Published online Apr 27, 2013. doi: 10.4254/wjh.v5.i4.189
Revised: October 20, 2012
Accepted: November 25, 2012
Published online: April 27, 2013
AIM: To investigate the pathogenesis of non-alcoholic fatty liver disease (NAFLD) after pancreatoduodenectomy (PD).
METHODS: A cohort of 82 patients who underwent PD at Okayama University Hospital between 2003 and 2009 was enrolled and the clinicopathological features were compared between patients with and without NAFLD after PD. Computed tomography (CT) images were evaluated every 6 mo after PD for follow-up. Hepatic steatosis was diagnosed on CT when hepatic attenuation values were 40 Hounsfield units. Liver biopsy was performed for 4 of 30 patients with NAFLD after PD who consented to undergo biopsies. To compare NAFLD after PD with NAFLD associated with metabolic syndrome, liver samples were obtained from 10 patients with NAFLD associated with metabolic syndrome [fatty liver, n = 5; non-alcoholic steatohepatitis (NASH), n = 5] by percutaneous ultrasonography-guided liver biopsy. Double-fluorescence immunohistochemistry was applied to examine CD14 expression as a marker of lipopolysaccharide (LPS)-sensitized macrophage cells (Kupffer cells) in liver biopsy specimens.
RESULTS: The incidence of postoperative NAFLD was 36.6% (30/82). Univariate analysis identified cancer of the pancreatic head, sex, diameter of the main pancreatic duct, and dissection of the nerve plexus as factors associated with the development of NAFLD after PD. Those patients who developed NAFLD after PD demonstrated significantly decreased levels of serum albumin, total protein, cholesterol and triglycerides compared to patients without NAFLD after PD, but no glucose intolerance or insulin resistance. Liver biopsy was performed in four patients with NAFLD after PD. All four patients showed moderate-to-severe steatosis and NASH was diagnosed in two. Numbers of cells positive for CD68 (a marker of Kupffer cells) and CD14 (a marker of LPS-sensitized Kupffer cells) were counted in all biopsy specimens. The number of CD68+ cells in specimens of NAFLD after PD was significantly increased from that in specimens of NAFLD associated with metabolic syndrome specimens, which indicated the presence of significantly more Kupffer cells in NAFLD after PD than in NAFLD associated with metabolic syndrome. Similarly, more CD14+ cells, namely, LPS-sensitized Kupffer cells, were observed in NAFLD after PD than in NAFLD associated with metabolic syndrome. Regarding NASH, more CD68+ cells and CD14+ cells were observed in NASH after PD specimens than in NASH associated with metabolic syndrome. This showed that more Kupffer cells and more LPS-sensitized Kupffer cells were present in NASH after PD than in NASH associated with metabolic syndrome. These observations suggest that after PD, Kupffer cells and LPS-sensitized Kupffer cells were significantly upregulated, not only in NASH, but also in simple fatty liver.
CONCLUSION: NAFLD after PD is characterized by both malnutrition and the up-regulation of CD14 on Kupffer cells. Gut-derived endotoxin appears central to the development of NAFLD after PD.
- Citation: Satoh D, Yagi T, Nagasaka T, Shinoura S, Umeda Y, Yoshida R, Utsumi M, Tanaka T, Sadamori H, Fujiwara T. CD14 upregulation as a distinct feature of non-alcoholic fatty liver disease after pancreatoduodenectomy. World J Hepatol 2013; 5(4): 189-195
- URL: https://www.wjgnet.com/1948-5182/full/v5/i4/189.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i4.189
The prevalence of non-alcoholic fatty liver disease (NAFLD) associated with metabolic syndrome is increasing worldwide[1,2]. NAFLD associated with metabolic syndrome is considered to be associated with chronic over-nutrition that results in the accumulation of visceral fat and obesity and is one of the most common forms of chronic liver disease[3]. NAFLD associated with metabolic syndrome is related to states such as severe obesity, impairment of glucose tolerance and hyperlipidemia[4]. Indeed, several studies have shown strong relationships among NAFLD associated with metabolic syndrome, hepatic insulin resistance and type 2 diabetes mellitus[5-7].
On the other hand, NAFLD sometimes develops after pancreatoduodenectomy (PD)[8]. Tanaka et al[9] recently reported that NAFLD after PD was characterized by non-obesity and a lack of both hyperlipidemia and insulin resistance. The pathogenesis of NAFLD after PD may thus differ from the pathogenesis of NAFLD associated with metabolic syndrome.
Recently, Tilg et al[10] proposed a new model, suggesting that many hits may act in parallel to finally result in liver inflammation, with gut and adipose tissue-derived factors in particular playing a central role. It was reported that the clinical features of the patients with NAFLD after PD are similar to those found in the murine non-alcoholic steatohepatitis (NASH) model induced by a methionine-choline-deficient (MCD) diet[9]. In mice fed the MCD diet, portal endotoxemia due to an impaired gut barrier caused by the MCD diet was observed[11]. We therefore hypothesized that gut-derived lipopolysaccharide (LPS) was associated with NAFLD after PD. LPS might trigger the release of inflammatory cytokines from Kupffer cells, in turn mediating severe hepatic steatosis and liver injury after PD. The process by which LPS activates Kupffer cells seems to be mediated by LPS-binding protein, CD14 and toll-like receptor 4[12]. We therefore focused on the CD14 expression on Kupffer cells from liver specimens in cases of NAFLD after PD.
We examined the prevalence, clinical and histological features, and expression of CD14 as a marker of LPS-sensitized Kupffer cells in liver tissues obtained from patients with either NAFLD associated with metabolic syndrome or NAFLD after PD.
Between February 2003 and August 2009, a total of 100 patients underwent PD at Okayama University Hospital, Okayama, Japan. Of these, 18 patients were excluded from the study because they were unavailable for regular follow-up computed tomography (CT) of the abdomen. The remaining 82 patients (49 men and 33 women) were enrolled in this study. There were no patients with preoperative NAFLD, based on CT and laboratory findings. The mean age at the time of surgery was 63 years (range 31-85 years). Histological diagnosis was pancreatic carcinoma in 32 patients, intraductal papillary-mucinous neoplasm in 27, bile duct carcinoma in 3, and others in 20. In terms of surgical procedures, conventional PD was employed for 73 patients and pylorus-preserving PD for 9 patients. For patients with adenocarcinoma of the pancreatic head, we routinely performed dissection of the nerve plexus around the superior mesenteric artery (SMA), leaving the left side of the SMA near the origin intact. A modification of Child’s method was employed for digestive reconstruction.
Body mass index (BMI) was determined for all patients and blood examinations were performed before and every 3 mo after PD. Follow-up continued for more than 12 mo in all cases. Routine blood examinations included fasting lipid, blood glucose and insulin levels, and levels of hemoglobin (HbA1c), asparate aminotransferase (AST), alanine aminotransferase, total protein, albumin and cholinesterase.
CT images were obtained using a 16-multidetector CT scanner (GE Yokogawa, Tokyo, Japan) without intravenous contrast medium. The raw data set was reconstructed at 5 mm thickness. CT images were evaluated every 6 mo after PD for follow-up. Hepatic steatosis was diagnosed on CT when hepatic attenuation values were 40 Hounsfield units. According to this criterion, 30 of 82 patients who underwent PD were found to have newly appearing hepatic steatosis.
Liver biopsy was performed for 4 of 30 patients with NAFLD after PD who consented to undergo biopsies (fatty liver, n = 2; NASH, n = 2); in these two men and two women, the mean age was 61 (52-73) years, histological diagnosis was pancreatic carcinoma in one patient, intraductal papillary-mucinous neoplasm in two, and serous cyst adenoma in one, and all of them underwent PD. To compare NAFLD after PD with NAFLD associated with metabolic syndrome, liver samples were obtained from 10 patients with NAFLD associated with metabolic syndrome (fatty liver, n = 5; NASH, n = 5) by percutaneous ultrasonography-guided liver biopsy; these four men and six women had a mean age of 52 (40-64) years. In 10 patients, NAFLD associated with metabolic syndrome was diagnosed by the following criteria: (1) the absence of regular intake of alcohol and past history of abdominal surgery; (2) negative results for hepatitis B virus surface antigen and anti-hepatitis C virus antibodies; and (3) the absence of other types of chronic liver disease.
Specimens were fixed in 40 g/L neutral-buffered formaldehyde, cut at 4 μm thickness and stained using hematoxylin and eosin or the Azan-Mallory method. Histological findings were assessed in a blinded fashion by an independent pathologist. Histological diagnosis of NASH was made based on the presence of macrovesicular steatosis, hepatocyte ballooning and lobular inflammation.
For double immunofluorescence, tissue array slides were deparaffinized and soaked in 0.01 mol/L citrate buffer (pH 6.0) at 90 °C for 30 min for antigen retrieval. Samples were treated with 10 mg/mL bovine serum albumin (BSA) to inhibit non-specific antibody binding, then incubated with primary murine monoclonal antibody to CD68 (Kp-1, dilution 1:500; Dako, Glostrup, Denmark) for 1 h at 37 °C. After washing three times with phosphate-buffered saline (PBS) (pH 7.2), samples were incubated with Cy5-labeled secondary rabbit polyclonal antibody to murine immunoglobulin G for 30 min at 37 °C. For the second immunoreaction, a similar procedure was used: samples were treated with 10 mg/mL BSA, then incubated with another primary antibody to CD14 (dilution 1:100; Zymed Laboratories, San Francisco, CA), then incubated with fluoresceinisothiocyanate isomer (FITC)-labeled secondary antibody. After washing with PBS, Cy5-labeled and FITC-labeled samples were examined using a fluorescence microscope (SZX12; Olympus, Tokyo, Japan).
Frequencies of double-positive cells were determined by histology experts counting these cells in entire specimens. Double-positive cells in the hepatic lobule were counted in five high-power fields (original magnification ×60). Cell counts are expressed as the mean ± SD in each specimen.
Statistical analysis were performed using SPSS for Windows version 11.0 software (SPSS, Chicago, IL, United States). Continuous variables are expressed as mean ± SD and the statistical significance of differences was determined using Student’s t test. Comparisons between groups were made using the χ2 test for categorical variables. Values of P < 0.05 were considered statistically significant.
The median follow-up for the 82 patients who underwent PD was 840 d (range, 183-2553 d). The frequency of NAFLD after PD was 36.6% (30/80). Clinical pre- and intraoperative data for patients with NAFLD (n = 30, 36.6%) and patients without NAFLD (n = 52, 63.4%) are summarized in Table 1. Univariate analysis identified cancer of the pancreatic head, sex, diameter of the main pancreatic duct (MPD) and dissection of the nerve plexus as factors associated with the development of NAFLD after PD.
| Non-NAFLD | NAFLD | P value1 | |
| (n = 52) | (n = 30) | ||
| BMI (kg/m2) | 21.7 ± 3.3 | 21.4 ± 2.6 | 0.622 |
| Age (yr) | 63.6 ± 10.9 | 63.4 ± 11.9 | 0.940 |
| Pancreatic head cancer (yes) | 31% | 53% | < 0.0012 |
| Sex (male) | 69% | 43% | 0.0352 |
| Diameter of MPD (mm) | 3.9 ± 3.2 | 5.6 ± 3.2 | 0.025 |
| Total cholesterol (mg/dL)3 | 188.2 ± 53.3 | 202.0 ± 39.9 | 0.238 |
| Triglycerides (mg/dL)3 | 119.8 ± 69.2 | 139.9 ± 74.8 | 0.363 |
| Total protein (g/dL)3 | 8.0 ± 0.9 | 8.3 ± 0.5 | 0.322 |
| Albumin (g/dL)3 | 4.0 ± 0.4 | 4.0 ± 0.5 | 0.761 |
| Cholinesterase (U/L)3 | 230.6 ± 88.2 | 244.5 ± 104.9 | 0.531 |
| Insulin (μU/mL)3 | 5.5 ± 2.3 | 7.7 ± 3.5 | 0.500 |
| HOMA-IR3 | 1.6 ± 1.0 | 3.2 ± 3.4 | 0.324 |
| HbA1c (%)3 | 5.5 ± 0.8 | 6.2 ± 1.8 | 0.060 |
| ALT3 | 65.6 ± 0.8 | 79.9 ± 107.3 | 0.506 |
| Operation (PPPD) | 15% | 10% | 0.7382 |
| Operation time (min) | 400.9 ± 49.0 | 394.6 ± 37.8 | 0.544 |
| Intraoperative blood loss (mL) | 425.2 ± 171.9 | 419.3 ± 155.6 | 0.878 |
| Patients with nerve plexus dissection | 15% | 93% | < 0.0012 |
| Pancreatic resection line (SMA) | 19% | 37% | 0.0812 |
Clinical postoperative data for patients with and without NAFLD are summarized in Table 2. The BMI, serum levels of total protein and albumin, and levels of cholinesterase, total cholesterol and insulin were significantly lower in patients with NAFLD after PD than in patients without NAFLD. Homeostasis model assessment for insulin resistance values, as an indicator of insulin resistance, and serum levels of triglycerides and HbA1c did not differ between these two groups.
| Non-NAFLD | NAFLD | P value1 | |
| (n = 52) | (n = 30) | ||
| BMI (kg/m2)2 | 20.1 ± 3.5 | 17.4 ± 2.8 | 0.004 |
| Total cholesterol (mg/dL)2 | 159.2 ± 40.4 | 133.3 ± 36.4 | 0.011 |
| Triglycerides (mg/dL)2 | 102.2 ± 36.4 | 84.9 ± 49.0 | 0.169 |
| Total protein (g/dL)2 | 7.0 ± 0.7 | 6.1 ± 0.7 | < 0.001 |
| Albumin (g/dL)2 | 4.0 ± 0.5 | 3.1 ± 0.7 | < 0.001 |
| Cholinesterase (U/L)2 | 215.5 ± 78.5 | 156.9 ± 88.1 | 0.006 |
| Insulin (μU/mL)2 | 9.7 ± 9.8 | 4.1 ± 3.8 | 0.009 |
| HOMA-IR2 | 3.1 ± 3.3 | 1.3 ± 1.2 | 0.117 |
| HbA1c (%)2 | 5.5 ± 0.9 | 5.6 ± 0.9 | 0.589 |
| ALT2 | 31.6 ± 18.5 | 52.0 ± 45.1 | 0.033 |
| Insulin treatment | 12% | 27% | 0.126 |
At 6 mo postoperatively, 17 of the 30 patients (57%) with NAFLD after PD showed increased blood levels of AST. Of these 17 patients, we performed liver biopsies to pathologically examine specimens from four patients [mean serum levels of AST was 67 (54-80) U/L] who consented to undergo biopsies. Histopathological examination diagnosed two cases as stage 2 NASH, according to the Brunt criteria[13], and two cases as moderate-to-severe macrovesicular steatosis.
In the 10 patients with NAFLD associated with metabolic syndrome, the mean serum level of AST was 56 (35-112) U/L. Five of 10 patients showed moderate-to-severe macrovesicular steatosis and the remaining five patients showed stage 2 NASH in histological examination.
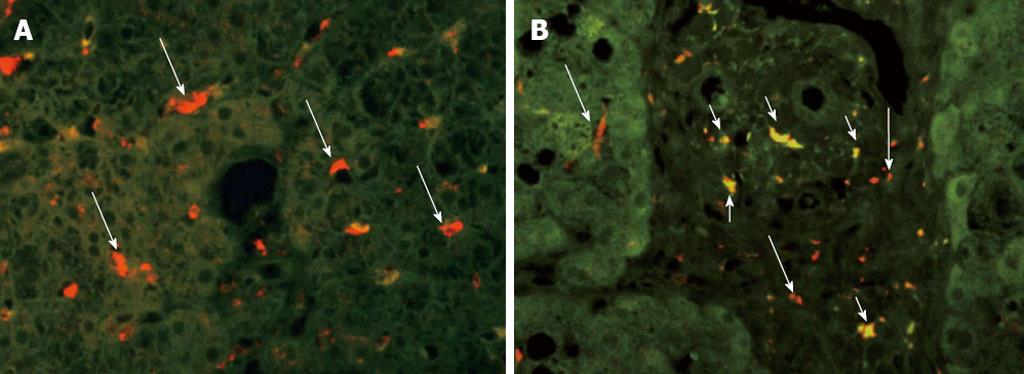
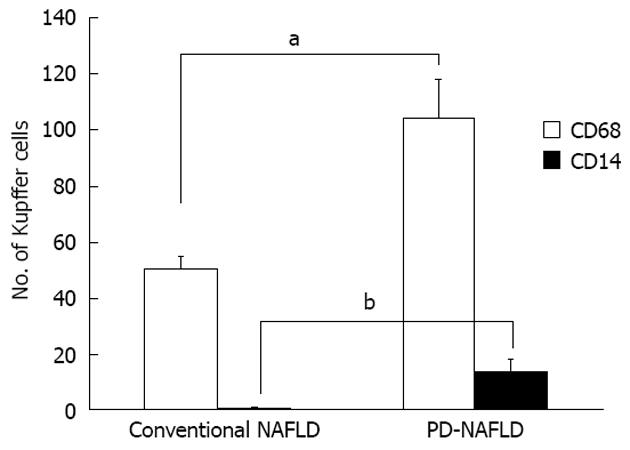
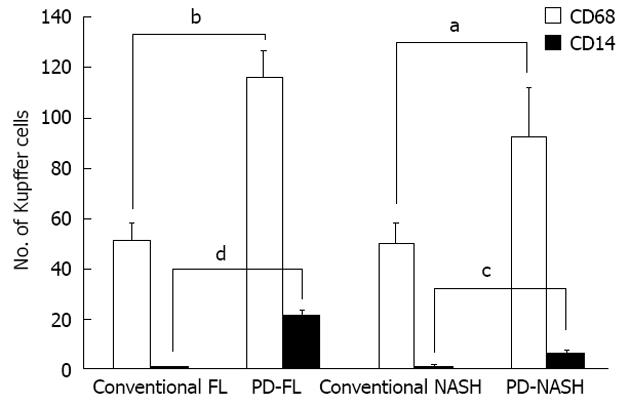
Numbers of cells positive for CD68 (a marker of Kupffer cells) and CD14 (a marker of LPS-sensitized Kupffer cells) were counted in all biopsy specimens (Figure 1). The mean number of CD68+ cells per individual was 50.6 ± 4.0 in specimens from NAFLD associated with metabolic syndrome and 104.3 ± 13.3 in specimens from NAFLD after PD, indicating the presence of significantly more Kupffer cells in NAFLD after PD than in NAFLD associated with metabolic syndrome (P < 0.001) (Figure 2). CD14+ cells were observed in specimens from NAFLD associated with metabolic syndrome and in specimens from NAFLD after PD, with mean positive cell counts of 0.6 ± 0.3 and 13.5 ± 4.2, respectively, showing more LPS-sensitized Kupffer cells in NAFLD after PD than in NAFLD associated with metabolic syndrome (P < 0.001) (Figure 2). We also attempted to classify NAFLD into simple fatty liver or NASH. As for simple fatty liver, mean numbers of CD68+ cells and CD14+ cells per individual were 51.0 ± 6.8 and 0.4 ± 0.2, respectively, in specimens from fatty liver associated with metabolic syndrome and 116.0 ± 10.7 and 20.5 ± 2.5, respectively, in specimens from fatty liver after PD. This indicated the presence of more Kupffer cells and more LPS-sensitized Kupffer cells in specimens from simple fatty liver after PD than in specimens from simple fatty liver associated with metabolic syndrome (P < 0.001, CD68+ cells; P < 0.001, CD14+ cells). Regarding NASH, the mean numbers of CD68+ cells and CD14+ cells per individual were 50.2 ± 7.4 and 0.8 ± 0.6, respectively, in specimens from NASH associated with metabolic syndrome and 92.5 ± 19.5 and 6.5 ± 0.5, respectively, in specimens from NASH after PD. This showed that more Kupffer cells and more LPS-sensitized Kupffer cells were present in specimens from NASH after PD than in specimens from NASH associated with metabolic syndrome (CD68+ cells, P < 0.05; CD14+ cells, P < 0.05) (Figure 3). These observations suggest that after PD, Kupffer cells and LPS-sensitized Kupffer cells were significantly upregulated, not only in NASH, but also in simple fatty liver.
Conventional NAFLD is thought to be caused by excessive nutrition, lipid metabolic disorder and insulin resistance, as a part of metabolic syndrome[4]. However, the present study demonstrated that NAFLD after PD was related to non-obese status, malnutrition and lack of hyperlipidemia and insulin resistance. These findings suggest that the mechanisms underlying NAFLD after PD differ from those causing NAFLD associated with metabolic syndrome. Recent studies have also suggested that pancreatic exocrine insufficiency may cause NAFLD after PD[8,9]. Although only univariate analyses were examined in our study, pancreatic cancer was associated with the development of NAFLD, which is considered to lead to impaired pancreatic exocrine functions due to obstruction of the MPD, in turn resulting in obstructive distal pancreatic atrophy and fibrosis.
The clinical features of patients with NAFLD after PD resemble those in mice with MCD-induced NASH, in terms of the absence of obesity, insulin resistance and hypocholesterolemia[14]. Increased fatty acid uptake and decreased hepatic export of triglycerides in the form of very-low-density lipoprotein (VLDL) represent two important mechanisms contributing to MCD-induced NASH[14,15]. Malabsorption of essential amino acids such as choline due to pancreatic exocrine insufficiency may result in the development of NAFLD after PD.
NAFLD is often observed in patients showing hypoinsulinemia. Insulin inhibits lipolysis in adipose tissue and decreases the flux of free fatty acids (FFA) into plasma. In the absence of adequate insulin secretion, plasma FFA levels could conceivably be elevated due to increased adipose tissue lipolysis and the liver would then be unable to adequately couple triglycerides to apoprotein B or secrete VLDL-triglyceride, resulting in hepatic steatosis[16]. In this study, serum insulin concentrations were significantly lower in NAFLD after PD compared with non-NAFLD after PD. Insufficiency of insulin may be another factor contributing to the development of NAFLD after PD.
We performed liver biopsy in four patients with NAFLD after PD, diagnosing two cases of NASH. Kupffer cell counts have been shown to play important roles in the pathogenesis of NASH[17]. Within the liver, Kupffer cells are major sources of tumor necrosis factor-alpha (TNF-α) produced in response to LPS[12,18,19]. The process by which LPS activates Kupffer cells seems to be mediated by LPS-binding protein, CD14 and toll-like receptor 4[20]. We therefore focused on CD14 expression in liver specimens from NAFLD after PD because the promoter polymorphism of CD14 has been reported as a risk factor for both alcoholic and non-alcoholic steatohepatitis[21]. CD14 expression on Kupffer cells is low in the healthy human liver[22,23] but increases in the presence of inflammatory liver disease[24]. Expression of CD14 on Kupffer cells can be upregulated with LPS[25,26]. A previous study reported that TNF-α production was decreased in genetically engineered CD14-deficient mice by downregulating sensitivity to LPS[27]. In contrast, the CD14 transgenic mice that overexpress CD14 on monocytes showed increased sensitivity to LPS[28]. These changes in CD14 expression could represent a mechanism regulating liver sensitivity to LPS toxicity.
The present study demonstrated that Kupffer cells were significantly more common and LPS-induced CD14+ Kupffer cells were upregulated in NAFLD after PD specimens compared to NAFLD associated with metabolic syndrome specimens. Furthermore, even in simple fatty liver after PD specimens, more Kupffer cells and more LPS-sensitized Kupffer cells were present than in specimens from simple fatty liver associated with metabolic syndrome. These findings indicate that LPS plays a significant role in the occurrence of NAFLD after PD, even from an early stage.
Previous reports have hypothesized that the overgrowth of small intestinal bacteria might play a contributory role in NASH pathogenesis, particularly via a component of the gram-negative bacterial population, through the production of LPS[29-33]. Among those patients who underwent PD, bacterial overgrowth may have occurred due to dissection around the SMA, leading to intestinal motor dysfunction and stasis, decreased secretion of gastric juices or blind loops.
The fatty liver is vulnerable to additional inflammatory insults, such as oxidative stress and gut-derived bacterial endotoxins, both of which can trigger hepatocellular inflammation and fibrosis[34,35]. Accumulation of FFA in the liver due to malabsorption of essential amino acids such as choline and gut-derived LPS perhaps from intestinal bacterial overgrowth are important in the pathogenesis of NAFLD after PD.
In conclusion, NAFLD after PD is characterized, not only by malnutrition, but also by up-regulation of CD14 on Kupffer cells with hepatic steatosis. Our results suggest that gut-derived endotoxin contributes to the development of NAFLD after PD.
Non-alcoholic fatty liver disease (NAFLD), which sometimes develops after pancreatoduodenectomy (PD), is characterized by non-obesity and a lack of both hyperlipidemia and insulin resistance. The pathogenesis of NAFLD after PD may thus differ from the pathogenesis of NAFLD associated with metabolic syndrome.
It was reported that the clinical features of the patients with NAFLD after PD are similar to those found in the murine non-alcoholic steatohepatitis model induced by a methionine-choline-deficient (MCD) diet. In mice fed the MCD diet, portal endotoxemia was observed. Thus, gut-derived factors such as lipopolysaccharide (LPS) appear to be related with NAFLD after PD. The authors hypothesized that gut-derived factors such as LPS might trigger the release of inflammatory cytokines from Kupffer cells, in turn mediating severe hepatic steatosis and liver injury after PD. The process by which LPS activates Kupffer cells seems to be mediated by LPS-binding protein, CD14 and toll-like receptor 4. The authors therefore focused on CD14 expression on Kupffer cells in liver specimens from cases of NAFLD after PD.
Unlike the patients with NAFLD associated with metabolic syndrome, the patients who developed NAFLD after PD showed significantly decreased levels of serum albumin, total protein, cholesterol and triglycerides, but no glucose intolerance or insulin resistance. Furthermore, the present study demonstrated that Kupffer cells were significantly more common and LPS-induced CD14+ Kupffer cells were upregulated in specimens of NAFLD after PD compared to specimens of NAFLD associated with metabolic syndrome. These findings indicate that LPS plays a significant role in the occurrence of NAFLD after PD. NAFLD after PD is characterized by both malnutrition and up-regulation of CD14 on Kupffer cells. Gut-derived endotoxin appears central to the development of NAFLD after PD.
Elucidation of the pathogenesis of NAFLD after PD may serve to prevent the development of NAFLD after PD.
CD14: CD14 is the main LPS-receptor that can activate monocytes in conjunction with serum LPS-binding protein. CD14 expression on Kupffer cells is low in the healthy human liver but increases in the presence of inflammatory liver disease. Expression of CD14 on Kupffer cells can be upregulated with LPS..
The authors investigated the pathogenesis of NAFLD after PD. The paper is well written overall.
P- Reviewers Sookoian SC, Hudacko RM S- Editor Song XX L- Editor Roemmele A E- Editor Li JY
| 1. | Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [DOI] [Full Text] |
| 4. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1915] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 5. | Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 542] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1507] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 7. | Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1525] [Article Influence: 101.7] [Reference Citation Analysis (1)] |
| 8. | Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, Usui M, Sakurai H, Tabata M. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1807] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 11. | Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 558] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 12. | Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431-1433. [PubMed] |
| 13. | Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 476] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 14. | Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Soliman AT, Alsalmi I, Asfour M. Hypoinsulinaemia has an important role in the development of oedema and hepatomegaly during malnutrition. J Trop Pediatr. 1996;42:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 685] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 18. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 353] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Kremer M, Thomas E, Milton RJ, Perry AW, van Rooijen N, Wheeler MD, Zacks S, Fried M, Rippe RA, Hines IN. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Cubero FJ, Nieto N. Ethanol and arachidonic acid synergize to activate Kupffer cells and modulate the fibrogenic response via tumor necrosis factor alpha, reduced glutathione, and transforming growth factor beta-dependent mechanisms. Hepatology. 2008;48:2027-2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Brun P, Castagliuolo I, Floreani AR, Buda A, Blasone L, Palù G, Martines D. Increased risk of NASH in patients carrying the C(-159)T polymorphism in the CD14 gene promoter region. Gut. 2006;55:1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Antal-Szalmás P. Evaluation of CD14 in host defence. Eur J Clin Invest. 2000;30:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Takai N, Kataoka M, Higuchi Y, Matsuura K, Yamamoto S. Primary structure of rat CD14 and characteristics of rat CD14, cytokine, and NO synthase mRNA expression in mononuclear phagocyte system cells in response to LPS. J Leukoc Biol. 1997;61:736-744. [PubMed] |
| 24. | Tomita M, Yamamoto K, Kobashi H, Ohmoto M, Tsuji T. Immunohistochemical phenotyping of liver macrophages in normal and diseased human liver. Hepatology. 1994;20:317-325. [PubMed] |
| 25. | Ziegler-Heitbrock HW, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121-125. [PubMed] |
| 26. | Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671-1676. [PubMed] |
| 27. | Haziot A, Ferrero E, Lin XY, Stewart CL, Goyert SM. CD14-deficient mice are exquisitely insensitive to the effects of LPS. Prog Clin Biol Res. 1995;392:349-351. [PubMed] |
| 28. | Ferrero E, Jiao D, Tsuberi BZ, Tesio L, Rong GW, Haziot A, Goyert SM. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:2380-2384. [PubMed] |
| 29. | Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010;55:560-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 30. | Shanab AA, Scully P, Crosbie O, Buckley M, O’Mahony L, Shanahan F, Gazareen S, Murphy E, Quigley EM. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1099] [Article Influence: 68.7] [Reference Citation Analysis (1)] |
| 32. | Wu WC, Zhao W, Li S. Small intestinal bacteria overgrowth decreases small intestinal motility in the NASH rats. World J Gastroenterol. 2008;14:313-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Nazim M, Stamp G, Hodgson HJ. Non-alcoholic steatohepatitis associated with small intestinal diverticulosis and bacterial overgrowth. Hepatogastroenterology. 1989;36:349-351. [PubMed] |
| 35. | Gao Y, Song LX, Jiang MN, Ge GY, Jia YJ. Effects of traditional chinese medicine on endotoxin and its receptors in rats with non-alcoholic steatohepatitis. Inflammation. 2008;31:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |