INTRODUCTION
Several case reports of alpha-fetoprotein (AFP) producing gastric cancer have been described[1-5]. It represents 3.9%-11% of all the neoplasia of the stomach collected in a Japanese series. Other than AFP production, gastric cancer and hepatocarcinoma (HCC) can share common histology features, as demonstrated in the case of the hepatoid adenocarcinoma (HAC) of the stomach. As described by Terracciano et al[6], HAC is an extrahepatic tumor with morphological features similar to HCC, often producing large amounts of AFP. The findings from Terracciano et al[6] suggest that HAC arises from gastric adenocarcinoma with an intestinal phenotype as a result of the further progression of the carcinogen process. The stomach is the organ where the tumor was most frequently described and is associated with a poor prognosis for vascular invasion, regional lymphadenopathy and frequent liver metastases[1-5]. Terracciano et al[6] showed positivity in virtually all HACs for AFP, cytokeratin-8 (CK-8), CK-18 and carcinoembryonic antigen, markers underlining its hepatoid nature, the contemporary positive staining for CK-19 and CK-20, and the frequent negativity of Hep Par1 in both primary tumors and their metastases. Furthermore, HAC differs from combined hepatocellular cholangiocarcinoma, because it is negative for CK-7[6]. Because HAC liver metastases and HCC cannot be differentiated on the basis of morphology alone, the described pattern of phenotypical markers is necessary for differential diagnosis.
Radiation induced liver disease (RILD), previously referred to as “radiation hepatitis”, can affect patients 4 to 8 wk after liver exposure to radiation[7-12]. RILD is a veno-occlusive disorder caused by a direct radiation-induced injury of the liver endothelium[10-12]. This condition can affect 6% to 66% of patients exposed to an excess of 30 to 35 Gy of radiation, depending on the volume of irradiated liver and hepatic functional reserve[10,12]. The typical clinical presentation is a triad of ascites, hepatomegaly and elevated liver enzymes[9]. Most patients recover completely after 3 to 5 mo but a minority will progress to a chronic phase, with hepatic fibrosis and liver failure; rare cases of fulminant liver failure have been described[10,12]. The diagnosis essentially emerges from the findings of the typical imaging features on contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI), affecting the liver in the irradiated field: “straight-border” sign[8], demarcated areas of hypo or hyper attenuation in a non anatomical distribution (contrasting with vascular lesions)[8], and alteration of the contrast uptake on CT[13].
We describe a case AFP producing liver metastatic gastric cancer in a patient affected by RILD and the implications in terms of diagnostic challenges and pathogenesis.
CASE REPORT
A 52-year-old male, heavy smoker (40 cigarettes per day for 30 years), 1.5 years before our first examination, underwent gastrectomy for gastric cancer of the angulus, stage II (pT2b, pN1, pMx) of the TNM Classification of Malignant Tumors[14], grade 3 (G3) according to the American Joint Commission on Cancer[15]. Blood analysis and liver imaging [contrast-enhanced CT, ultrasound sonography (US)] performed during hospital stay for gastric surgery demonstrated the absence of risk factors for liver disease and a normal liver structure and function. Preoperative serum tumor markers were not evaluated. Three months after surgery, he underwent adjuvant therapy: 5-fluoruracil (5-FU) ev infusion 225 mg/mq per die (35 d) plus 45 Gy irradiation on the epigastric region. During follow-up 6 mo after surgery, a diffuse alteration of the contrast medium uptake affecting the left hepatic lobe was evidenced by a control CT performed without an emerging clinical indication. CT showed band-like hypo attenuation in the liver with parenchymal swelling corresponding to the radiation field. Interestingly, the CT showed a thin layer of ascites. On MRI, the diseased area appeared like a rectangular shape affecting the left liver lobe and the hilar region, which resulted in hypo intense on T1-weighted images, iso and hyper intense on T2-weighted images in the pre-contrast phase, and hypo intense in the post-contrast images (Figure 1). No alteration of liver serum enzymes was detected. The contrast enhanced imaging follow-up, through both CT and MRI, showed a complete recovery of the liver parenchyma 10 mo after irradiation, suggesting the diagnosis of RILD. The patient did not show any alteration of serological liver parameters, except for high AFP serum levels [13 fold above normal values (NV)], whose first evaluation dated back to a period of the post surgical follow-up when the liver lesion was disappearing. After upper and lower endoscopy examinations which resulted negative for neoplasia, the patient was referred to our gastroenterology unit in May 2008 due to a further increase of the AFP level (21 fold above NV). In the same time period, other tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19-9 were normal. At this time, HCV-Ab and hepatitis B surface antigen were negative and transaminases, indexes of cholestasis and prothidosynthesis were in the normal range. No evidence of metabolic disease or iron accumulation emerged. The patient had an average intake of three standard alcohol drinks per day (12 g of ethanol per standard alcohol drink) and was treated with ramipril for mild essential hypertension. Finally, no instrumental signs of portal hypertension by US were found. On physical examination, the liver was palpable (2 cm under the costal arch in inspiration), with parenchymatous consistency and a smooth surface, the spleen was not palpable, no peripheral lymphadenopathies were discovered and the testes were normal. Thus, the patient underwent an abdominal and testis US, total body dynamic contrast-enhanced CT and upper endoscopy with gastric mucosa biopsies. All these examinations failed to detect alterations, except for a 1.5 cm lymph node at the site of the surgical anastomosis (Figure 2A). As a consequence of a dramatic increase of AFP serum levels (200-fold the NV) in September 2008, an US examination detected a 3 cm × 2 cm, hypoechogenic lesion of the left hepatic lobe which was biopsied. The total body dynamic contrast-enhanced CT confirmed a single 4 cm hypo vascular lesion affecting the left hepatic lobe, in the same region previously affected by RILD (Figures 1 and 2). The histology showed an extensively necrotic, poorly differentiated epithelial tumor (Figure 3A). The neoplastic cells were immunoreactive for MOC-31 (cell surface glycoprotein), CK-8 and CK-18 (Figure 3B and C) and negative for CK-7, CK-20 and Hep Par 1. Sparse cells were immunoreactive for AFP as well (Figure 3D). Since the upper endoscopic examination excluded pathology of the residual stomach mucosa, a presumptive diagnosis of a primary poorly differentiated liver tumor originated at the site affected by RILD was taken into consideration. However, a positron emission tomography examination showed 2 areas of increased metabolic activity localized in correspondence to a parasternal lymph node and in the left hepatic lobe. Those alterations were referred to as metastatic lesions. Other areas with a less pronounced metabolic activity were described as affecting the epigastric region and the mesenteric-pancreatic region. Therefore, the primitive gastric cancer (Figure 3E) was re-evaluated by immunohistochemistry. The analysis revealed diffuse positivity of the neoplastic cells for MOC-31, CK-8, CK-18 and AFP (Figure 3F-H), focal positivity for Hep Par-1 and negative for CK-7 and CK-20. The comparative histology analysis of the liver lesion and the gastric cancer allowed a definitive conclusion of liver metastasis of an AFP-producing gastric cancer. The patient was enrolled to receive systemic chemotherapy for gastric cancer metastases. The patient deteriorated from progressive hepatic metastases and expired 12 mo after the detection of the first liver metastatic lesion. No autopsy was performed.
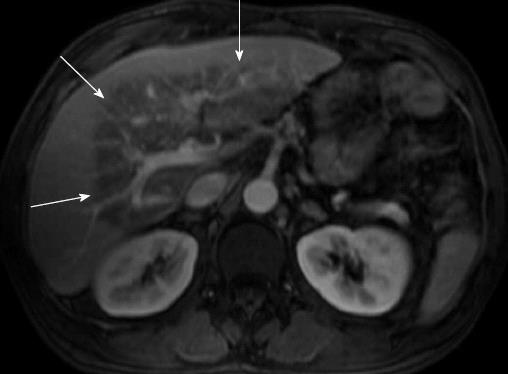
Figure 1 Spoiled gradient recalled-echo and fat-suppressed T1-weighted magnetic resonance imaging after iv gadolinium administration.
The venous phase on the axial portal plane shows, at the level of the left hepatic lobe and of the liver hilum region, a triangular hypo intense area (arrows) with hyper intense small areas within; vascular structures are preserved (May 2007).
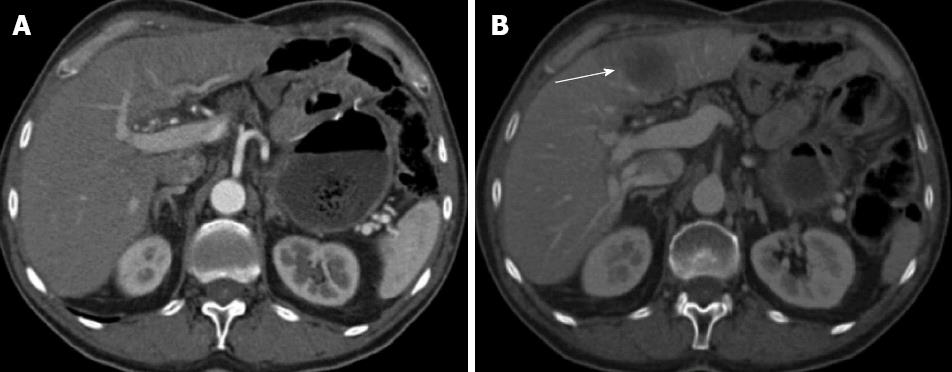
Figure 2 Axial computed tomography scan after iv iodinated contrast medium administration in the arterial hepatic phase (A) and portal phase (B).
A: The computed tomography study does not show focal lesions in the liver parenchyma (Jul 2008); B: A focal nodular hypo attenuated lesion is present affecting the left hepatic lobe (arrow); it presents with regular margins and appears mildly vascularised (Nov 2008).
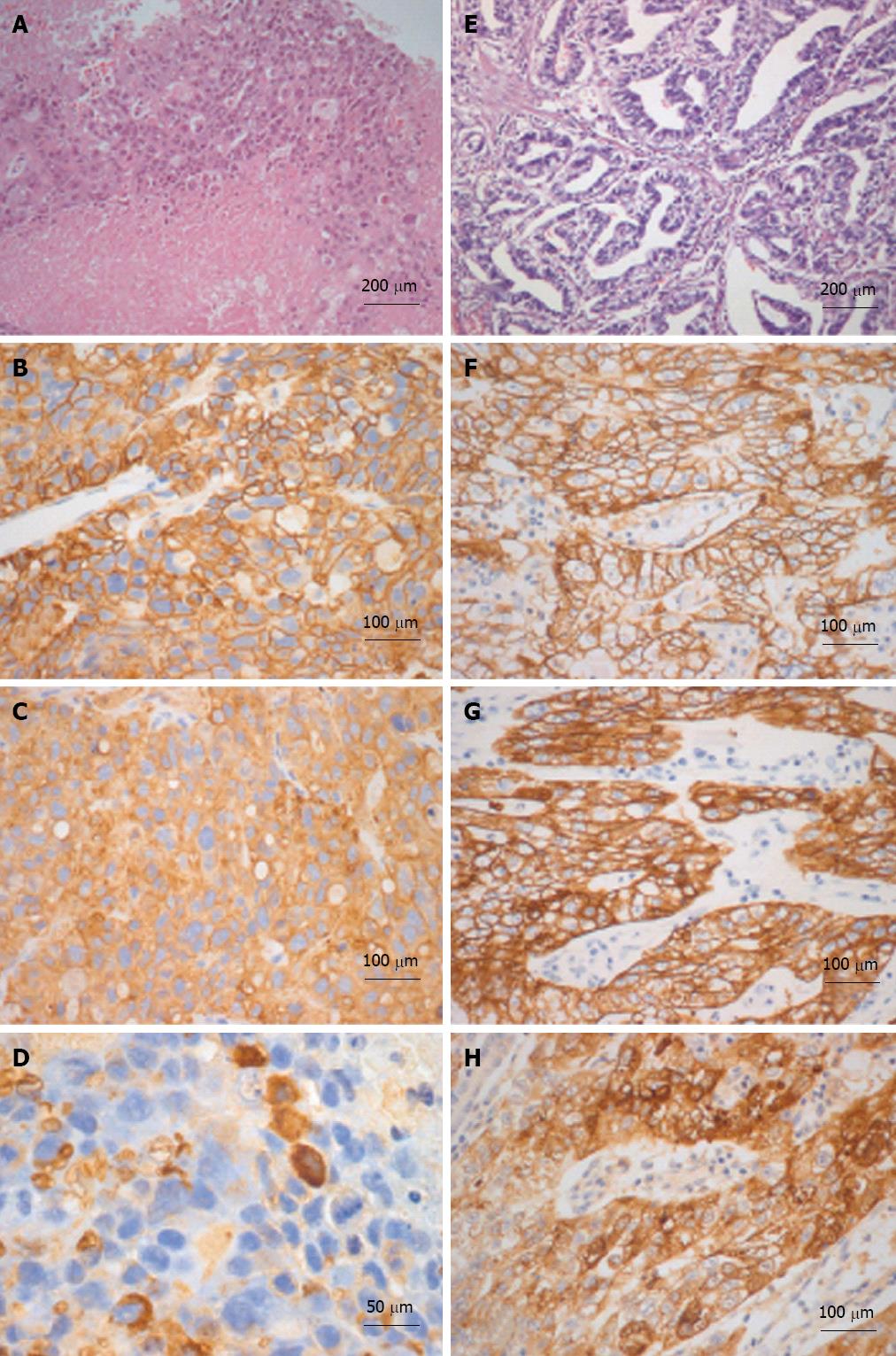
Figure 3 Histological-immunohistochemical characterizations of liver and gastric tumors are illustrated in A-D and E-H respectively.
The liver tumor is extensively necrotic (A) and neoplastic cells are immunoreactive for MOC-31 (B) and cytokeratin (CK)-18 (C). Sparse cells are immunoreactive for alpha-fetoprotein (AFP) as well (D). The gastric adenocarcinoma (E) is immunoreactive for MOC-31 (F), CK-18 (G) and AFP (H) as well. A and E: Hematoxylin and eosin.
DISCUSSION
This case report engaged us in a differential diagnosis between HCC and AFP-producing gastric cancer metastasis. The final diagnosis of the liver lesion was achieved only by immunohistochemistry, where MOC-31 (cell surface glycoprotein), CK-8 and CK-18 positivity, focal positivity for AFP and negativity for CK-7, CK-20 of both liver lesion and gastric cancer allowed the definitive conclusion of liver metastasis of AFP-producing, and likely endodermal stem cell-derived, gastric cancer. Moreover, this phenotype is in accordance with the features of hepatoid adenocarcinoma of the stomach, as described by Terracciano et al[6]. As a differential diagnosis, we hypothesized a case of primitive liver cancer originating on RILD. This hypothesis was sustained by the potential mutagen effect of radiation[16] and chemotherapy, other than the absence of other metastatic lesions and the lack of local gastric recurrence. In contrast, the immunohistochemical analyses and the comparison with the primitive gastric cancer allowed the definitive conclusion of liver metastasis. Therefore, the message for clinicians from this case report could be that, in the presence of high AFP serum levels and history of gastric cancer, the immunohistochemical characterization of primitive cancer and liver lesion is absolutely necessary for a definitive diagnosis. Moreover, in our opinion, the accurate evaluation of a pre-existing chronic liver disease[17] is of relevance because its absence, as in our case, decreases the probability of primitive liver cancer, reinforcing the accuracy of the diagnostic process. Indeed, theoretically primitive liver cancer occurrence could be increased by eventual radiation administration during the activation of resident stem cell compartment occurring in chronic liver diseases[17-24]. The second point in the discussion is the relationship between RILD and the emergence of liver metastases. In our patient, the diagnosis of RILD was based on strictly accepted criteria, including a contrast-enhanced CT and MRI that allowed us to follow the evolution of the characteristic liver lesion until its resolution[7-9]. Moreover, the dose of radiation was compatible with RILD occurrence, similar to the timing of a RILD occurrence after radiation exposure. As RILD clinical presentation depends on the volume of irradiated liver and hepatic functional reserve, a localized exposure of the liver parenchyma in a patient with a good hepatic functional reserve could lead to a RILD without elevated liver enzymes or massive ascites, as in our patient. In addition, we performed all necessary clinical, biochemical and imaging procedures to exclude underlying liver diseases. Whether AFP-producing gastric cancer is associated with frequent liver metastases[1-5], no cases of gastric cancer liver metastases emerging in a site of the liver previously affected by RILD were described. However, we were encouraged to describe the case because, in our opinion, the localization of metastasis in the site of the liver involved by RILD could be not casual but could involve specific pathogenetic mechanisms. In fact, several clinical and experimental evidences suggested an increased incidence of metastases after radiation doses that were insufficient to control the primary tumor[25-28]. Furthermore, in vivo studies in mice demonstrated an increased susceptibility of the lung and the liver irradiated prior to i.v. administration of tumor cells, to be affected by cancer cell engraftment[29-31]. Radiation could favor metastases seeding since vascular damage facilitates tumor cell intravasation[29] and because cell death caused by radiation may release soluble factors, promoting the engraftment and growth of circulating cancer cells[32]. A specific role could be suggested for vascular endothelial growth factor (VEGF) generated in the site of RILD-induced vascular and cell damage. In fact, obstruction of the hepatic microcirculation, as occurs during RILD, leads to an increased hepatic VEGF expression[33]. This growth factor might induce proliferation of previously dormant liver microtumors[34] or may exert chemo-attractant function on VEGFR (VEGF receptors) expressing circulating cells, leading to enhanced metastases seeding[35,36]. Interestingly, gastric carcinoma cells express different types of VEGF and relative receptors, including VEGF-C and VEGFR-3, which may play a role in the seeding and growth of cells in the site where enhanced VEGF production occurs[37]; in our case, the left liver lobe affected by RILD. In conclusion, it could be rational to consider that RILD increased the probability of gastric cancer metastases seeding due to the occurrence of vascular damage facilitating the tumor cells intravasation and due to the increased production of chemo-attractant molecules for malignant circulating cells, such as VEGF.
ACKNOWLEDGMENTS
We thank Dr. Gianluca Maria Varano for technical assistance in RM images procurement.
P- Reviewer Sazci A S- Editor Li JY L- Editor Roemmele A E- Editor Li JY