Published online Apr 27, 2013. doi: 10.4254/wjh.v5.i4.182
Revised: December 26, 2012
Accepted: January 29, 2013
Published online: April 27, 2013
Processing time: 219 Days and 2.5 Hours
AIM: To examine the epidemiological data, hematological safety and treatment responses of peginterferon-alpha 2a plus ribavirin therapy for hepatitis C.
METHODS: Between March 2008 and February 2011, 196 hepatitis C virus (HCV) genotype 1 infected Japanese (127 treatment-naive and 69 treatment-experienced patients) patients treated with peginterferon-alpha 2a plus ribavirin were enrolled. We examined the epidemiological data and treatment responses were retrospectively analyzed in terms of hematological safety. HCV RNA was measured by the COBAS TaqMan HCV test.
RESULTS: Overall sustained virological response (SVR) rates of treatment-naive and treatment-experienced patients were 56% and 39%, respectively. Multivariate logistic regression analysis showed that SVR was attained independently of early virological response in both treatment-naive and treatment-experienced patients. SVR rates did not differ between the pretreatment hemoglobin < 13 g/dL and ≥ 13 g/dL groups. However, in treatment-naive patients, the SVR rate of the pretreatment platelet count < 130000/µL group was significantly lower than that of the pretreatment platelet count ≥ 130000/µL group.
CONCLUSION: Attention should be paid to potential thrombocytopenia in the treatment of chronic hepatitis C patients.
- Citation: Kanda T, Kato K, Tsubota A, Takada N, Nishino T, Mikami S, Miyamura T, Maruoka D, Wu S, Nakamoto S, Arai M, Fujiwara K, Imazeki F, Yokosuka O. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol 2013; 5(4): 182-188
- URL: https://www.wjgnet.com/1948-5182/full/v5/i4/182.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i4.182
Chronic hepatitis C virus (HCV) infection leads to cirrhosis and hepatocellular carcinoma[1]. The combination of pegylated interferon alpha-2a or alpha-2b plus ribavirin is the standard of care (SOC) for HCV-infected patients[2]. This therapy leads to sustained virological response (SVR) in approximately 50% of patients[2]. In 2011, two HCV NS3/4A protease inhibitors, boceprevir and telaprevir, became available for HCV genotype 1 patients in United States and some other countries[3-6]. The addition of boceprevir or telaprevir to standard therapy with pegylated interferon plus ribavirin, compared with standard therapy alone, significantly increased the SVR rates in patients infected with HCV genotype 1[3-6].
Thrombocytopenia occasionally accompanies advanced chronic liver diseases[7] and is associated with the natural history of HCV infection and anti-viral therapy[8]. Thrombocytopenia is also one of the major obstacles when treating patients infected with HCV by pegylated interferon plus ribavirin with or without direct-acting antivirals, including boceprevir and telaprevir[9,10]. Diagnosis of thrombocytopenia in chronic hepatitis C patients is associated with increased incidences of certain comorbidities, complications and medical interventions, significantly increasing medical resource utilization[11].
Genome-wide association studies have recently revealed that interleukin 28B (IL28B) single nucleotide polymorphisms are significantly associated with the response to pegylated interferon-alpha plus ribavirin therapy for chronic hepatitis C[12-15] and that inosine triphosphatase (ITPA) gene variant protects against anemia during pegylated interferon-alpha plus ribavirin therapy for chronic hepatitis C[16]. However, severe hemoglobin decline, which is mainly found in ITPA-CC patients, was inversely correlated with thrombocytopenia, contributing to the association between severe anemia and a relative reactive increase in platelet count[17,18].
It is well known that improved adherence to medication will favorably affect SVR rates in pegylated interferon-alpha 2a plus ribavirin therapy for chronic hepatitis C[19,20]. Because the use of erythropoietin or hematopoietic growth factors was not allowed in these treatments by Japanese health insurance plans, hematological adverse events are the most common laboratory abnormalities, leading to dose modification or discontinuation. In the present study, we retrospectively analyzed the epidemiological data and treatment responses were retrospectively analyzed in terms of hematological safety.
From March 2008 through October 2011, patients were recruited from Chiba University and 30 hospitals in Chiba, Ibaraki and Saitama prefectures[21-23]. Patients were eligible if they met the following inclusion criteria: (1) infected with HCV genotype 1 alone; (2) age ≥ 20 years; (3) diagnosis of chronic hepatitis C based on positive HCV RNA; (4) negative for HBs antigen; (5) negative for human immunodeficiency viral antibody; (6) no high autoantibody titers; (7) no severe renal disease; (8) no severe heart disease; (9) no mental disorders; (10) no current intravenous drug abuse; and (11) no pregnancy[21].
The design of this study has been partly described[21-23]. 196 patients, who could be judged with SVR or non-SVR, were enrolled. In this study, 180 μg of pegylated interferon-alpha 2a per week plus 400-1200 mg of ribavirin daily comprised the usual treatment protocol for as long as 48 or 72 wk. Clinical and laboratory assessments were performed at least every 4 wk during treatment and a 24 wk follow-up period[22]. Adverse reactions were documented by oral inquiry, physical examinations and laboratory tests.
The COBAS TaqMan HCV test (Roche Diagnostics, Tokyo, Japan), with a range from 1.2 to 7.8 log IU/mL, was used for the measurement of HCV RNA levels every 4 wk before, during and for 24 wk after the end of treatment.
Serum alanine aminotransferase, other liver function and hematological tests were carried out by standard methods every 4 wk before, during and for 24 wk after the end of treatment.
SVR was defined as undetectable serum HCV RNA at 24 wk after the end of treatment. Patients with undetectable HCV RNA within the initial 4 wk of treatment were considered to have demonstrated a rapid virological response (RVR). Patients who had undetectable HCV RNA within the initial 12 wk of treatment were considered to have had a complete early virological response (cEVR) (described as EVR here).
This work was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The Ethics Committee of Chiba University School of Medicine approved the study protocol. Informed consent was obtained from all patients prior to enrollment.
Data were expressed as mean ± SD. Differences were evaluated by Student’s t test, χ2 test or Fisher’s exact test. P < 0.05 was considered statistically significant. Multivariate logistic regression analysis was used to determine factors that significantly contributed to SVR. Statistical analysis was performed using the Excel statistics program for Windows ver.7 (SSRI, Tokyo, Japan) and DA Stats software (O. Nagata, Nifty Serve: PAF01644).
Baseline characteristics of the patients are shown in Figure 1 and Table 1. Of 196 patients, 127 were treatment-naive and 69 had a history of interferon therapy with or without ribavirin. Higher HCV viral load (HCV RNA ≥ 5.0 log IU/mL) was seen in 95.2% (121/127) treatment-naive and 98.5% (68/69) treatment-experienced patients. In the 69 patients previously treated, 4 had received pegylated interferon-alpha 2a monotherapy, 14 standard interferon monotherapy, 6 standard interferon plus ribavirin, 38 pegylated interferon-alpha 2b plus ribavirin, 2 pegylated interferon-alpha 2a plus ribavirin, and 5 with unknown details. Concerning the virological response of the 69 patients to their previous treatment, 25 were relapsers, 26 were null-responders, and 18 were unknown. In the present study, of the 127 treatment-naive patients, 89 and 38 patients were treated for as long as 48 and 72 wk, respectively, and of the 69 treatment-experienced patients, 36 and 33 patients were treated for as long as 48 and 72 wk, respectively.
| Previous treatment | P value1 | ||
| (-) | (+) | ||
| Number of patients | 127 | 69 | |
| Age (yr) | 56.1± 10.7 | 59.0 ± 10.1 | 0.064 |
| Gender (male/female) | 62/65 | 34/35 | NS |
| Body mass index (kg/m2) | 23.4 ± 3.2 | 23.3 ± 3.9 | NS |
| LDL cholesterol (mg/dL) | 107 ± 52.3 | 102 ± 31.5 | NS |
| ALT (IU/L) | 72.0 ± 53.7 | 66.4 ± 48.5 | NS |
| Gamma-glutamyl transferase (IU/L) | 55.6 ± 74.8 | 67.0 ± 77.2 | NS |
| Alpha-fetoprotein (ng/mL) | 12.7 ± 30.0 | 25.9 ± 79.5 | NS |
| HCV RNA (log IU/mL) | 6.5 ± 0.7 | 6.4 ± 0.7 | NS |
| White blood cells (/mL) | 5213 ± 1519 | 4619 ± 1273 | 0.006 |
| Neutrophils (/mL) | 2752 ± 924 | 2474 ± 981 | 0.058 |
| Hemoglobin (g/dL) | 14.0 ± 1.4 | 13.7 ± 1.6 | NS |
| Platelets (× 104/mL) | 16.5 ± 5.0 | 15.6 ± 5.2 | NS |
| RVR (+/-) | 18/109 | 7/62 | NS |
| EVR (+/-) | 66/61 | 24/45 | 0.021 |
In the 127 treatment-naive patients, SVR was achieved in 56.6% (72/127) and non-SVR was seen in 43.3% (55/127) (27 relapsers, 11 null-responders and 17 stopped treatment due to adverse events) (Table 2). In these treatment-naive patients, there were significantly more male patients and higher neutrophil and platelet counts in the SVR group than in the non-SVR group at baseline (Table 2). Lower HCV viral load (HCV RNA < 5.0 log IU/mL) was seen in 8.3% (6/72) treatment-naive and 0% (0/55) treatment-experienced patients. RVR and EVR were significantly higher in the SVR group [25.0% (18/72) and 77.7% (56/72), respectively] than in the non-SVR group [0% (0/55) and 18.1% (10/45), respectively].
| Previous treatment | ||||||
| (-) | (+) | |||||
| SVR | Non-SVR | P value1 | SVR | Non-SVR | P value1 | |
| Number of patients | 72 | 55 | 27 | 42 | ||
| Age (yr) | 54.6 ± 10.9 | 58.0 ± 10.3 | NS | 58.5 ± 9.9 | 59.3 ± 10.3 | NS |
| Gender (male/female) | 41/31 | 21/34 | 0.036 | 11/16 | 23/19 | NS |
| Body mass index (kg/m2) | 23.4 ± 3.2 | 23.3 ± 3.9 | NS | 23.6 ± 3.6 | 22.8 ± 2.6 | NS |
| LDL cholesterol (mg/dL) | 107 ± 52.3 | 102 ± 31.5 | NS | 100 ± 31.3 | 92.8 ± 26.7 | NS |
| ALT (IU/L) | 67.6 ± 42.0 | 77.8 ± 65.9 | NS | 52.9 ± 35.4 | 75.1 ± 54.0 | NS |
| Gamma-glutamyl transferase (IU/L) | 45.1 ± 43.1 | 69.8 ± 102 | NS | 50.2 ± 51.1 | 76.9 ± 88.0 | NS |
| Alpha-fetoprotein (ng/mL) | 8.8 ± 9.7 | 17.4 ± 43.1 | NS | 13.3 ± 26.3 | 32.6 ± 96.5 | NS |
| HCV RNA (log IU/mL) | 6.4 ± 0.7 | 6.6 ± 0.6 | NS | 6.3 ± 0.9 | 6.4 ± 0.6 | NS |
| White blood cells (/mL) | 5363 ± 1582 | 5015 ± 1423 | NS | 4561 ± 1175 | 4657 ± 1345 | NS |
| Neutrophils (/mL) | 2910 ± 1006 | 2546 ± 767 | NS | 2337 ± 813 | 2562 ± 1074 | NS |
| Hemoglobin (g/dL) | 14.2 ± 1.5 | 13.8 ± 1.4 | NS | 13.8 ± 1.1 | 13.7 ± 1.9 | NS |
| Platelets (× 104/mL) | 17.5 ± 4.9 | 15.3 ± 4.8 | 0.013 | 16.5 ± 4.9 | 15.0 ± 5.3 | NS |
| RVR (+/-) | 18/54 | 0/55 | < 0.001 | 7/20 | 0/42 | < 0.001 |
| EVR (+/-) | 56/16 | 10/45 | < 0.001 | 8/19 | 5/37 | < 0.001 |
In the 69 patients previously treated, SVR was achieved in 39.1% (27/69) and non-SVR was seen in 60.8% (42/69) (23 relapsers, 14 null-responders and 5 stopped treatment due to adverse events) (Table 2). In these previously treated patients, the baseline backgrounds between the SVR and non-SVR groups did not differ (Table 2). RVR and EVR were significantly higher in the SVR group [25.9% (7/27) and 70.3% (19/27), respectively] than in the non-SVR group [0% (0/42) and 11.9% (5/42), respectively].
Multivariate analysis showed that EVR was significantly associated with SVR in treatment-naive patients and in treatment-experienced patients. For the EVR in the treatment-naive patients, odds ratio (OR) is 15.01 (95%CI: 5.72-44.56, P < 0.001); for the EVR in the treatment-experienced patients, OR is 21.7 (95%CI: 6.12-96.35, P < 0.001). Category is the same in the two groups. In treatment-naive patients, platelet count tended to be an independent factor in multivariate analysis. For the platelet counts, category > 16.1 × 104/μL, OR is 2.79 (95%CI: 0.98-8.57, P = 0.061).
Hematological adverse events are the most common laboratory abnormalities leading to dose modification or discontinuation[24]. First, we examined the effects of hemoglobin at baseline on the SVR rates.
In the 127 treatment-naive patients, there were no differences in SVR rates between the hemoglobin < 13 g/dL and hemoglobin ≥ 13 g/dL groups [55.2% (16/29) and 57.1% (56/98), respectively]. We also did not observe any difference in SVR rates between the hemoglobin < 13 g/dL and hemoglobin ≥ 13 g/dL groups in 48 wk treatment [55.6% (10/18) and 57.7% (41/71), respectively] or 72 wk treatment [54.5% (6/11) and 55.6% (15/27), respectively] in these patients.
In the 69 previously treated patients, there were no differences in SVR rates between the hemoglobin < 13 g/dL and hemoglobin ≥ 13 g/dL groups [30.0% (6/20) and 42.9% (21/49), respectively]. We also did not observe any difference in SVR rates between the hemoglobin < 13 g/dL and hemoglobin ≥ 13 g/dL groups in 48 wk treatment [38.5% (5/13) and 39.1% (9/23), respectively] or 72 wk treatment [14.3% (1/7) and 46.2% (12/26), respectively] (P = 0.126) in these patients.
Next, we examined the effects of platelet counts at baseline on the SVR rates. In the 127 treatment-naive patients, the SVR rate of the platelet count < 130000/μL group [37.0% (10/27)] was significantly lower than that of the platelet count ≥ 130000/μL group [62.0% (62/100)] (Table 3). We also observed a significantly lower SVR rate in the platelet count < 130000/μL group [35.0% (7/20)] than in the platelet count ≥ 130000/μL group [63.8% (44/69)] with 48 wk treatment. The RVR rate of the platelet count < 130000/μL group [15.0% (3/20)] was similar to that of the platelet count ≥ 130000/μL group [18.8% (13/69)] with 48 wk treatment, but the EVR rate of the platelet count < 130000/μL group [30.0% (6/20)] was significantly lower than that of the platelet count ≥ 130000/μL group [69.5% (48/69)] with 48 wk treatment (P = 0.0033). In contrast, there were no differences in SVR rates between the platelet count < 130000/μL [42.9% (3/7)] and ≥ 130000/µL groups with 72 wk treatment [58.1% (18/31)] (Table 3). The RVR and EVR rates of the platelet count < 130000/μL group [14.2% (1/7) and 57.1% (4/7), respectively] were similar to those of the platelet count ≥ 130000/μL group [3.2% (1/31) and 25.8% (8/31), respectively] with 72 wk treatment.
| < 130000/μL | ≥130000/μL | P value | |
| Proportion of SVR-archived in treatment-naive patients | |||
| Total patients | 10/27 (37.0%) | 62/100 (62.0%) | 0.020 |
| 48 wk treatment | 7/20 (35.0%) | 44/69 (63.8%) | 0.022 |
| 72 wk treatment | 3/7 (42.9%) | 18/31 (58.1%) | NS |
| Treatment-experienced patients | |||
| Total patients | 7/24 (29.2%) | 20/45 (44.4%) | NS |
| 48 wk treatment | 7/20 (33.3%) | 9/21 (42.9%) | NS |
| 72 wk treatment | 2/9 (22.2%) | 11/24 (45.8%) | NS |
In the 69 previously treated patients, there were no differences in SVR rates between the platelet count < 130000/µL and ≥ 130000/μL groups [29.2% (7/24) and 44.4% (20/45), respectively] (Table 3). We also did not observe any differences in SVR rates between the platelet count < 130000/μL and ≥ 130000/μL groups with 48 wk [33.3% (5/15) and 42.9% (9/21), respectively] or 72 wk treatment [22.2% (2/9) and 45.8% (11/24), respectively] in these patients (Table 3).
After HCV NS3/4A protease inhibitors began to be used in clinical practice, resulting side effects of their application for chronic hepatitis C were also expected to appear[6,24]. In fact, hematological adverse events became the most common laboratory abnormalities, leading to dose modifications or even discontinuation during SOC for chronic hepatitis C[25-30]. In the present study, we observed that EVR was significantly associated with SVR in both treatment-naive and treatment-experienced patients. According to multivariate analysis, RVR was not associated with SVR in either of the patient types, although the reason for this might be that RVR was obtained in only 25 patients. We also examined the epidemiological data and treatment responses were retrospectively analyzed in terms of hematological safety. We observed that the SVR and EVR rates of the platelet count ≥ 130000/μL group were better than those of the platelet count < 130000/μL group in treatment naive-patients (Table 3).
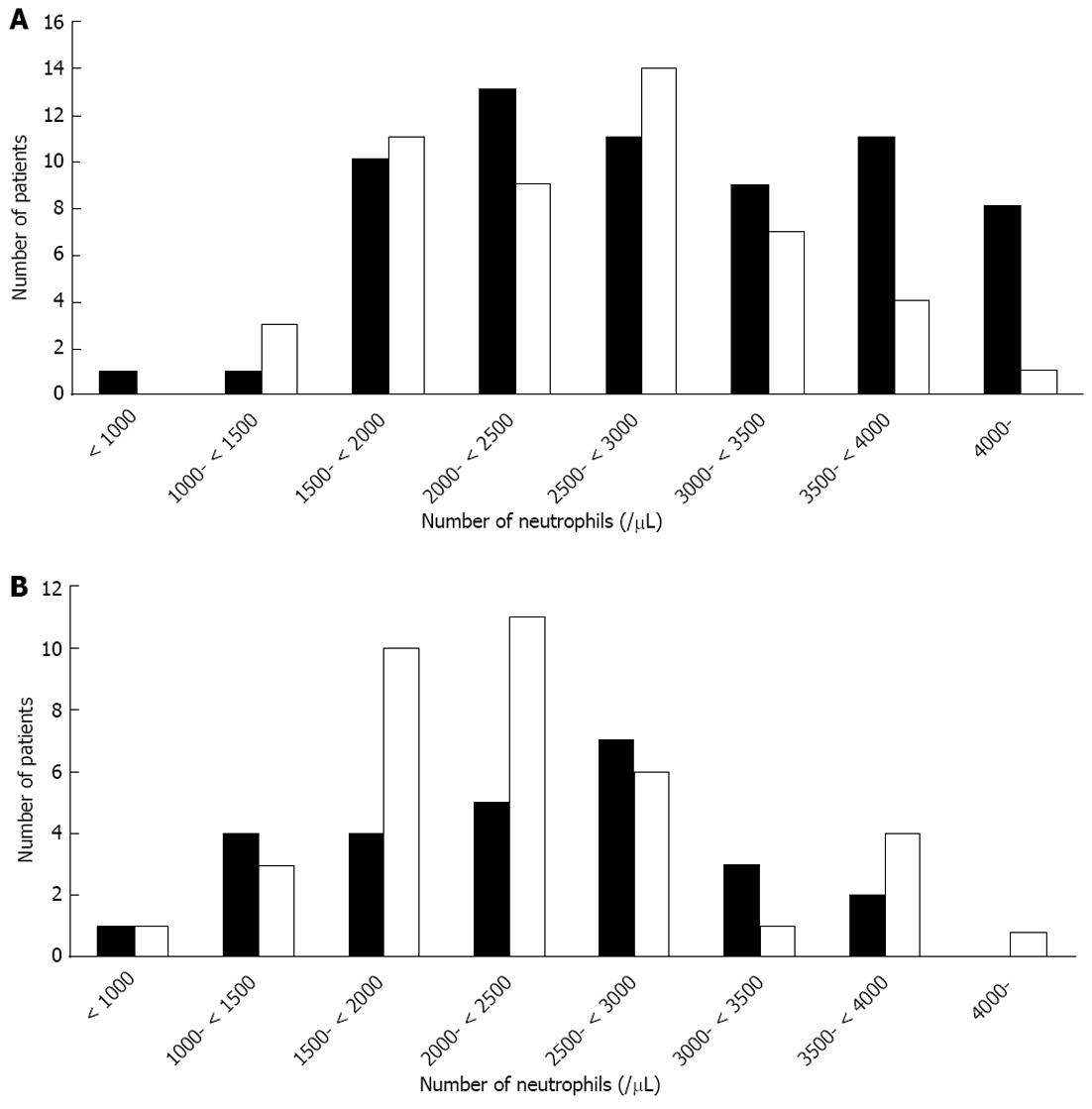
Unexpectedly, we did not observe any difference in SVR rates between the hemoglobin < 13 and ≥ 13 g/dL groups (see Results section) or according to neutrophil counts (Figure 1), although the hemoglobin level or WBC level was supposedly an important factor affecting adherence to treatment. We did not observe any association between baseline platelet count below 130000/μL and SVR in the treatment-experienced patients in the present study (Table 3). It may be possible that thrombocytopenia reflects the fact that patients with low platelet counts are more prone to being cirrhotic and therefore should have a lower response rate to therapy.
The significant lower SVR in patients with baseline platelet counts below 130000/μL in treatment-naive patients treated for 48 wk, but not for 72 wk, likely reflects the major role of liver stage in patients with presumably favorable viral kinetics (considering that patients treated for 48 wk will have had an EVR), whereas in patients with slower decay of viral load and presumably treated for 72 wk, liver stage could have had a lower impact on SVR. In the present study, liver biopsy was performed in 67 patients and fibrosis stages 1, 2, 3 and 4 were seen in 30, 11, 5 and 3 of the platelet count ≥ 130000/μL group and 3, 7, 6, 2 of the < 130000/μL group, respectively. We also observed that 3 of 5 cirrhotic patients obtained SVR. Perhaps IL28B polymorphism played a major role in these patients, possibly explaining why some cirrhotics achieved SVR. Further studies will be needed to clarify this point.
So far, anemia and neutropenia are well-recognized effects of higher-dose peginterferon alpha plus ribavirin regimens, but it was also reported that no patient had to discontinue therapy owing to thrombocytopenia[27]. We observed a greater number of lower SVR rates in the low platelet group [35.2% (12/34); P = 0.037] of the higher hemoglobin group than in the high platelet group [57.5% (65/113)] of the higher hemoglobin group (hemoglobin ≥ 13 g/dL). We also observed that lower SVR rates tended to occur more in the low platelet group [29.4% (5/17)] than in the high platelet group [53.1% (17/32)] of the lower hemoglobin group (hemoglobin < 13 g/dL). Thus, the present study suggested that thrombocytopenia is an important factor for SVR.
In the present study, we also experienced only two treatment-naive patients discontinuing treatment due to neutropenia. One was a 70-year old male who discontinued treatment at 1 wk after its commencement, and the other was a 51-year old female who discontinued treatment at 9 wk (Figure 1). Further study will be needed as the numbers of samples in the current study were limited.
In conclusion, SVR was attained independently of EVR in both treatment-naive and treatment-experienced patients. The SVR rates between the pretreatment hemoglobin < 13 and ≥ 13 g/dL groups did not differ. However, in treatment-naive patients, the SVR rate of the pretreatment platelet count < 130000/μL group was significantly lower than that of the pretreatment platelet count ≥ 130000/μL group. Patients with low platelet counts were subject to dose and/or treatment duration reductions. In fact, if these subjects required such treatment adjustments, this may provide a partial explanation for the difference in SVR rates between naive and experienced patients. Attention should be paid to thrombocytopenia in the treatment of chronic hepatitis C patients.
We thank Dr. Yutaka Yonemitsu, Dr. Hiroshige Kojima, Dr. Michikazu Abe, Dr. Kenichi Fukai, Dr. Fumihiko Kanai, Dr. Susumu Nakahori, Dr. Shigenobu Kawai, Dr. Yasushi Maru, Dr. Takeshi Nihei, Dr. Norio Kikuchi, Dr. Noritomo Shimada, Dr. Yasuo Hirai, Dr. Shuuichi Saito, Dr. Shinichi Hino, Dr. Masaaki Saito, Dr. Kazuhiko Kita, Dr. Shinichi Sato, Dr. Yutaka Natsuki, Dr. Hidetaka Terabayashi, Dr. Masahiko Sanada, Dr. Noriaki Suzuki, Dr. Ryosaku Azemoto, Dr. Hideki Takanashi, Dr. Michio Kimura, Dr. Nobuyuki Sugiura, Dr. Motohide Takashi, Dr. Shinnen Kin, Dr. Satoru Kaneda, Dr. Hikaru Nagahara, Dr. Kinki Rin, Dr. Ei Itobayashi and all other investigators for coordinating this work.
It is well known that improved adherence to medication will favorably affect sustained virological response (SVR) rates in peginterferon-alpha 2a plus ribavirin therapy for chronic hepatitis C. Because the use of erythropoietin or hematopoietic growth factors was prohibited in these treatments by Japanese health insurance plans, hematological adverse events are the most common laboratory abnormalities, leading to dose modification or discontinuation.
Peginterferon-alpha 2a plus ribavirin therapy for chronic hepatitis C leads to hematological adverse events, some of which are unknown. The authors examined the epidemiological data and treatment responses were retrospectively analyzed in terms of hematological safety. In this study, the authors demonstrate that attention should be paid to potential thrombocytopenia in the treatment of chronic hepatitis C patients.
Recent reports have highlighted the importance of inosine triphosphatase gene variants that protect against anemia in patients treated for chronic hepatitis C. In treatment-naive patients, the SVR rate of the pretreatment platelet count < 130000/μL group was significantly lower than that of the pretreatment platelet count ≥ 130000/μL group. The authors also observed that 3 of 5 biopsy-proven cirrhotic patients obtained SVR.
With the use of standard of care, attention should be paid to thrombocytopenia in the treatment of chronic hepatitis C patients.
It is well known that improved adherence to medication will favorably affect SVR rates in pegylated interferon-alpha 2a plus ribavirin therapy for chronic hepatitis C. In the present study, the authors retrospectively analyzed the epidemiological data and treatment responses were retrospectively analyzed in terms of hematological safety. In treatment-naive patients, the SVR rate of the pretreatment platelet count < 130000/μL group was significantly lower than that of the pretreatment platelet count ≥ 130000/μL group, despite the existence of cirrhosis. Of particular interest was the fact that the results suggested that attention should be paid to thrombocytopenia in the treatment of chronic hepatitis C patients.
P- Reviewer Perrella A S- Editor Song XX L- Editor Roemmele A E- Editor Li JY
| 1. | Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 293] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1980] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 4. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 5. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 6. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Tejima K, Masuzaki R, Ikeda H, Yoshida H, Tateishi R, Sugioka Y, Kume Y, Okano T, Iwai T, Gotoh H. Thrombocytopenia is more severe in patients with advanced chronic hepatitis C than B with the same grade of liver stiffness and splenomegaly. J Gastroenterol. 2010;45:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kauf TL, Nelson DR, Schelfhout J, Delaney JA, Wang PF. Trends in the prevalence of thrombocytopenia among individuals infected with hepatitis C virus in the United States, 1999-2008. BMC Res Notes. 2012;5:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Giannini EG, Marenco S, Fazio V, Pieri G, Savarino V, Picciotto A. Peripheral blood cytopaenia limiting initiation of treatment in chronic hepatitis C patients otherwise eligible for antiviral therapy. Liver Int. 2012;32:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Cooper CL, Druyts E, Thorlund K, Nachega JB, El Khoury AC, O’Regan C, Mills EJ. Boceprevir and telaprevir for the treatment of chronic hepatitis C genotype 1 infection: an indirect comparison meta-analysis. Ther Clin Risk Manag. 2012;8:105-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Poordad F, Theodore D, Sullivan J, Grotzinger K. Medical resource utilisation and healthcare costs in patients with chronic hepatitis C viral infection and thrombocytopenia. J Med Econ. 2011;14:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2722] [Article Influence: 170.1] [Reference Citation Analysis (0)] |
| 13. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1774] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 14. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1503] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 15. | Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;46:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Fellay J, Thompson AJ, Ge D, Gumbs CE, Urban TJ, Shianna KV, Little LD, Qiu P, Bertelsen AH, Watson M. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Tanaka Y. Interleukin28B and inosine triphosphatase help to personalize hepatitis C treatment. Digestion. 2011;84:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Tanaka Y, Kurosaki M, Nishida N, Sugiyama M, Matsuura K, Sakamoto N, Enomoto N, Yatsuhashi H, Nishiguchi S, Hino K. Genome-wide association study identified ITPA/DDRGK1 variants reflecting thrombocytopenia in pegylated interferon and ribavirin therapy for chronic hepatitis C. Hum Mol Genet. 2011;20:3507-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 736] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 20. | Miyauchi T, Kanda T, Imazeki F, Mikata R, Tawada A, Arai M, Fujiwara K, Nakamoto S, Wu S, Tanaka T. Response to peginterferon-alpha 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 1. Hepatol Int. 2013;7:144-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kanda T, Imazeki F, Yonemitsu Y, Mikami S, Takada N, Nishino T, Takashi M, Tsubota A, Kato K, Sugiura N. Quantification of hepatitis C virus in patients treated with peginterferon-alfa 2a plus ribavirin treatment by COBAS TaqMan HCV test. J Viral Hepat. 2011;18:e292-e297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kanda T, Imazeki F, Wu S, Nakamoto S, Yokosuka O. The assessment of serum hepatitis C virus RNA 12 wk after the end of treatment using TaqMan polymerase chain reaction is less relevant than after 24 wk for predicting sustained virological response. Hepatology. 2011;54:1482; author reply 1482-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Kanda T, Imazeki F, Mikami S, Kato K, Shimada N, Yonemitsu Y, Miyauchi T, Arai M, Fujiwara K, Tsubota A. Occurrence of hepatocellular carcinoma was not a rare event during and immediately after antiviral treatment in Japanese HCV-positive patients. Oncology. 2011;80:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124:1711-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 26. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 28. | Miyamura T, Kanda T, Nakamoto S, Wu S, Jiang X, Arai M, Fujiwara K, Imazeki F, Yokosuka O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses. 2012;4:1264-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Nakagawa M, Sakamoto N, Watanabe T, Nishimura-Sakurai Y, Onozuka Y, Azuma S, Kakinuma S, Nitta S, Kiyohashi K, Kusano-Kitazume A, Murakawa M, Yoshino K, Itsui Y, Tanaka Y, Mizokami M, Watanabe M; Ochanomizu Liver Conference Study Group. Association of ITPA gene variation and serum ribavirin concentration with a decline in blood cell concentrations during pegylated interferon-alpha plus ribavirin therapy for chronic hepatitis C. Hepatol Int. 2013;7:153-161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Omata M, Kanda T, Yu ML, Yokosuka O, Lim SG, Jafri W, Tateishi R, S . Hamid S, Chuang WL, Chutaputti A, Wei L, Sollano J, Sarin SK, Kao JH, W. McCaughan G. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409-435. [RCA] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |