (≤ 3 cm) HCCs[1].
RF ablation: RF ablation (RFA) is the most widely used ablation therapy. RFA ablation consists of placing a needle electrode directly into the tumor, by US or CT guidance, and heating tissue to temperatures exceeding 60 °C[10] to obtain the coagulative necrosis of the tumor. Similarly to resected area, ablation zone should be 5-10 mm larger in comparison to the preexisting tumor[11]. Due to a coagulative necrosis and hemorrhagic products, treated HCC is typically hypoattenuating or heterogeneously hyperattenuating on unenhanced CT. Contrast enhanced images help differentiate viable from necrotic tumor. In general, successfully treated HCC shows absence of arterial enhancement (Figure 2), whereas any nodular arterially enhancing area within or along the margin of the ablated zone is suspicious of viable tumor[10]. Moreover, patients treated with RFA are considered at higher risk in comparison to the general cirrhotic population for the occurrence of new foci of HCC (Figure 3). However, absence of arterial enhancement at CT does not imperatively correspond to absence of viable tissue[11]. Lu et al[11] reported 100% specificity and 36% sensitivity of CT for the depiction of residual or recurrent tumor. In their study, only 4/11 (37%) HCCs with positive histological findings were detected at CT[11]. These observations are in agreement with a study by Dromain et al[12] who also found that MRI allowed earlier detection of residual tumor than does CT. At MR, successfully treated HCC shows T2-hypointensity and strong T1-hyperintensity[12]. The inclusion of subtraction images in the MR protocol increases detection of residual arterial enhancement in those cases of HCC with spontaneuous T1 hyperintensity[13]. At initial follow-up studies, treated HCC may show a thin and peripheral arterially enhancing rim, due to inflammatory reaction to thermal necrosis[14,15]. This rim sometimes shows hypoattenuation on unenhanced CT and mild hypertintensity on T2-weighted images. More rarely, needle electrode placing can cause formation of arterio-venous shunts[14,15]. These shunts can be easily diagnosed on the basis of wedge-shaped morphology, peripheral location and lack of wash out on portal venous phase (Figure 4). However, if the arterially enhancing zone is small and nodular, a correct characterization may be difficult and a 3-6 mo follow-up is recommended[16]. Size increase of arterially enhancing area, or the appearance of wash-out may suggest presence of viable tumor. Other complications include biloma, hepatic abscess, portal vein thrombosis (Figure 5), arterial pseudoaneurysm (Figure 6), tumor seeding (Figure 7) and adjacent organ injury[17].
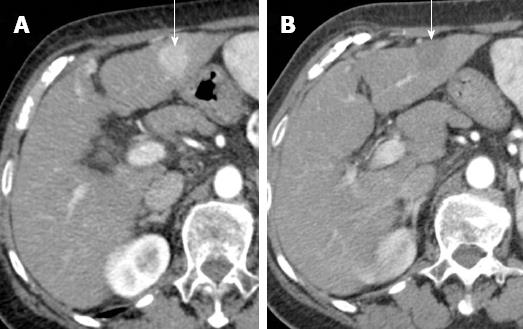
Figure 2 Complete necrosis after radiofrequency ablation for hepatocellular carcinoma.
A: Pretreatment arterial phase computed tomography (CT) shows hypervascular hepatocellular carcinoma (arrow); B: Arterial phase CT obtained 1 mo after treatment shows ablated area (arrow). Absence of arterial enhancement suggests complete tumor necrosis.
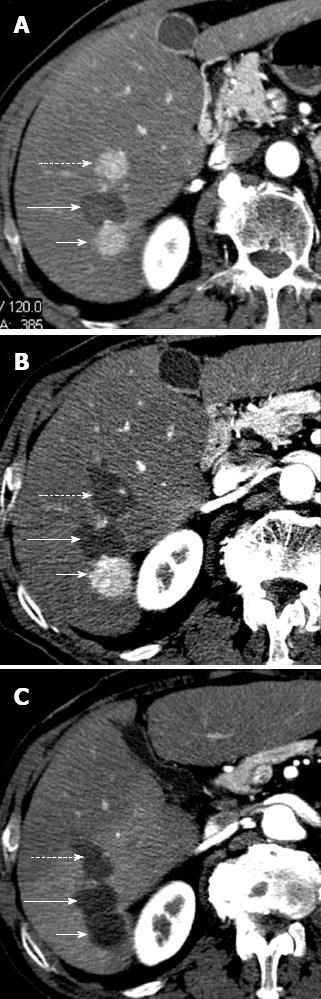
Figure 3 Hepatocellular carcinoma after multiple radiofrequency ablations.
A: Arterial phase computed tomography (CT) obtained 8 mo after radiofrequency ablation shows two new enhancing hepatocellular carcinoma (HCC) nodules located anteriorly (dotted arrow) and posteriorly (short arrow) to ablated HCC (long arrow). These findings suggest occurrence of new HCC nodules; B: Arterial phase CT obtained 2 mo after additional radiofrequency (RF) ablation shows that HCC nodule located anteriorly (dotted arrow) has been replaced by a hypoattenuating, non enhancing area (arrow) that is larger than preexisting tumor. These findings suggest complete necrosis. Posteriorly located HCC (short arrow) increased in size; C: Arterial phase CT obtained 2 mo after posterior. HCC had been replaced by a hypoattenuating, nonenhancing ablation area as a result of additional RF ablation. This example shows that RF ablation is a repeatable procedure.
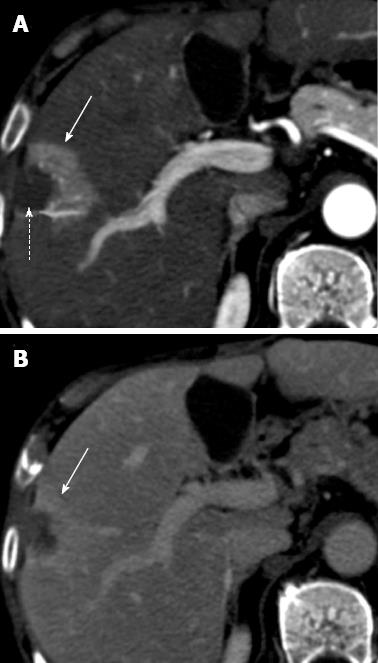
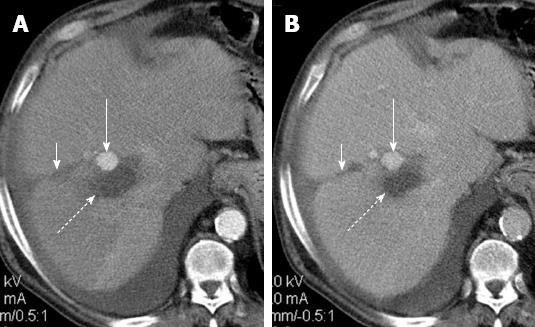
Figure 4 Perfusion alteration after radiofrequency ablation for hepatocellular carcinoma.
A: Arterial phase computed tomography (CT) obtained 1 mo after treatment shows ablated zone (dotted arrow) and a semilunar enhancing area (solid arrow) medial and anterior to ablated zone; B: Delayed phase CT shows persistent enhancement of the semilunar area (solid arrow), suggesting that the arterial enhancement is due to perfusion alteration rather than residual tumor.
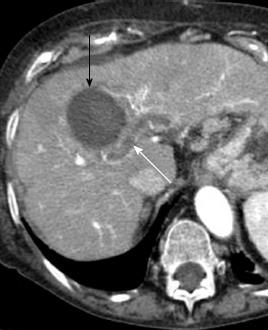
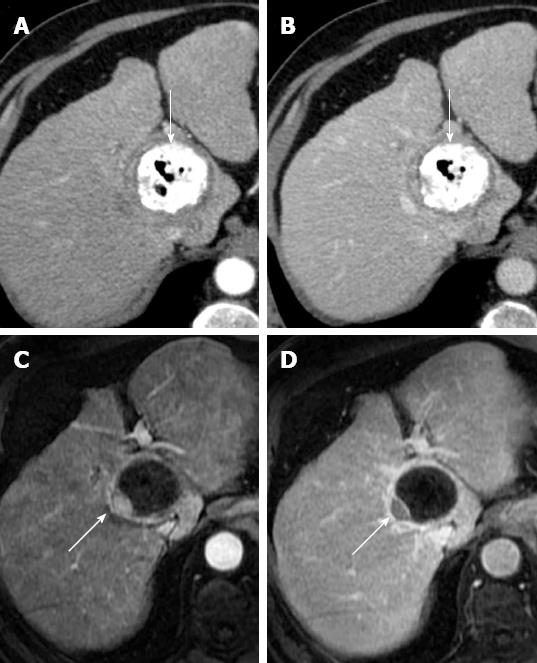
Figure 5 Portal vein thrombosis after radiofrequency ablation for hepatocellular carcinoma.
Arterial phase computed tomography obtained 1 mo after radiofrequency ablation shows a non enhancing thrombus in intrahepatic portal vein (white arrow), in proximity of ablated area (black arrow).
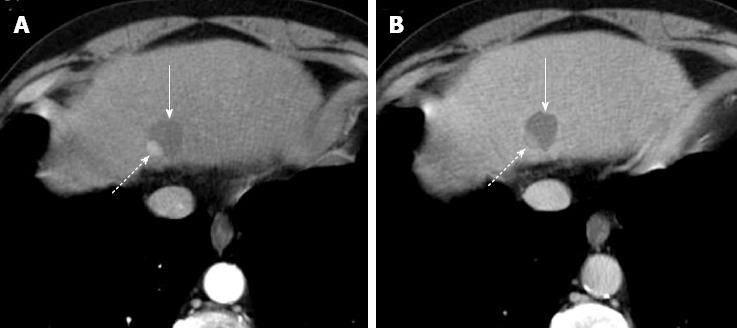
Figure 6 Arterial pseudoaneurysm after radiofrequency ablation for hepatocellular carcinoma.
Post-treatment arterial phase (A) computed tomography (CT) shows a round enhancing area (long arrow) anterior to ablated zone (dotted arrow) with persistent enhancement on portal venous phase (B) CT. Round shape, isoattenuation to aorta and absence of wash-out suggest diagnosis of iatrogenic pseudoaneurysm. Note probe track (short arrow on A and B).
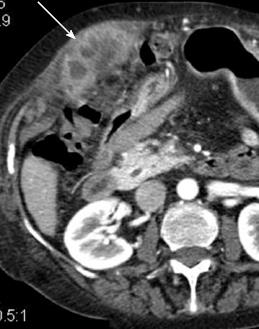
Figure 7 Tumor seeding after radiofrequency ablation for hepatocellular carcinoma.
Arterial phase computed tomography shows heterogeneously enhancing hepatocellular carcinoma tissue (arrow) invading muscles of the anterior abdominal wall along needle tract.
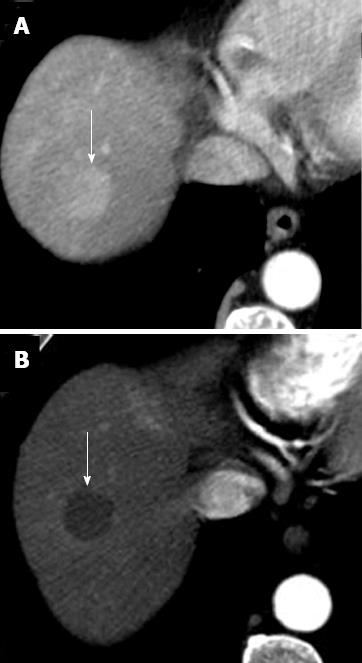
Percutaneous ethanol injection: Percutaneous ethanol injection (PEI) consists of percutaneous ethanol instillation into the HCC by sonographic or CT guidance. It is a well-tolerated, inexpensive procedure with few adverse effects. This alternative procedure may be performed in those patients with small HCCs, in whom RFA is not suitable to be performed due to tumor location[18]. In fact, some tumors are located at risky sites (defined as less than 5 mm from a large vessel or an extrahepatic organ, near gallbladder, or in subphrenic locations) and RFA treatment can cause severe complications. In addition, in tumors larger than 2 cm in size, initial RF may leave a tiny nest of viable tissue that will easily be ablated by ethanol with a relevant saving of resources. However, several studies demonstrated that PEI provides a lower rate of complete necrosis in comparison to RFA[19-21]. CT and MR post-treatment imaging features are similar to those obtained after RFA[22] (Figure 8).
Figure 8 Incomplete hepatocellular carcinoma necrosis after percutaneous ethanol injection.
Arterial (A) and portal venous (B) computed tomography obtained 1 mo after treatment shows that approximately 10%-20% of the tumor, located in the dorsal and lateral portion of the treated, hypoattenuating area, is still viable as demonstrated by the presence of enhancement in arterial phase and hypoattenuation (“washout”) on portal venous phase (dotted arrow on A and B). The majority of tumor (solid arrow) does not show enhancement as a result of the treatment.
Transarterial chemoembolization
Transarterial chemoembolization (TACE) consists of transarterial administration of a mixture of chemotherapy (Doxorubicin or Cisplatin in most cases) and iodized oil (Lipiodol, Guerbert, France), followed by embolizing particles[23]. Since HCC receives blood supply almost completely from the hepatic artery (as opposed to normal liver), these agents accumulate preferentially into HCC lesions. Iodized oil acts as chemotherapy carrier, while particles occlude tumor feeding arteries. Therefore, TACE combines delivery of high dose chemotherapy to the tumor with embolization of its feeding arteries. The major indications for TACE are multiple HCCs without vascular invasion or extrahepatic spread and HCCs for which percutaneous ablation is precluded by position (e.g., pericholecistic, subphrenic, etc.) or size[1]. TACE is also indicated in those patients in whom previous procedures have failed[1,24]. At initial post-treatment examinations, treated HCC usually presents the same size of the preexisting tumor. The evaluation of CT images is based on the assumption that tumor portion that retains iodized oil is necrotic, while enhancing foci represent viable tissue[10]. However, it is sometimes difficult to detect viable tissue, because beam hardening artefacts produced by iodized oil can impair evaluation of arterial enhancement[10] (Figure 9A and B). Kim et al[25] reported that use of unenhanced phase in conjunction with biphasic CT could improve the detection of additional foci of viable tumor. According to this study, an HCC treated with TACE could be considered viable if it showed hyperattenuation or isoattenuating on hepatic arterial phase and hypoattenuation on unenhanced and portal venous phases. MRI is known to be superior to CT for the evaluation of HCC after performing TACE[26] (Figure 9C and D). Completely necrotic HCC usually shows variable signal intensity on unenhanced T1-weighted images and T2-weighted images and lack of enhancement after gadolinium injection[27]. MR accuracy is however relatively low. Hunt et al[28] reported an overall accuracy rate of 55%, with 43% sensitivity and 75% specificity. Treated HCC sometimes shows a thin and peripheral pseudocapsule that enhances on hepatic arterial and delayed phases. Moreover, iodized oil can sometimes injury small hepatic arteries and cause formation of arterio-portal shunts. Absence of wash-out is crucial to differentiate these pseudolesions from residual viable tumor. Other complications include hepatic artery injuries (dissection or thrombosis), biloma, hepatic abscess and embolization of non target vessels[29]. Non target vessels include arteries that arise from the hepatic circulation (gastroduodenal, right gastric, accessory left gastric, cystic arteries)[30]. Embolization of these vessels results in gastrointestinal ulcers, skin ulcerations, and, rarely, ischemic cholecystitis[29,30]. Furthermore, although the purpose of TACE is to obtain a selective intratumoral delivery of chemioterapy, several studies demonstrated that many patients may have higher plasma levels of chemotherapeutic agents with systemic toxic effects[31]. Therefore, alternative techniques that increase the precision of drug delivery are required. TACE with drug eluting beads (DEB) is recently emerging as an alternative option to conventional TACE. TACE with DEB consists of transarterial injection of microspheres that sequester chemotherapy immediately before administration and release it into the tumor in a sustained and controlled manner[18]. In addition, absence of iodized oil does not mask arterial enhancement[32] (Figure 10), allowing easier assessment of residual, viable tumor in comparison to traditional TACE performed with iodized oil.
Figure 9 Incomplete hepatocellular carcinoma necrosis after transarterial chemoembolization.
Arterial (A) and portal venous (B) phase computed tomography (CT) obtained 1 mo after transarterial chemoembolization (TACE) shows that hepatocellular carcinoma (HCC) is entirely replaced by Lipiodol accumulation (arrow). No evidence of residual tumor was found. Arterial (C) and portal venous (D) phase T1-weighted gradient-echo magnetic resonance (MR) obtained 1 wk after CT shows residual viable tumor (arrow) in the posterolateral portion of the tumor. This case shows higher accuracy of MR in comparison to CT in assessing HCC response after TACE.
Figure 10 Complete hepatocellular carcinoma necrosis after transarterial chemoembolization with drug-eluting beads.
A: Pretreatment arterial phase computed tomography (CT) shows a hypervascular hepatocellular carcinoma (HCC) (arrow); B: Arterial phase CT obtained 3 mo after transarterial chemoembolization shows a hypoattenuating, non enhancing nodule (arrow). Absence of arterial enhancement suggests complete HCC necrosis.
Radioembolization
An alternative technique to treat advanced HCC is radioembolization. It consists of transarterial administration of yttrium-90 microspheres that are preferentially deposited within hypervascular tumors and that emit lethal beta radiation[18]. This induces tumor coagulative necrosis and avascularity. Post-treatment HCC appearance is similar to that obtained following RFA and PEI[33].
Targeted therapy
Targeted therapies are a new generation of anticancer drugs designed to interfere with tumor growth and progression. Sorafenib, a multikinase inhibitor, is the only drug that has been demonstrated to significantly improve survival in patients with untreatable, unresectable HCCs in a large randomized controlled trial[3]. Sorafenib reduces tumor vascularization and subsequently induces tumor necrosis and hemorrhage[3] (Figure 11). For these reasons, the traditional WHO and RECIST criteria based on evaluation of tumor size may not be ideal to determine tumor response. Therefore, different criteria based on assessment of viable and enhancing tumor are needed. Modified RECIST (mRECIST) differ from RECIST criteria, since they consider only the diameter of viable tumor, defined as the portion of the tumor that shows arterial enhancement and venous wash-out[5,34]. Recent studies have reported the potential of perfusion CT and MR[35,36]. However, high CT radiation dose, MR breathing, cardiac motion and high costs limit the use of these techniques[35,37,38].
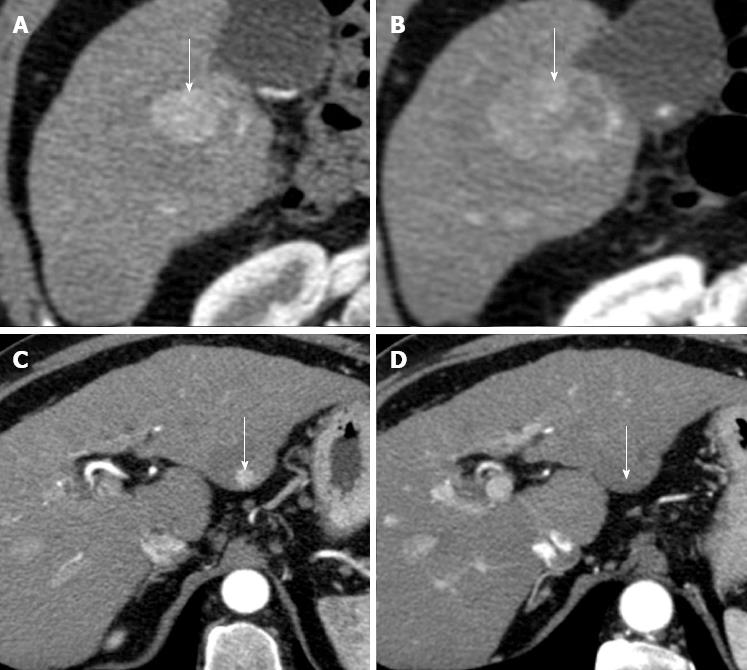
Figure 11 Hepatocellular carcinomas after sorafenib.
A: Pretreatment arterial phase computed tomography (CT) shows a large hypervascular hepatocellular carcinoma (HCC) (arrow) in right hepatic lobe; B: Arterial phase CT obtained at the same level of A, 9 mo after the start of sorafenib, shows increase in size of HCC (arrow); C: Pretreatment arterial phase CT image shows a second, small hypervascular HCC (arrow) arising within a larger hypoattenuating dysplastic nodule in left hepatic lobe; D: Arterial phase CT obtained at the same level of C, 9 mo after the start of sorafenib, shows disappearance of HCC enhancement (arrow). This case shows that different HCC response to sorafenib may occur in the same patient.