Published online Apr 27, 2013. doi: 10.4254/wjh.v5.i4.160
Revised: December 13, 2012
Accepted: January 17, 2013
Published online: April 27, 2013
Processing time: 182 Days and 15 Hours
AIM: To investigate melatonin’s preventive action in oxidative stress in a rat model with high fat diet-induced non-alcoholic fatty liver disease (NAFLD).
METHODS: NAFLD was induced by high fat diet (HFD) in adult, male, Wistar rats, weighing 180-230 g. After acclimatization for one week, they were randomly assigned to 6 experimental groups that comprised animals on regular diet plus 5 or 10 mg/kg melatonin, for 4 or 8 wk; animals on HFD, with or without 5 or 10 mg/kg melatonin, for 4 or 8 wk; and animals on HFD for 8 or 12 wk, with melatonin 10 mg/kg for the last 4 wk. Liver damage was assessed biochemically by the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and histologically. Lipid peroxidation and oxidative stress were assessed by malondialdehyde and glutathione levels in liver tissue. Lipidemic indices and portal vein pressure were also measured.
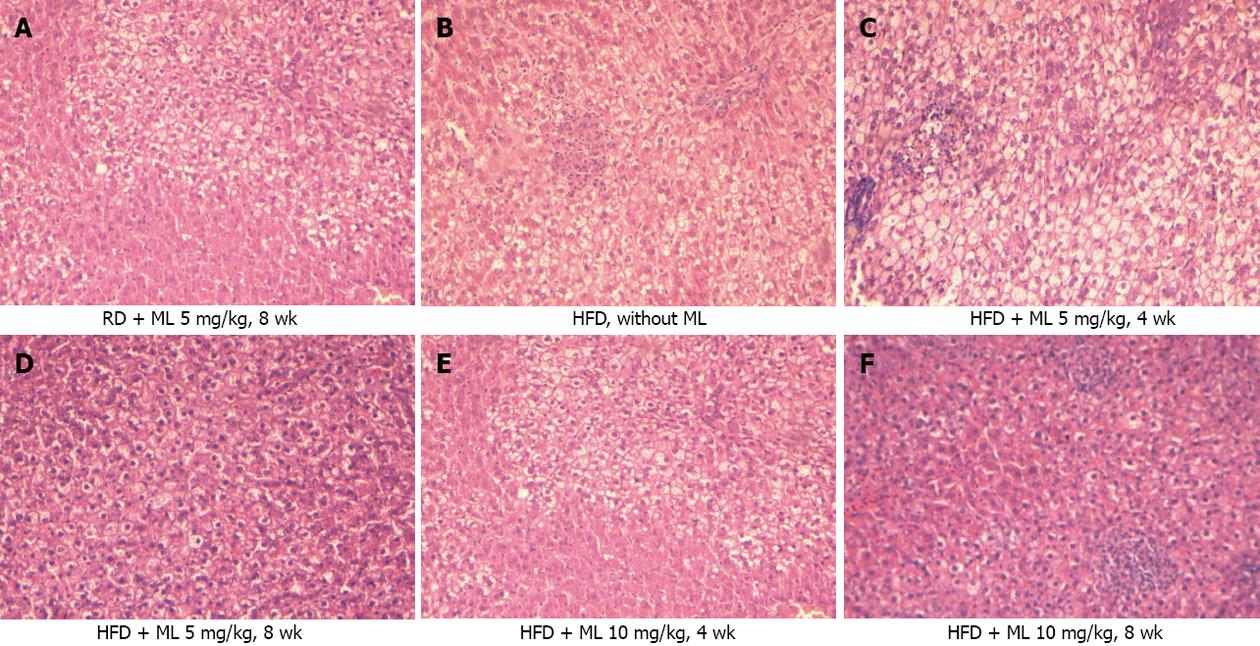
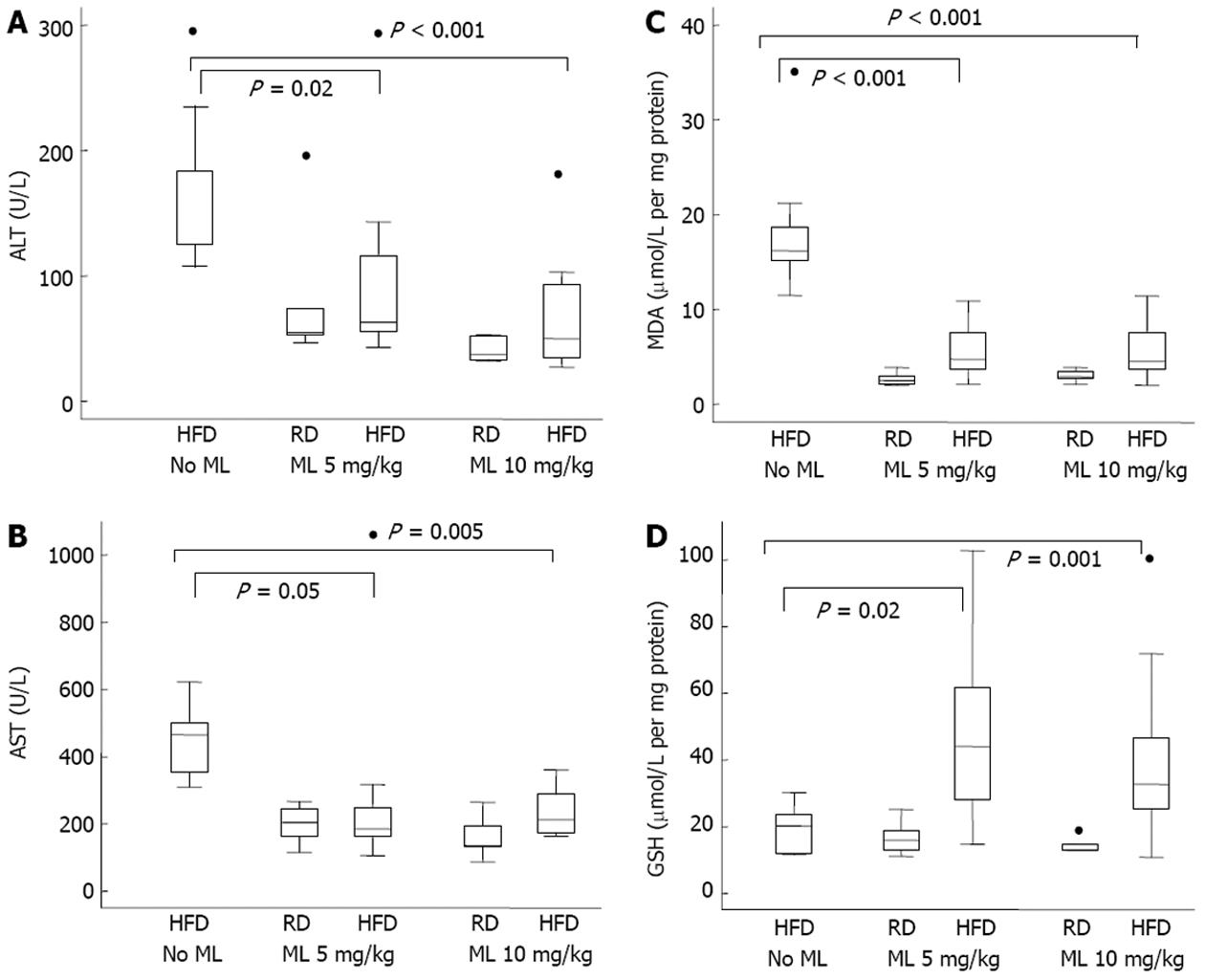
RESULTS: Compared to rats not receiving melatonin, rats on 5 or 10 mg/kg of melatonin had lower mean liver weight (-5.0 g and -4.9 g) (P < 0.001) and lower liver weight to body weight ratio (-1.0%) (P < 0.001), for the two doses, respectively. All rats fed HFD without melatonin developed severe, grade III, steatosis. Rats on HFD with concurrent use of melatonin showed significantly less steatosis, with grade III steatosis observed in 1 of 29 (3.4%) rats on 10 mg/kg melatonin and in 3 of 27 (11.1%) rats on 5 mg/kg melatonin. Melatonin was ineffective in reversing established steatosis. Melatonin also had no effect on any of the common lipidemic serum markers, the levels of which did not differ significantly among the rats on HFD, irrespective of the use or not of melatonin. Liver cell necrosis was significantly less in rats on HFD receiving melatonin than in those not on melatonin, with the AST levels declining by a mean of 170 U/L (P = 0.01) and 224 U/L (P = 0.001), and the ALT levels declining by a mean of 62.9 U/L (P = 0.01) and 93.4 U/L (P < 0.001), for the 5 and 10 mg/kg melatonin dose, respectively. Melatonin mitigated liver damage due to peroxidation and oxidative stress in liver tissue as indicated by a significant decline in MDA production by 12.7 (P < 0.001) and 12.2 (P < 0.001) μmol/L /mg protein /mg tissue, and a significant increase in glutathione by 20.1 (P = 0.004) and 29.2 (P < 0.001) μmol/L /mg protein /mg tissue, for the 5 and 10 mg/kg melatonin dose, respectively.
CONCLUSION: Melatonin can attenuate oxidative stress, lessen liver damage, and improve liver histology in rats with high fat diet-induced NAFLD, when given concurrently with the diet.
- Citation: Hatzis G, Ziakas P, Kavantzas N, Triantafyllou A, Sigalas P, Andreadou I, Ioannidis K, Chatzis S, Filis K, Papalampros A, Sigala F. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J Hepatol 2013; 5(4): 160-169
- URL: https://www.wjgnet.com/1948-5182/full/v5/i4/160.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i4.160
Non-alcoholic fatty liver (NAFL) and its progressive form nonalcoholic steatohepatitis (NASH) is a common non-alcoholic fatty liver disease (NAFLD). The NAFL-NASH complex can be viewed as a pathological spectrum, with steatosis without inflammation or necrosis at one end (NAFL), and its progression with inflammation, necrosis and fibrosis or cirrhosis at the other end (NASH)[1].
Recent data show that NAFLD is a hepatic manifestation of the metabolic syndrome that is associated with insulin resistance and encompasses a cluster of disorders, such as obesity, dyslipidemia, type 2 diabetes mellitus, and hypertension[2]. Many of the risk factors for the NAFL-NASH complex are well defined, but the underlying pathogenesis is not well understood. At present, therapy is aimed at modifying the risk factors, but there are no proven therapies for the prevention or even treatment of NAFLD. Work on the pathogenesis of NAFLD suggests that the amelioration or prevention of oxidative stress may be an effective treatment of NAFLD in humans eschewing a healthy lifestyle[2].
Of the many biological targets of oxidative stress, lipids figure most prominently. Lipid peroxidation generates a number of byproducts, with malondialdehyde (MDA) being the principal product of fatty acid peroxidation, a highly toxic molecule that is used as a biomarker of lipid peroxidation and oxidative stress[3]. The biological effects of oxidative stress are neutralized in vivo by antioxidative defense mechanisms that include vitamins C and E, carotenoids, antioxidant enzymes, and glutathione (GSH). The latter in its reduced form is the single most important protective and regulatory antioxidant. Since lipid peroxidation markedly lowers its level, glutathione together with MDA are used as indicators of oxidative stress in the liver[4].
Melatonin is a serotonin-derived neurohormone formed primarily in the brain by the pineal gland of all mammals, including man[5]. In humans, melatonin is secreted mostly nocturnally. Melatonin exerts its many physiological actions via specific cell membrane and nuclear receptors, although many of its actions are receptor-independent, including the scavenging of free radicals and the interaction with cytosol proteins, like calmodulin and tubulin-associated proteins[6,7]. More importantly, melatonin as an active substance in the neuro-immune-endocrine system expresses numerous biological functions concerning the circadian rhythm, sleep, the stress response, the process of aging, and immunity[8]. Furthermore, melatonin has natural direct or indirect antioxidant effects[6,9].
We hypothesized that the use of melatonin could complement and amplify the antioxidant-defense system and thereby reduce or prevent the severity of liver damage associated with NAFLD. We tested our hypothesis, in a rat model of NAFLD induced by a high fat diet, by studying the effects of pharmacological doses of melatonin on liver function, liver histopathology, portal vein pressure, lipid metabolism, and oxidative stress.
Adult, male, Wistar rats weighing 180-230 g were supplied by the Hellenic Pasteur Research Institute. They were housed in a climate-controlled room at 24 ± 1.5 °C, with a 12-h light (8 am-8 pm)/dark (8 pm-8 am) cycle, and free access to food and tap water. The animals were treated according to the “Principles of Laboratory Animal Care” of the National Society for Medical Research, and the Guidelines for the Care and Use of Laboratory Animals, prepared by the Academy of Sciences and published by the National Institutes of Health (Institute of Laboratory Animal Resources Commission on Life Sciences, 1996). At the end of the study, the rats were anaesthetized with ether to measure the portal vein pressure, collect blood via cardiac puncture, and remove the liver upon sacrifice.
Melatonin was purchased from Sigma Aldrich Chemical Co. (St Louis, MO, United States), dissolved in a minimum volume of absolute ethanol, and diluted to 5 mg/mL with 0.9% NaCl. The ethanol concentration in the final solution was 0.8%.
Melatonin was injected intraperitoneally, consistently between 7 pm and 8 pm, at a dose of 5 or 10 mg/kg body weight. Rats that did not receive melatonin were injected with an equivalent amount of saline/alcohol fluid (0.9% NaCl containing 0.8% ethanol).
High fat diet (HFD) in pellet form was obtained from Mucedola s.rl (Milano, Italy). It contains 19% protein, 17.5% fat, 3.5% fibre, 3.5% ashes, vitamins and minerals. Regular diet (RD) was obtained from Kounker Keramaris Bros and Co, (Athens, Greece). It contains 16.5% protein, 8% fibre, 8% ashes, 2% fat, 1% NaCl, vitamins and minerals.
Eighty-two rats were used in the study. After acclimatization for one week, they were randomly assigned to 6 experimental groups. Group 1 rats (n = 12) were fed HFD for 4 wk (subgroup 1A, n = 6) or 8 wk (subgroup 1B, n = 6). Group 2 rats (n = 10) were fed RD plus 5 mg/kg melatonin for 4 wk (subgroup 2A, n = 5) or 8 wk (subgroup 2B, n = 5). Group 3 rats (n = 10) were fed RD plus 10 mg/kg melatonin for 4 wk (subgroup 3A, n = 5) or 8 wk (subgroup 3B, n = 5). Group 4 rats (n = 20) were fed HFD plus 5 mg/kg melatonin for 4 wk (subgroup 4A, n = 10) or 8 wk (subgroup 4B, n = 10). Group 5 rats (n = 20) were fed HFD plus 10 mg/kg melatonin for 4 wk (subgroup 5A, n = 10) or 8 wk (subgroup 5B, n = 10). Group 6 rats (n = 10) were fed HFD for 8 wk (subgroup 6A, n = 5) or 12 wk (subgroup 6B, n = 5) plus 10 mg/kg melatonin for the last 4 wk.
The abdomen was entered via a midline incision and the portal vein was cannulated through the mesenteric vein with a PE-50 catheter. The portal pressure was recorded on a multichannel recorder through a highly sensitive transducer (SpaceLabs Medical Inc. Model 11-14-15)[10]. The zero reference point was determined to be 1 cm above the operating table.
Blood was collected via cardiac puncture in vacutainer tubes (Becton Dickinson Hellas, Athens, Greece), centrifuged at room temperature for 15 min at 3000 g, and serum stored in cryotubes at -70 °C. Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using a multianalyzer (Cobas Miras, Roche, Basel, Switzerland). Serum total cholesterol (TC), triglycerides (TG), low-density (LDL), and high-density (HDL) lipoproteins were measured using an online dual-enzymatic method for the simultaneous quantification of TC and TG, employing high-performance liquid chromatography.
Upon sacrifice, livers were quickly removed, washed with ice-cold 0.9% NaCl, dried on a filter paper, weighed, and samples were taken from the left anterior lobe. These were divided in two parts, one part was fixed immediately in 10% neutral-buffered solution with 4% formaldehyde for 24 h before being embedded in paraffin, the other part was immediately stored at -70 °C for measurement of tissue MDA and GSH levels.
Serial liver sections, 4 μm thick, were processed routinely for hematoxylin-eosin staining. The histopathological features were scored by one pathologist who was unaware of the specific dietary regimen of the animals. Each section was evaluated for grading of steatosis according to the criteria reported by Brunt et al[11].
Before the assay, tissue samples were washed in ice-cold NaCl 0.9%, blotted on absorbent paper, and weighed. Each sample was then minced in a small volume of ice-cold 20 mm Tris-HCl buffer, pH 7.4, in a 1:10 w/v ratio, and homogenized. After centrifugation at 3000 g for 10 min at 4 °C, the clear homogenate supernatant was used for biochemical assay. For the determination of MDA, 0.65 mL of 10.3 mmol/L N-methyl-2-phenyl-indole in acetonitrile was added to 0.2 mL of tissue sample. After vortexing for 3-4 s, 0.15 mL of 15.4 mol/L methanesulfonic acid was added and samples were mixed well, closed with a tight stopper, and incubated at 45 °C for 40 min. The samples were then cooled on ice, centrifuged, and the absorbance was measured spectrophotometrically at 586 nm. A calibration curve, comprising accurately prepared standard MDA solutions (from 2 to 20 nmol/mL), was also run for quantitation[12]. Measurements were performed in triplicate. MDA levels were expressed as μmol/L/mg protein/mg tissue.
GSH levels were measured in homogenized liver tissue using the colorimetric assay for GSH, according to the manufacturer’s instructions (Bioxytec GSH-400, Oxis Research, Portland United States)[13]. GSH concentrations were determined using a standard curve of absorbance units versus GSH concentrations. GSH levels were expressed as μmol/L /mg protein /mg tissue.
In the analysis of data, continuous variables were reported as mean ± SD and compared using the Student t test. Categorical variables were reported as relative frequencies (%) and compared using the χ2 test. Treatment effects on histology grading were estimated using logistic regression models.
The odds ratios (ORs) were estimated and presented with their 95%CI. A two-sided P < 0.05 was considered statistically significant. Stata v8 package was used for data analysis.
The results pertain to seventy-eight rats, since four of the eighty-two used rats (two, one, and one from subgroups 4A, 4B and 5A, respectively) died upon completion of the study during anaesthesia.
Rats on HFD (groups 1, 4 and 5) had a significantly higher mean liver weight (+4.2 g) (P < 0.001), higher liver weight over body weight ratio (+1.1%) (P < 0.001), and higher mean portal vein pressure (+3.9 mmHg) (P < 0.001) compared to rats on RD (groups 2 and 3). Rats receiving melatonin (groups 2, 3, 4 and 5) had lower mean liver weight [-5.0 g at 10 mg/kg (P < 0.001) and -4.9 g at 5 mg/kg melatonin (P < 0.001)], lower liver weight over body weight ratio (-1.0%) (P < 0.001) for both melatonin doses, and lower mean portal vein pressure [-2.5 mmHg (P = 0.03) and -2.7 mmHg for 5 and 10 mg/kg melatonin, respectively], compared to rats not receiving melatonin (group 1). All comparisons between subgroups with 5 mg/kg vs 10 mg/kg melatonin were not statistically significant. The duration of melatonin use (4 wk vs 8 wk) had an insignificant effect on liver weight and liver weight over body weight ratio; however it was associated with a statistically significant decline in mean portal vein pressure (-1.9 mmHg) (P = 0.02) in groups treated for 8 wk compared to 4 wk (Table 1). After adjusting for HFD and duration of treatment, melatonin was associated with a significant decline in liver weight and liver weight over body weight ratio, but had no significant effect on portal vein pressure (Table 2). Comparisons between groups on high (10 mg/kg) vs low (5 mg/kg) melatonin yielded statistically insignificant results for both liver weight and liver weight over body weight ratio.
| Liver weight (g) | P value | Liver weight/body weight (%) | P value | Portal vein pressure (mmHg) | P value | |
| Diet | ||||||
| HFD | 13.3 ± 2.8 | < 0.001 | 3.5 ± 0.5 | < 0.001 | 11.9 ± 2.7 | < 0.001 |
| RD | 9.1 ± 1.3 | BV | 2.4 ± 0.3 | BV | 8.0 ± 1.7 | BV |
| Melatonin | ||||||
| 10 mg/kg | 11.2 ± 2.5 | < 0.001 | 3.0 ± 0.5 | < 0.001 | 10.5 ± 2.6 | 0.01 |
| 5 mg/kg | 11.3 ± 2.3 | < 0.001 | 3.0 ± 0.6 | < 0.001 | 10.3 ± 2.8 | 0.03 |
| 0 mg/kg | 16.2 ± 2.3 | BV | 4.0 ± 0.5 | BV | 13.0 ± 3.5 | BV |
| Duration | ||||||
| 8 wk | 12.0 ± 3.6 | 0.86 | 3.2 ± 0.7 | 0.71 | 10.1 ± 2.5 | 0.02 |
| 4 wk | 12.2 ± 2.6 | BV | 3.2 ± 0.7 | BV | 12.0 ± 3.3 | BV |
| Melatonin | 5 mg/kg | P value | 10 mg/kg | P value |
| Liver weight (g) | -3.9 | < 0.001 | -3.9 | < 0.001 |
| Liver weight/body weight ratio (%) | -0.7 | < 0.001 | -0.7 | < 0.001 |
| Portal vein pressure (mmHg) | -1.6 | 0.09 | -1.4 | 0.14 |
Of the 48 rats on HFD (groups 1, 4 and 5), all but one (in subgroup 5B) (47/48, 98%) developed steatosis grade I to grade III, against none among the 20 rats on RD (groups 2 and 3) (P < 0.001). All 12 rats (100%) on HFD without melatonin (group 1) developed grade III steatosis. By contrast, rats on HFD for 4 or 8 wk with concurrent use of 5 or 10 mg/kg melatonin (groups 4 and 5) showed a significant mitigation of liver steatosis, with only 4 of 36 rats developing grade III steatosis (P < 0.001). When all rats receiving melatonin were grouped together, grade III steatosis was evident in 1 of 29 (3.4%) rats receiving 10 mg/kg melatonin (groups 3 and 5) (P < 0.001) and in 3 of 27 (11.1%) rats (groups 2 and 4) (P < 0.001) receiving 5 mg/kg melatonin (Figure 1).
Overall, 1 mg/kg increase in melatonin dose had a protective effect on steatosis in rats on HFD, reducing its risk (grade I or worse steatosis) by OR 0.85 (95%CI: 0.72-0.99). The duration of melatonin use (4 wk vs 8 wk) had no significant effect on the severity of steatosis (Table 3). After adjusting for duration of melatonin use, melatonin showed again a protective effect toward grade I or worse steatosis in rats on HFD, reducing the risk by OR 0.69 per 1 mg/kg increase in melatonin dose (95%CI: 0.59-0.83) (Table 4).
| n = 68 | Grade 0 | Grade 1 | Grade 2 | Grade 3 | OR (95%CI) | P value |
| Diet | ||||||
| HFD (n = 48) | 1 (2.10) | 19 (39.60) | 12 (25) | 16 (33.30) | NE | < 0.001 |
| RD (n = 20) | 20 (100) | 0 (0) | 0 (0) | 0 (0) | BV | |
| Melatonin | ||||||
| 10 mg/kg (n = 29) | 11 (37.90) | 9 (31) | 8 (27.60) | 1 (3.40) | 0.851 (0.72-0.99) | 0.04 |
| 5 mg/kg (n = 27) | 10 (37) | 10 (37) | 4 (14.90) | 3 (11.10) | ||
| 0 mg/kg (n = 12) | 0 (0) | 0 (0) | 0 (0) | 12 (100) | ||
| Duration | ||||||
| 8 wk (n = 35) | 11 (31.40) | 10 (28.60) | 7 (20) | 7 (20) | 0.95 (0.34-2.66) | 0.92 |
| 4 wk (n = 33) | 10 (30.30) | 9 (27.30) | 5 (15.10) | 9 (27.30) | BV | |
| n = 48 | OR (95%CI) | P value |
| Melatonin (per 1 mg/kg increase) | 0.69 (0.59-0.83) | < 0.001 |
| Duration (8 wk vs 4 wk) | 0.65 (0.20-2.10) | 0.47 |
All rats (10/10, 100%) on HFD for 8 or 12 wk receiving 10 mg/kg melatonin in the last 4 wk (subgroups 6A and 6B) showed grade III steatosis, as against 4 of 36 rats (11.1%) on HFD that concurrently received 5 mg/kg or 10 mg/kg melatonin (groups 4 and 5) (P < 0.001).
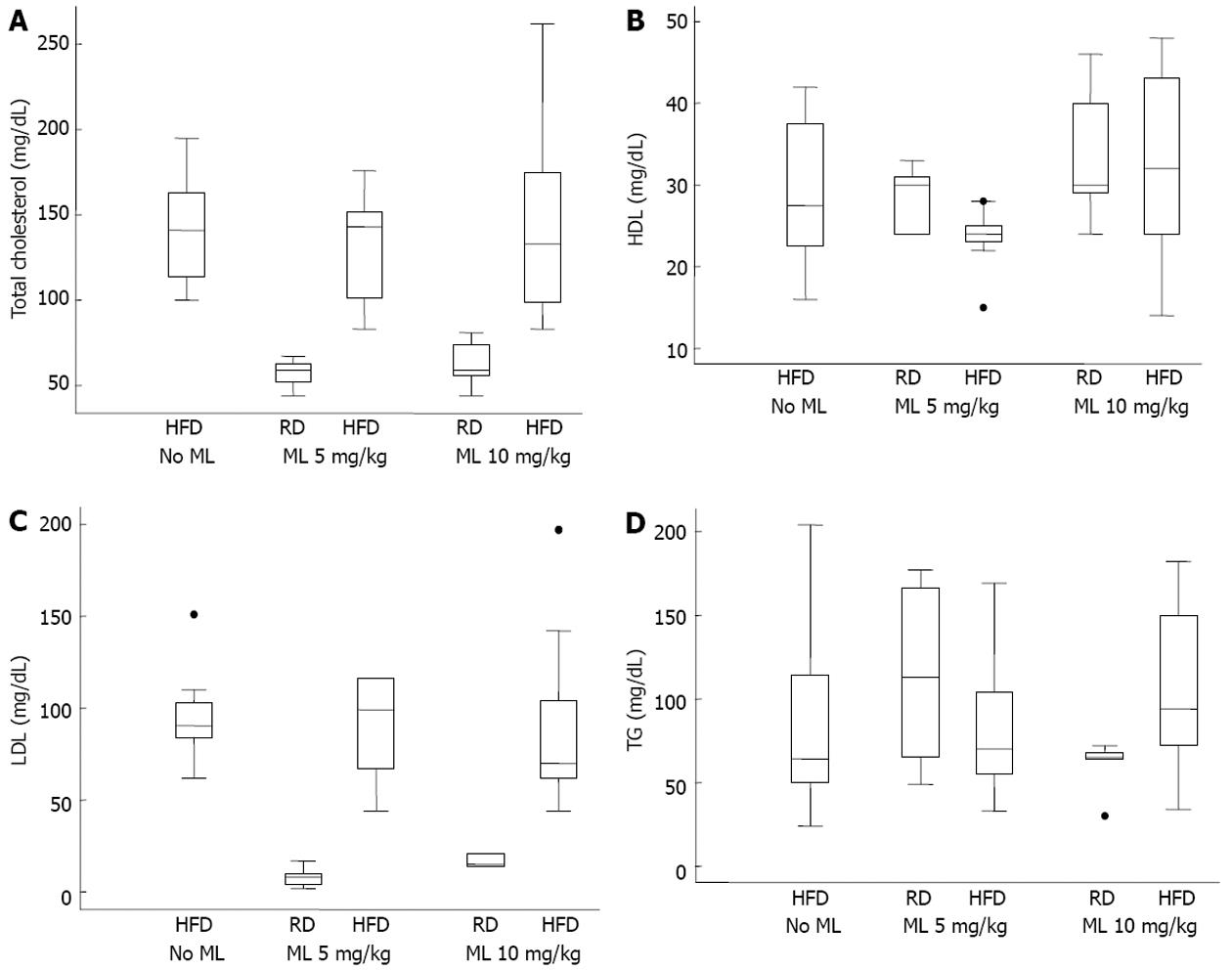
The levels of TC, LDL, HDL and TG did not differ significantly among the rats on HFD, irrespective of the use or not of melatonin (Figure 2). After adjusting for HFD and duration of melatonin use, melatonin had no effect on any of the common lipidemic serum markers (Table 5).
| Melatonin (5 mg/kg) | P value | Melatonin (10 mg/kg) | P value | |
| Lipidemic profile | ||||
| TC (mg/dL) | -10.7 | 0.45 | -2.2 | 0.87 |
| LDL (mg/dL) | -6.3 | 0.60 | -3.9 | 0.74 |
| HDL (mg/dL) | -5.3 | 0.10 | 1.9 | 0.52 |
| TG (mg/dL) | 6.7 | 0.71 | -5.2 | 0.76 |
| Hepatic function | ||||
| AST (U/L) | -170 | 0.01 | -224 | 0.001 |
| ALT (U/L) | -62.9 | 0.01 | -93.4 | < 0.001 |
| Oxidative profile | ||||
| MDA | -12.7 | < 0.001 | -12.2 | < 0.001 |
| GSH | 20.1 | 0.004 | 29.2 | < 0.001 |
There was a significant aminotransferase decrease in rats on HFD receiving melatonin as compared with those not on melatonin (Figure 3A and B). Specifically, AST and ALT levels declined by a mean of 170 and 62.9 U/L, respectively, in the 5 mg/kg melatonin group, and by 224 and 93.4 U/L, respectively, in the 10 mg/kg melatonin group (Table 5). However, differences between the 5 mg/kg vs 10 mg/kg melatonin dose were not significant for either the AST (P = 0.30) or the ALT (P = 0.12) (Table 5).
Compared to animals not on melatonin, melatonin use led to a significant decline in MDA production in liver tissue by 12.7 and 12.2 for the 5 and 10 mg/kg melatonin dose, respectively, and a significant increase in GSH in liver tissue by 20.1 and 29.2 for the 5 and 10 mg/kg melatonin dose, respectively, in multivariable analysis adjusting for diet and duration of melatonin use (Figure 3C and D) (Table 5). The MDA and GSH differences in liver tissue between rats receiving 5 mg/kg vs 10 mg/kg melatonin were not significant.
The validity of our experimental model of NAFLD in rats on HFD was confirmed by the histopathological findings of liver steatosis. Liver steatosis was absent in rats on regular diet.
In this rat NAFLD model, administration of 5 or 10 mg/kg melatonin, for 4 or 8 wk, was effective in mitigating the course of NAFLD. In particular, melatonin showed a strong tendency to attenuate liver steatosis and to curtail the rise of liver weight, portal vein pressure, and serum aminotransferases. By contrast, rats on HFD without melatonin showed significantly higher liver weight, liver weight over body weight ratio, portal vein pressure, serum aminotransferases, and higher TC, TG, LDL and HDL.
These results are in keeping with previous studies indicating a hepatoprotective effect of melatonin in rat models of diet-induced NAFLD[14,15].
The putative mechanism of the beneficial effect of melatonin on NAFLD in rats on HFD is the decrease of lipid peroxidation and the limitation or prevention of oxidative stress. Melatonin’s antioxidant repertoire includes: its stimulation of antioxidant enzymes[16], the regulation of gene transcription for antioxidant enzymes[17], its direct free radical scavenger action[18], the stimulation of glutathione synthesis[19], its ability to augment the activities of other antioxidants[20], the protection of antioxidative enzymes from oxidative damage[21], its action on the mitochondrial respiratory chain activity whereby melatonin lowers the electron leakage and reduces the generation of free radicals[22], and finally its significant attenuation of LPS-induced sterol regulatory element-binding protein (SREBP)-1c activation and expression of SREBP-1c target genes that prevents LPS-induced hepatic lipid accumulation[23].
Lipid peroxidation, and peroxidation of membrane lipids, links steatosis to steatohepatitis, with its attendant necroinflammation, liver cell necrosis, increased ALT and AST levels, and fibrosis[24]. The aldehyde product of lipid peroxidation, MDA, induces hepatic stellate cell activation[25], these being the main collagen-producing cells within the liver, leading to enhanced extracellular matrix protein deposition. MDA may also contribute to inflammation by activating nuclear factor-kappaB (NF-κB), a transcription factor regulating the expression of several proinflammatory cytokines and adhesion molecules, including tumour necrosis factor-alpha, intercellular adhesion molecule 1, and E-selectin[26,27].
However, steatosis by itself (first hit) is not sufficient for the development of steatohepatitis and fibrosis, as the latter would require the action of additional factors (second hit)[28]. Such factors would include a source of free radicals capable of inducing oxidative stress[29], the inhibition of electron transfer along the respiratory chain that would lead to the generation of superoxide anions capable of initiating lipid peroxidation[30], and enhanced formation of reactive oxygen species (ROS) leading to increased oxidative stress. In this respect, melatonin has additive or multiplier effects on the reduction of lipid peroxidation[16,21], being twice as effective as vitamin E at protecting cell membranes from lipid peroxidation[31]. The observation in our study of significantly reduced MDA levels in livers of rats on HFD and melatonin, but not in rats on HFD alone might suggest that melatonin may have efficiently reduced lipid peroxidation and its end products, such as MDA.
In addition, melatonin, as a free radical scavenger, is effective against oxidative stress by reducing the production of free radicals and ROS and by improving the function of the mitochondrial respiratory chain[18,22,32]. Furthermore, melatonin increases the levels of several antioxidative enzymes, including superoxide dismutase, glutathione peroxidase, and glutathione reductase.
Significantly, our study provided support for the protective effect of melatonin against oxidative stress by showing increased glutathione levels in the liver of rats on HFD and melatonin when compared to rats on HFD without melatonin. It would appear that melatonin does stimulate the production of glutathione, with the ensuing protective effect against oxidative stress.
Moreover, besides its free radical scavenging and antioxidative functions, melatonin’s receptor-mediated local functions may contribute to its ability to preserve cell function and limit cell death from apoptosis or necrosis due to oxidative damage[33-35]. Melatonin’s protective effect against liver cell necrosis was supported in our study by the significantly lower level of serum aminotransferases in rats on HFD receiving melatonin.
In contrast to the protective action of melatonin when given synchronously with HFD, this effect was lost in rats receiving melatonin at a late phase of the experiment, with melatonin failing to reverse or reduce the severity of steatosis. Our study is the first to indicate melatonin’s possible ineffectiveness at reversing established steatosis. Clearly, further study is needed to test if delayed melatonin administration for more than 4 wk might be effective in reversing steatosis.
In our study, melatonin had no significant effect on the lipidemic markers. After adjusting for HFD and duration of melatonin use, the lipidemic serum markers (TC, TG, LDL and HDL) were higher in rats on HFD without melatonin than in rats on HFD receiving melatonin, but this difference did not reach statistical significance. This is in contrast to the findings of Pan et al[14] and Chen et al[23] who found significantly reduced TC and TG levels in rats given a moderate or high dose of melatonin (5 or 10 mg/kg), and also to the findings of Hoyos et al[36] who found significantly reduced levels of TC and LDL in the melatonin groups. However, our findings match those of the latter study as it concerns the TG levels.
We don’t have a clear explanation for this discrepancy. Suffice to say that melatonin does not protect against the consequences of a high fat diet, including increased intestinal lipid absorption leading to an increased supply of free fatty acids to the liver, the increased de novo synthesis of fatty acids in the liver, or the increased VLDL-TG discharge from the liver. On the other hand, melatonin may have a hypocholesterolemic[36] and hypolipidemic effect[23,37]. This may partly explain our findings of no statistical difference in the lipidemic profile between the groups. Or, possibly, the conflicting results may correlate with the duration of melatonin administration or the dose used.
The extrapolation of our data to humans requires further investigation. Recently, Gonciarz et al[38] conducted a pilot study of a 3-mo course of melatonin treatment of patients with non-alcoholic steatohepatitis, with encouraging results. Based on our experimental evidence, the case might be made for the use of melatonin in conditions with characteristics of the metabolic syndrome, where excessive free radical generation and oxidative stress may occur. This view is supported by the findings of a recent study where melatonin improved the metabolic syndrome induced by high doses of fructose in rats, as showed by a decrease in the insulin resistance, together with a decrease in the concentration of serum tumour necrosis factor-α, hepatic lipid peroxide, and hepatic reduced glutathione[39].
Oral administration of melatonin is safe, and without serious acute or chronic toxicity[38,40]. However, its daytime use may cause feelings of sleepiness and fatigue, which can adversely affect performance[41].
In summary, melatonin was shown to counter lipid peroxidation and oxidative stress, thus providing a degree of protection against the development or the severity of NAFLD in rats concurrently on HFD and melatonin. However, in our experimental model, melatonin was not effective in reversing a state of established steatosis. Interestingly, melatonin administration was not associated with a significant change in the lipid metabolic profile.
The non-alcoholic fatty liver and its progressive form, nonalcoholic steatohepatitis, is a common non-alcoholic fatty liver disease (NAFLD). Recent data show that non-alcoholic fatty liver disease is a hepatic manifestation of the metabolic syndrome that is associated with insulin resistance and encompasses a cluster of disorders, such as obesity, dyslipidemia, type 2 diabetes mellitus, and hypertension. NAFLD is widely prevalent and is clinically being seen with greater regularity because of the high fat and calorie diets consumed in many countries, the disease being present in approximately 30% of the United States population, with the risk that as many as 15% to 20% of subjects with non-alcoholic steatohepatitis will develop cirrhosis. While much new information on the pathogenesis and natural history of non-alcoholic fatty liver disease is available, proven therapies for the prevention or even treatment of this common disease remain to be established.
Work on the pathogenesis of NAFLD suggests that lipid peroxidation, peroxidation of membrane lipids and oxidative stress, are its main risk factors. In the area of prevention of NAFLD, the research hotspot is the use of melatonin, with its direct or indirect antioxidant effects, to complement and amplify the natural antioxidant-defenses-system of humans and thereby reduce or prevent the severity of liver damage associated with non-alcoholic liver disease.
Recent data show that lipid peroxidation, fatty acid peroxidation, and oxidative stress generate a number of byproducts that are highly toxic to liver cells leading to NAFLD. At present, therapy is aimed at modifying the risk factors, but there are no proven therapies for the prevention or even treatment of NAFLD. The biological effects of oxidative stress are neutralized in vivo by antioxidative defense mechanisms that include vitamins C and E, carotenoids, antioxidant enzymes, and glutathione. The use of melatonin could complement and amplify the natural antioxidant-defenses-system and thereby reduce or prevent the severity of liver damage associated with oxidative stress, in NAFLD. In the present experimental study, the authors provide ample evidence that melatonin counters against lipid peroxidation and oxidative stress, thus providing a degree of protection against the development or the severity of NAFLD. However, in this experimental model it was shown for the first time that melatonin was not effective in reversing a state of already established NAFLD.
Melatonin counters against oxidative stress and its use can ameliorate or prevent oxidative stress and may prevent or retard the development of NAFLD in humans not adopting a healthy life-style, by decreasing lipid peroxidation and by limiting or preventing oxidative stress. Based on the experimental evidence of this study, the case might be made for the use of melatonin in conditions with characteristics of the metabolic syndrome, where excessive free radical generation and oxidative stress may occur.
Melatonin is a neurohormone formed primarily in the brain of all mammals, being secreted mostly nocturnally in humans. Melatonin expresses numerous actions such as natural direct or indirect antioxidant effects and scavenging of free radicals. Humans can benefit from melatonin’s actions, especially if they have a tendency to develop the metabolic syndrome.
This is a well designed and conducted study. Results are clearly presented, and represent a possible contribution for the prevention and management of NAFLD. This report documents that melatonin prevents or retards the development of fatty liver in rats fed a high fat diet. Moreover, the authors report that once fatty liver develops, melatonin does not reverse this condition. These are important findings with clear clinical implications.
P- Reviewers Reiter RJ, Herrero JI, Faintuch J S- Editor Song XX L- Editor Hughes D E- Editor Li JY
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3713] [Article Influence: 161.4] [Reference Citation Analysis (2)] |
| 2. | Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1845] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 4. | Videla LA, Fernandez V, Ugarte G, Valenzuela A. Effect of acute ethanol intoxication on the content of reduced glutathione of the liver in relation to its lipoperoxidative capacity in the rat. FEBS Lett. 1980;111:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebestény T, Maronde E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res. 2011;51:17-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1-16. [PubMed] [DOI] [Full Text] |
| 7. | Cardinali DP, Golombek DA, Rosenstein RE, Cutrera RA, Esquifino AI. Melatonin site and mechanism of action: single or multiple? J Pineal Res. 1997;23:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 563] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 9. | Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 777] [Article Influence: 31.1] [Reference Citation Analysis (1)] |
| 10. | Fernández M, Pizcueta P, García-Pagán JC, Feu F, Cirera I, Bosch J, Rodés J. Effects of ritanserin, a selective and specific S2-serotonergic antagonist, on portal pressure and splanchnic hemodynamics in rats with long-term bile duct ligation. Hepatology. 1993;18:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2873] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 12. | Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2417] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 13. | Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5027] [Cited by in RCA: 4852] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 14. | Pan M, Song YL, Xu JM, Gan HZ. Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J Pineal Res. 2006;41:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Tahan V, Atug O, Akin H, Eren F, Tahan G, Tarcin O, Uzun H, Ozdogan O, Tarcin O, Imeryuz N. Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J Pineal Res. 2009;46:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1419] [Cited by in RCA: 1449] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 17. | Steinhilber D, Brungs M, Werz O, Wiesenberg I, Danielsson C, Kahlen JP, Nayeri S, Schräder M, Carlberg C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J Biol Chem. 1995;270:7037-7040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 181] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin’s interaction with reactive species. J Pineal Res. 2003;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 513] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 19. | Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T. Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic Biol Med. 1999;27:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, Cuzzocrea S, Fulia F, Barberi I. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol. 2001;53:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ. Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic Res. 2003;37:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Solís-Muñoz P, Solís-Herruzo JA, Fernández-Moreira D, Gómez-Izquierdo E, García-Consuegra I, Muñoz-Yagüe T, García Ruiz I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J Pineal Res. 2011;51:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Chen X, Zhang C, Zhao M, Shi CE, Zhu RM, Wang H, Zhao H, Wei W, Li JB, Xu DX. Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J Pineal Res. 2011;51:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 25. | Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 406] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 26. | Jaeschke H, Wang Y, Essani NA. Reactive oxygen species activate the transcription factor NF-kB in the liver by induction of lipid peroxidation. Hepatology. 1996;24:238A. |
| 27. | Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3598] [Cited by in RCA: 3636] [Article Influence: 117.3] [Reference Citation Analysis (1)] |
| 28. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (36)] |
| 29. | Berson A, De Beco V, Lettéron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 257] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 30. | García-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernández-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825-834. [PubMed] |
| 31. | Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F. Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994;55:PL271-PL276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 484] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57-60. |
| 33. | Barrett P, Conway S, Morgan PJ. Digging deep--structure-function relationships in the melatonin receptor family. J Pineal Res. 2003;35:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Cabrera J, Quintana J, Reiter RJ, Loro J, Cabrera F, Estévez F. Melatonin prevents apoptosis and enhances HSP27 mRNA expression induced by heat shock in HL-60 cells: possible involvement of the MT2 receptor. J Pineal Res. 2003;35:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Hoyos M, Guerrero JM, Perez-Cano R, Olivan J, Fabiani F, Garcia-Pergañeda A, Osuna C. Serum cholesterol and lipid peroxidation are decreased by melatonin in diet-induced hypercholesterolemic rats. J Pineal Res. 2000;28:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M. The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J Physiol Pharmacol. 2010;61:577-580. [PubMed] |
| 38. | Gonciarz M, Gonciarz Z, Bielanski W, Mularczyk A, Konturek PC, Brzozowski T, Konturek SJ. The pilot study of 3-month course of melatonin treatment of patients with nonalcoholic steatohepatitis: effect on plasma levels of liver enzymes, lipids and melatonin. J Physiol Pharmacol. 2010;61:705-710. [PubMed] |
| 39. | Kitagawa A, Ohta Y, Ohashi K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J Pineal Res. 2012;52:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Seabra ML, Bignotto M, Pinto LR, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 201] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci USA. 1994;91:1824-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 392] [Article Influence: 12.6] [Reference Citation Analysis (0)] |