Published online Jun 27, 2013. doi: 10.4254/wjh.v5.i6.336
Revised: September 30, 2012
Accepted: November 11, 2012
Published online: June 27, 2013
Processing time: 330 Days and 13.2 Hours
A 63-year-old Caucasian man presented with a cholestatic syndrome, renal failure and arthralgias. A laboratory examination revealed high immunoglobulin G (IgG) and IgG4 levels (5.95 g/L; normal range: 0.08-1.4 g/L), pointing to a diagnosis of systemic IgG4-related disease, with definite radiological evidence of biliary and pancreatic expression, and plausible renal, articular, salivary and lacrimal glands involvement. Due to the rarity of the condition, there are currently no random control trials to point to the optimal therapeutic approach. The patient has been on steroid therapy with the subsequent introduction of azathioprine, with a complete resolution of all symptoms, a rapid reduction to normalization of all blood tests, and a complete regression of the radiological picture. Our experience underlines the complexity of IgG4-related disease and its variable and sometimes progressive presentation, while pointing out the need for a careful and complete assessment for possible multi-organ involvement.
- Citation: Maida M, Macaluso FS, Cabibbo G, Lo Re G, Alessi N. Progressive multi-organ expression of immunoglobulin G4-related disease: A case report. World J Hepatol 2013; 5(6): 336-339
- URL: https://www.wjgnet.com/1948-5182/full/v5/i6/336.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i6.336
Immunoglobulin G4-related disease (IgG4-RD) is a recently recognized disease[1] with a presumed autoimmune pathogenesis, characterized by high levels of IgG4 and a good response to immunosuppressive therapy[2]. Its clinical pattern is variable and may present with single organ expression (pancreatitis, cholangitis, sialadenitis, dacryoadenitis, nephritis, inflammatory pseudotumor and retroperitoneal fibrosis; other organs may occasionally be affected) or with multi-organ involvement[3,4]. All these localizations of IgG4-RD have a homogenous histological pattern, i.e., lymphoplasmacytic infiltration with abundant IgG4-positive cell and storiform fibrosis. The etiology remains unknown, although a modified Th2 response with activation of regulatory T cells and overexpression of transforming growth factor beta and interleukin-4, 5, 10, 13 could be involved[5]. We report a patient with an insidious onset of IgG4-RD who proceeded to a full multi-organ expression.
A 63-year-old Caucasian man was admitted to our unit in July 2011 for a cholestatic syndrome of 4 mo duration. His medical history included heavy smoking (about 50 cigarettes/d) and an active consumption of 6 alcohol units/d, a previous diagnosis of “rheumatoid arthritis” treated with intermittent steroid therapy for several years, a histological diagnosis of interstitial nephritis three years before, and no history of allergic disease. In March 2011, at the onset of jaundice, he had been admitted to another hospital. At that time, cholestatic tests [total/direct bilirubin 6.3/5.98 mg/dL, alkaline phosphatase 3 × upper limit of normal (ULN), gamma-glutamyltransferase (GGT) 6 × ULN] and pancreatic enzymes (amylase and lipase 2 × ULN) were raised (Table 1). Abdominal ultrasound (US) and computed tomography (CT) scans of the abdomen revealed a dilatation of the intrahepatic and extrahepatic biliary tract, without evidence of gallstones or biliary sludge, and an increased volume of the pancreas with loss of the physiological lobular appearance. A diagnosis of obstructive jaundice secondary to chronic pancreatitis was made and a plastic endoprosthesis was placed in the common bile duct by endoscopic retrograde cholangiopancreatography. During the following weeks, a progressive reduction of biochemical markers of cholestasis occurred (Table 1).
| Parameter | Normal range | Values | |||||
| March | April | July 2011 | August 2011 | October 2011 | March 2012 | ||
| 2011 | 2011 | (admission) | (4th week of follow-up) | (12th week of follow-up) | (36th week of follow-up) | ||
| Alkaline phosphatase (U/L) | 40-129 | 401 | 190 | 490 | 97 | 83 | 74 |
| GGT (U/L) | 8-61 | 380 | 91 | 506 | 32 | 25 | 21 |
| Bilirubin total/direct (mg/dL) | < 1 | 6.3/5.98 | 1/0.8 | 4.5/4.3 | 0.7/0.2 | 0.5/0.1 | 0.4/0.1 |
| Amylase (U/L) | 28-100 | 210 | 66 | 230 | 85 | 99 | 76 |
| Lipase (U/L) | 13-60 | 121 | 60 | 130 | 50 | 58 | 36 |
| AST (U/L) | < 37 | 56 | 30 | 110 | 16 | 15 | 20 |
| ALT (U/L) | < 41 | 64 | 35 | 130 | 19 | 13 | 19 |
| Creatinine (mg/dL) | 0.67-1.17 | 1.7 | 1.65 | 2 | 1.1 | 1 | 1.1 |
| Gamma-globulins (g/dL) | 0.67-1.56 | - | - | 2.94 | 1.09 | 1.5 | 1.45 |
| IgG4 (g/L) | 0.08-1.4 | - | - | 5.95 | 1.2 | - | - |
In July 2011, he was admitted to our unit for recurrence of jaundice and elevated markers of cholestasis.
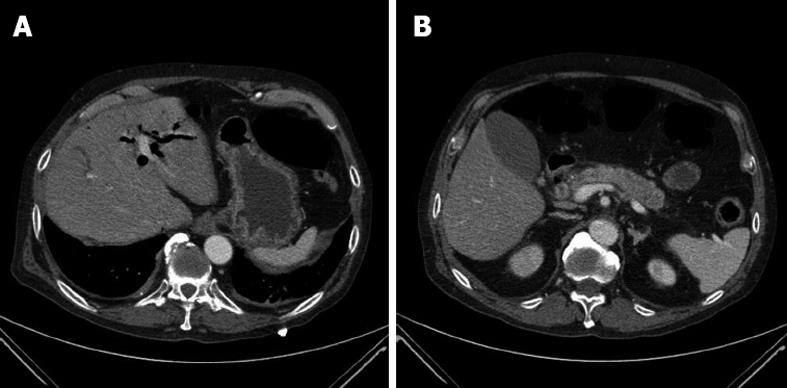
Physical examination on admission was unremarkable, except for mild bilateral submandibular and lacrimal glands swelling. Laboratory tests showed hyperbilirubinemia (direct/total 4.5/4.3 mg/dL), alkaline phosphatase 3 × ULN, GGT 10 × ULN, aspartate aminotransferase and alanine aminotransferase 3 × ULN, pancreatic amylase and lipase 2 × ULN, gamma-globulins 3 × ULN, and creatinine 2 mg/dL (Table 1). Anti-hepatitis C virus and hepatitis B surface antigen and non organ specific autoantibodies (antinuclear antibodies, anti-mitochondral antibodiy, smooth-muscle antibodies, liver kidney microsomal antibody, liver cytosolic-1) were negative. Abdominal US revealed a hypoechoic lesion of 2.3 cm in the uncinate process of the pancreas, dilatation of intrahepatic and extrahepatic bile ducts (up to 9 mm for the common bile duct) and of Wirsung’s duct (4 mm), with no gallstones or biliary sludge. CT scans of the abdomen showed an increased volume of the pancreas, especially in the head, loss of physiological lobular appearance, dilatation of intrahepatic and extrahepatic bile ducts, with wall thickening and contrast enhancement in the arterial phase of distal common bile duct (Figure 1), and a diffuse bilateral enlargement of the kidneys. The patient declined to have a liver and/or pancreatic biopsy performed.
IgG4 levels were 5.95 g/L (normal range: 0.08-1.4 g/L), pointing to a diagnosis of IgG4-related pancreatitis and possibly cholangitis. Suspecting the coexistence of IgG4-related kidney disease (IgG4-RKD), the etiology of renal failure was re-evaluated. Laboratory tests confirmed a creatinine value of 2 mg/dL, normal serum and urinary electrolytes, the absence of proteinuria and hematuria, and normal values of immunoglobulin free light chains in serum and urine. It was not possible to reassess the previous renal biopsy, performed elsewhere.
In view of the mild bilateral submandibular and lacrimal glands swelling and the worsening of previously present xerostomia and xerophthalmia, a salivary flow test and Schirmer’s test were performed, showing a deficiency of both salivary and lacrimal secretion. Tests for anti-Sjögren syndrome A and B antibodies and anti-extractable nuclear antibodies were negative. A biopsy of the salivary glands showed the absence of lymphocyte and plasma cell infiltration.
Finally, the previous diagnosis of rheumatoid arthritis was ruled out because of no involvement of small joints, a normal X-ray of the hand, negative serology (rheumatoid factor and anticitrullinated peptide antibody) and normal acute-phase reactants (C-reactive protein and erythrocyte sedimentation rate).
The patient was given oral prednisone (0.6 mg/kg per day for 4 wk, then tapered over a period of 12 wk to a maintenance dose of 10 mg/d). After the first 4 wk, there was a marked general improvement, with regression of xerophthalmia and xerostomia and a rapid reduction to normalization of aminotransferases, alkaline phosphatase, GGT, bilirubin, creatinine, amylase, lipase, gamma-globulins and IgG4 levels (Table 1).
At the 12th week of follow up, abdominal US was negative and magnetic resonance imaging showed a resolution of the radiological picture, with persistence of minimal dilation of the left biliary branch (Figure 2).
Considering the coexistence of diabetes and osteoporosis, the dose of prednisone was reduced to 5 mg/d and azathioprine 1.5 mg/kg per d added as a glucocorticoid-sparing agent. At the 36th week of follow-up, abdominal US and biochemical tests were persistently normal (Table 1) and the patient is currently asymptomatic.
IgG4-RD may involve several organs, including the pancreas, biliary duct, salivary glands, kidneys, lungs, retroperitoneum and lymph nodes. The peculiarity of our patient lies in the progression towards a systemic multi-organ expression of the disease.
Diagnostic criteria for systemic IgG4-RD are not fully established. To date, only the criteria for IgG4-related pancreatitis, cholangitis, nephritis and glandular disease are available. However, they are not suitable for the diagnosis of other organ involvement. In this case, the diagnosis of pancreatitis was confirmed by both Asian diagnostic criteria[6] and HISORt criteria for autoimmune pancreatitis[7]. Diagnosis of IgG4-related cholangitis was confirmed by HISORt criteria for IgG4-associated cholangitis[8]. Liver and/or pancreatic biopsies were not performed; they cannot be deemed essential for diagnosis, since two out of three for Asian diagnostic criteria and two out of five for HISORt criteria were satisfied. For evidence of renal damage and CT-documented enlargement of the kidneys in a context of an IgG4-RD, the etiology of renal failure was also re-evaluated. According to the criteria proposed by the Japanese Society of Nephrology[9], the diagnosis of IgG4-RKD was labeled as “possible”. Because of the presence of xerostomia and xerophthalmia, a salivary glands biopsy was performed, showing a non-specific chronic inflammation. In the absence of specific histology, it was not possible to confirm the diagnosis of IgG4-related glandular disease because all diagnostic criteria were not satisfied[10]. Nevertheless, the probability of a false negative due to the previous long-term steroid therapy should be considered. The diagnosis of rheumatoid arthritis was finally ruled out and the persistent arthralgias may be related to IgG4-RD.
Despite the absence of histological confirmation, IgG4-related involvement of kidneys, salivary and lacrimal glands was strengthened by the full clinical and biochemical remission of respective manifestations after steroidal therapy.
Treatment with immunosuppressive agents is effective in IgG4-RD. Due to the rarity of the condition, there are currently no RCTs to point to the optimal therapeutic approach. Following a consensus statement from 17 referrals centers in Japan[11], we treated the patient with prednisone as first line therapy, with subsequent introduction of azathioprine, obtaining a complete clinical, biochemical and radiological response with good tolerability.
Our experience underlines the complexity of IgG4-RD and its variable and sometimes progressive presentation, while pointing out the need for a careful and complete assessment for possible multi-organ involvement.
P- Reviewers Ishibashi H, Ozaslan E S- Editor Gou SX L- Editor Roemmele A E- Editor Li JY
| 1. | Takahashi H, Yamamoto M, Suzuki C, Naishiro Y, Shinomura Y, Imai K. The birthday of a new syndrome: IgG4-related diseases constitute a clinical entity. Autoimmun Rev. 2010;9:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1863] [Article Influence: 143.3] [Reference Citation Analysis (83)] |
| 6. | Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 416] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 656] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 8. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 9. | Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, Yamanaka N, Inoue D, Yamamoto M, Takahashi H. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 10. | Masaki Y, Sugai S, Umehara H. IgG4-related diseases including Mikulicz’s disease and sclerosing pancreatitis: diagnostic insights. J Rheumatol. 2010;37:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |