Published online Nov 27, 2013. doi: 10.4254/wjh.v5.i11.635
Revised: October 7, 2013
Accepted: November 2, 2013
Published online: November 27, 2013
Processing time: 86 Days and 22 Hours
AIM: To examine the association between the interleukin 28B (IL-28B) genotype and treatment response in hepatitis C virus (HCV)-infected patients with persistently normal alanine aminotransferase (PNALT).
METHODS: We compared the treatment response of HCV-infected patients with PNALT to that of patients with non-PNALT. Between February 2010 and April 2013, 278 patients infected with HCV were enrolled in this study. All of the patients were treated with peginterferon-alpha 2a or 2b plus ribavirin. In addition, 180 μg of peginterferon alpha-2a or 1.5 μg/kg peginterferon alpha-2b per week plus weight-based ribavirin (600-1000 mg/d) were typically administered for 24 wk to HCV genotype 2-infected patients or for 48-72 wk to HCV genotype 1-infected patients. In all of the patients, the IL-28B rs8099917 genotype was determined using a TaqMan single-nucleotide polymorphism assay. HCV RNA was measured using the COBAS TaqMan HCV test.
RESULTS: Female patients were dominant in the PNALT group (P < 0.0001). Among 72 HCV genotype 1-infected patients with PNALT, the early virologic response (EVR) rates (P < 0.01) and the sustained virologic response (SVR) rates (P < 0.01) were higher in patients with the IL-28B TT genotype than in those with the IL-28B TG/GG genotype. In HCV genotype 1-infected patients with PNALT, multivariate logistic-regression analysis showed that SVR was independently predicted by the IL-28B rs8099917 TT type (P < 0.05) and having an EVR (P < 0.01). The IL-28B rs8099917 TT genotype strongly correlated with treatment response in HCV genotype 1-infected Asian patients with PNALT.
CONCLUSION: The IL-28B genotype may be useful for selecting HCV genotype 1-infected patients with PNALT who should receive interferon-based treatment.
Core tip: Whether the interleukin 28B (IL-28B) genotype affects the treatment response to peginterferon plus ribavirin in hepatitis C virus (HCV)-infected patients with persistently normal alanine aminotransferase (PNALT) is unclear. We examined the association between the IL-28B genotype and treatment response in HCV-infected patients with PNALT. Opinions about the appropriate treatment method for HCV-infected patients with PNALT differ. In the present study, we found that IL-28B rs8099917 TT was associated with SVR in HCV genotype 1-infected Asian patients with PNALT. The determination of IL-28B genotype is important for the successful treatment of HCV genotype 1-infected patients with PNALT.
-
Citation: Miyamura T, Kanda T, Nakamura M, Jiang X, Wu S, Nakamoto S, Mikami S, Takada N, Imazeki F, Yokosuka O.
IL-28B polymorphisms and treatment response in hepatitis C virus patients with persistently normal alanine aminotransferase. World J Hepatol 2013; 5(11): 635-641 - URL: https://www.wjgnet.com/1948-5182/full/v5/i11/635.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i11.635
Hepatitis C virus (HCV) is a causative agent of acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC)[1-3]. Peginterferon-alpha 2a or 2b plus ribavirin treatment leads to sustained virologic response (SVR) rates of approximately 50% and 80% in patients infected with HCV genotype 1 and genotype 2 or 3, respectively[4-6]. The standard of care has been peginterferon plus ribavirin until the recent approval of combination therapies, including telaprevir and boceprevir. Until the development of an interferon-free regimen, peginterferon alpha plus ribavirin will play a critical role in the eradication of this virus.
Persistently normal alanine aminotransferase (PNALT) is present in 25%-40% of patients with chronic HCV infection[7,8]. Although an elevated alanine aminotransferase (ALT) level suggests progressive liver damage in chronic HCV infection, normal ALT levels do not always exclude significant liver damage. Zeuzem et al[9] reported that the SVR rates in patients with PNALT were similar to those in patients with abnormal ALT. However, opinions about the appropriate treatment method for HCV-infected patients with PNALT differ[7-11].
Genome-wide association studies have revealed a strong relationship between single-nucleotide polymorphisms (SNPs) near interleukin 28B (IL-28B) on chromosome 19 and the virologic response to peginterferon plus ribavirin treatment in patients worldwide who are infected with HCV genotype 1[12-14] as well as an association with the natural clearance of this virus[15,16]. IL-28B has antiviral properties and can interact with human interferon responses[17-21]. Associations between IL-28B variants and HCC development[22] and recurrence[23] have recently been reported. Moreover, an association between the IL-28B rs12979860 CC genotype and higher ALT levels has also been described[24]. It is possible that the IL-28B genotype is associated with inflammatory activity in the liver and the progression of hepatic fibrosis.
In clinical practice, it is difficult to make the decision to treat HCV-infected patients with PNALT. In the present study, we investigated whether IL-28B rs8099917 genetic variations were useful for the prediction of treatment response in HCV-infected patients with PNALT.
This work was conducted in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Written informed consent was obtained from each patient participating in this study. The study was approved by the ethics committee of Chiba University, Japan (permission number 282 and 1462), and conformed to the tenets of the Declaration of Helsinki.
Between February 2010 and April 2013, 278 patients infected with HCV were enrolled in this study. All patients were treated with peginterferon-alpha 2a or 2b plus ribavirin at Chiba University Medical School Hospital, Kikkoman General Hospital, or Toho University, Sakura Medical Center. The patients were eligible if they met the following inclusion criteria: (1) infection with HCV; (2) age ≥ 20 years; (3) no absolute contraindications for peginterferon plus ribavirin therapy such as pregnancy, severe heart disease, abnormal hemoglobinemia, chronic renal failure, mental disorders, severe liver failure, or autoimmune diseases; (4) absence of HIV infection; (5) no currently active drug abuse; and (6) no drug allergy to interferon or nucleos(t)ide analogues. Some of these patients had previously been included in other studies[19,25,26].
Among these 278 HCV RNA-positive patients, 178 had ALT elevation (each value exceeding the higher limit of the normal range was considered abnormal)[7], and 100 exhibited normal ALT levels at least 3 times during a 24-mo period (considered as PNALT patients).
In the present study, 180 μg of peginterferon alpha-2a or 1.5 μg/kg of peginterferon alpha-2b per week plus weight based ribavirin (600-1000 mg/d) were typically administered for 24 wk to HCV genotype 2-patients or for 48-72 wk to HCV genotype 1-patients.
HCV RNA was measured using the COBAS TaqMan HCV test (Roche Diagnostics, Tokyo, Japan). The linear dynamic range of this assay was 1.2 to 7.8 log IU/mL. HCV genotypes were determined using the antibody serotyping method of Tsukiyama-Kohara et al[27], and Tanaka et al[28]. Serum ALT measurement and other liver function tests were performed according to standard methods. The normal range of serum ALT was considered 8-42 IU/L.
SNP rs8099917 was examined in plasma by allelic discrimination using TaqMan minor groove binding (MGB) probes as described previously[26]. Briefly, we used DNA Extract All Reagents Kit (Applied Biosystems Inc., Foster City, CA, United States) to prepare the DNA sample from fresh plasma. Probes for the TaqMan MGB assay were manufactured by Applied Biosystems. Thermal cycling was performed in an ABI Step One Real-Time PCR system (Applied Biosystems) according to the manufacturer’s protocol. Activation of TaqMan GTXpress Master Mix (Applied Biosystems) and the initial denaturation cycle were at 95 °C for 20 s, followed by 40 cycles at 95 °C for 3 s and 60 °C for 20 s. We analyzed SNP rs8099917 TT as the major genotype and TG/GG as the minor genotype in the present study.
SVR was defined as undetectable serum HCV RNA at 24 wk after the end of treatment. Patients who had undetectable HCV RNA within the initial 4 wk of treatment were considered to have had a rapid virologic response (RVR). Patients with undetectable HCV RNA within the initial 12 wk were considered to have had a complete early virologic response (cEVR) (described as EVR in this study).
The results are expressed as the mean ± SD. Student’s t-test or the χ2 test was used to determine statistical significance. Variables with P < 0.05 in univariate analyses were retained for multivariate logistic regression analysis. For all tests, two-sided P-values were calculated, and the results were considered statistically significant at P < 0.05. The statistical analysis was performed using the Excel Statistics program for Windows, version 7 (SSRI, Tokyo, Japan).
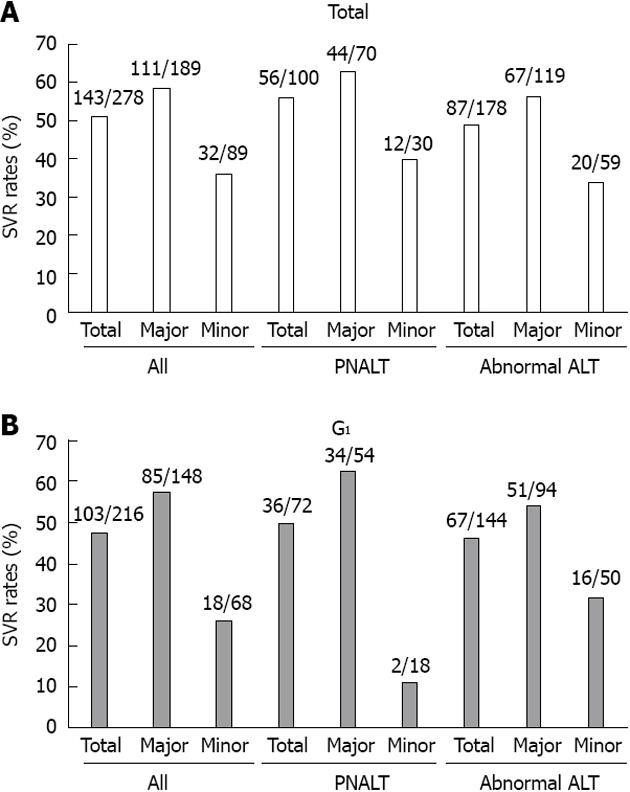
The baseline characteristics are shown in Table 1. Of the 278 total patients, 100 (36.0%) and 178 (64.0%) were in the PNALT and abnormal ALT groups, respectively. Female patients were dominant in the PNALT group (P < 0.0001), whereas male patients were dominant in the abnormal ALT group (P < 0.0001). The AST, ALT, and γ-GT levels in the PNALT group were lower compared with those in the abnormal ALT group (P < 0.0001) (Table 1). In the PNALT group, 15 patients had relapsed after treatment, and 10 patients were null responders. In the abnormal ALT group, 23 patients had relapsed after treatment, and 19 patients were null responders. Of the 278 patients, 215 (77.3%), 60 (21.5%), and 3 (1.0%) were classified into HCV genotypes 1, 2, and unknown, respectively. HCV genotype 1 patients in PNALT and abnormal ALT groups were 72 (72.0%) and 143 (80.3%), respectively. The proportions of IL-28B genotypes did not differ between the PNALT and abnormal ALT groups (Table 1).
| Total | PNALT | Abnormal ALT | P value | |
| Number of patients | 278 | 100 | 178 | |
| Age (yr) | 55.6 ± 11.4 | 56.2 ± 10.9 | 55.3 ± 11.6 | 0.526 |
| Gender (male/female) | 136/142 | 31/69 | 105/73 | < 0.0001 |
| AST (IU/L) | 56.6 ± 44.7 | 29.8 ± 12.1 | 71.3 ± 49.1 | < 0.0001 |
| ALT (IU/L) | 70.5 ± 64.3 | 27.6 ± 7.2 | 94.6 ± 69.4 | < 0.0001 |
| γ-GT (IU/L) | 50.7 ± 57.3 | 26.2 ± 22.1 | 64.1 ± 65.7 | < 0.0001 |
| WBC (/mm3) | 5190 ± 1500 | 5060 ± 1490 | 5260 ± 1510 | 0.28 |
| Hemoglobin (g/dL) | 14.4 ± 7.1 | 13.5 ± 1.2 | 15.0 ± 8.9 | 0.094 |
| Platelets (× 104/mm3) | 17.1 ± 5.6 | 17.7 ± 5.9 | 16.8 ± 5.5 | 0.20 |
| Previous treatment (-/+) | 211/67 | 75/25 | 136/42 | 0.90 |
| IL-28B SNP(Maj/Min) | 189/89 | 70/30 | 119/59 | 0.68 |
| VR/Null response | 203/65 | 84/16 | 129/49 | 0.042 |
| RVR (+/-) | 32/200 | 12/70 | 20/130 | 0.93 |
| EVR (+/-) | 116/118 | 46/36 | 70/82 | 0.18 |
| SVR (+/-) | 143/135 | 56/44 | 87/91 | 0.30 |
Of the 278 total patients, 211 (75.8%) and 67 (24.1%) were treatment naïve and retreated, respectively. SVR was obtained in 143 (51.4%) of the 278 patients. Within treatment groups, SVR was achieved in 120 (56.8%) of 211 treatment-naive and 23 (34.3%) of 67 retreated patients, respectively. The age of SVR patients (53.1 ± 12.8 years) was lower than that of non-SVR patients (58.3 ± 8.9 years) (P = 0.00011).
We next compared the virologic responses (VR) between the PNALT and abnormal ALT groups, and the proportions of treatment-naïve and retreated patients did not differ between these 2 groups (Table 1 and Figure 1). Additionally, the proportion of each IL-28B SNP rs8099917 did not differ between the 2 groups. Of interest, significantly fewer null responders were included in the PNALT group than in the abnormal ALT group (P = 0.042). However, the proportions of patients with an RVR, EVR, or SVR did not differ between the 2 groups (Table 1).
Among the 100 PNALT patients, 70 and 30 patients had the IL-28B rs8099917 major and minor genotypes, respectively (Table 2). In this PNALT group, patients with the IL-28B rs8099917 major genotype were older than those with the IL-28B rs8099917 minor genotype. The γ-GT levels in the IL-28B rs8099917 major group were lower compared with those in the IL-28B rs8099917 minor group. However, we observed lower hemoglobin levels in the IL-28B rs8099917 major group compared with those in the IL-28B rs8099917 minor group. In the PNALT group, other than RVRs, virologic responses were better in the IL-28B rs8099917 major group (Table 2).
| IL-28B rs8099917 | PNALT group (n = 100) | Abnormal ALT group (n = 178) | ||||
| Major | Minor | P value | Major | Minor | P value | |
| Number of patients | 70 | 30 | 119 | 59 | ||
| Age (yr) | 57.7 ± 10.8 | 52.6 ± 10.5 | 0.031 | 55.3 ± 11.3 | 55.4 ± 12.3 | 0.95 |
| Gender (male/female) | 21/49 | 10/20 | 0.92 | 68/51 | 37/22 | 0.58 |
| AST (IU/L) | 29.3 ± 13.1 | 30.9 ± 9.7 | 0.58 | 69.4 ± 51.4 | 75.4 ± 44.1 | 0.47 |
| ALT (IU/L) | 26.8 ± 7.2 | 29.5 ± 7.0 | 0.086 | 92.1 ± 70.4 | 99.5 ± 67.8 | 0.5 |
| γ-GT (IU/L) | 21.0 ± 11.5 | 38.4 ± 33.8 | 0.00069 | 54.0 ± 41.3 | 85.1 ± 95.7 | 0.0056 |
| WBC (/mm3) | 5130 ± 1440 | 4900 ± 1640 | 0.52 | 5280 ± 1,680 | 5230 ± 1070 | 0.84 |
| Hemoglobin (g/dL) | 13.3 ± 1.0 | 13.9 ± 1.5 | 0.02 | 15.3 ± 10.8 | 14.4 ± 1.1 | 0.52 |
| Platelets (× 104/mm3) | 17.2 ± 5.7 | 18.8 ± 6.3 | 0.21 | 17.0 ± 5.3 | 16.4 ± 5.8 | 0.49 |
| Previous treatment (-/+) | 54/16 | 21/9 | 0.61 | 93/26 | 43/16 | 0.55 |
| VR/Null response | 63/7 | 21/9 | 0.027 | 98/21 | 31/23 | 0.000059 |
| RVR (+/-) | 9/50 | 3/20 | 0.92 | 17/84 | 3/46 | 0.12 |
| EVR (+/-) | 38/21 | 8/15 | 0.029 | 59/44 | 11/38 | 0.00011 |
| SVR (+/-) | 44/26 | 12/18 | 0.058 | 67/52 | 20/39 | 0.0079 |
Among the 178 abnormal ALT patients, 119 and 59 had the IL-28B rs8099917 major and minor genotypes, respectively (Table 2). The γ-GT levels in the IL-28B rs8099917 major group were lower than those in the IL-28B rs8099917 minor group. In the abnormal ALT group, other than RVRs, virologic responses were better in the IL-28B major group (Table 2).
Among 72 PNALT patients infected with HCV genotype 1, 54 and 18 had the IL-28B rs8099917 major and minor genotypes, respectively (Table 3). Among the patients with the IL-28B rs8099917 major genotype, 8 had relapsed after treatment, and 4 were null responders. In patients with the IL-28B rs8099917 minor genotype, 1 had relapsed after treatment, and 5 were null responders. The ALT and γ-GT levels in the IL-28B rs8099917 major group were lower than those in the IL-28B rs8099917 minor group (Table 3). In HCV genotype 1-infected patients with PNALT, virologic responses were better in the IL-28B major group (Table 3). However, among the HCV genotype 2-infected patients with PNALT, virologic responses did not differ between the IL-28B major and minor groups although the number of HCV genotype 2-infected patients was smaller in the present study (data not shown). Among these patients, an SVR occurred in 71.4%, 62.5%, and 83.3% of all, IL-28B major, and IL-28B minor patients, respectively.
| IL-28B rs8099917 | Major | Minor | P value | SVR | Non-SVR | P value |
| Number of patients | 54 | 18 | 36 | 36 | ||
| Age (yr) | 58.4 ± 10.9 | 54.6 ± 11.0 | 0.20 | 56.6 ± 13.4 | 58.4 ± 8.0 | 0.49 |
| Gender (male/female) | 17/37 | 7/11 | 0.77 | 14/22 | 10/26 | 0.45 |
| AST (IU/L) | 30.3 ± 14.5 | 34.5 ± 10.3 | 0.29 | 13.2 ± 1.1 | 32.0 ± 6.3 | < 0.00010 |
| ALT (IU/L) | 27.5 ± 6.7 | 31.7 ± 5.6 | 0.019 | 27.3 ± 7.4 | 29.8 ± 5.7 | 0.11 |
| γ-GT (IU/L) | 21.7 ± 11.9 | 47.3 ± 39.3 | 0.00024 | 19.7 ± 10.6 | 39.2 ± 32.7 | 0.0011 |
| WBC (/mm3) | 5020 ± 1410 | 4570 ± 1350 | 0.27 | 5000 ± 1350 | 4780 ± 1470 | 0.51 |
| Hemoglobin (g/dL) | 13.3 ± 0.9 | 13.7 ± 1.5 | 0.17 | 13.2 ± 1.1 | 13.5 ± 1.1 | 0.25 |
| Platelets (× 104/mm3) | 16.6 ± 6.0 | 17.0 ± 5.6 | 0.80 | 17.1 ± 6.0 | 16.2 ± 5.8 | 0.51 |
| Previous Treatment (-/+) | 42/12 | 12/6 | 0.52 | 29/7 | 25/11 | 0.41 |
| VR/Null response | 46/8 | 9/9 | 0.0064 | 36/0 | 19/17 | < 0.00010 |
| RVR (+/-) | 6/38 | 0/16 | 0.28 | 6/27 | 0/27 | 0.057 |
| EVR (+/-) | 25/19 | 1/15 | 0.0013 | 23/10 | 3/24 | < 0.00010 |
| SVR (+/-) | 34/20 | 2/16 | 0.00040 | |||
| IL-28B SNP rs8099917 | ||||||
| (Maj/Min) | 34/2 | 20/16 | 0.00040 |
To clarify the predictors of SVR, we compared pretreatment and treatment factors between SVR and non-SVR-HCV genotype 1-infected patients with PNALT (Table 3). In HCV genotype 1-infected patients with PNALT, univariate analysis showed that AST, γ-GT, IL-28B SNP rs8099917, virologic response, and having an EVR contributed to the achievement of SVR. Factors significantly associated with SVR by univariate analysis were included in a multivariate logistic regression analysis. In HCV genotype 1-infected patients with PNALT, SVR was independently predicted by the IL-28B rs8099917 major genotype and having an EVR (Table 4).
| Factor | Category | Odds ratio | 95%CI | P value |
| IL-28B rs8099917 | Major/Minor | 7.11 | 1.305-38.799 | 0.023 |
| EVR | (+/-) | 13.28 | 3.242-54.399 | 0.0003 |
The main finding of the present study evaluating IL-28B SNP rs8099917 was that this genotype may be a useful predictors of SVR following treatment with peginterferon-alpha plus ribavirin in HCV genotype 1-infected patients with PNALT. This finding is in line with previous reports indicating that IL-28B SNPs rs1297986 and rs8099917 could predict hepatitis C treatment-induced viral clearance[12-15]. Importantly, the present study results indicated that IL-28B SNP rs8099917 and EVR are useful surrogate markers of SVR even in HCV genotype 1-infected patients with PNALT.
Nunnari et al[7] reported that the frequency of IL-28B SNP rs12979860 did not differ between the hyper-ALT and PNALT groups. Furthermore, the natural history of HCV carriers with PNALT is most likely not always benign and could reflect a more severe evolution of liver disease[29]. Controversies exist regarding the appropriate treatment method for HCV-infected patients with PNALT[29]. The most recent guidelines recommended that HCV-infected PNALT-patients with moderate or severe fibrosis should be treated[8,10]. Tanaka et al[14] has shown that the IL-28B SNP rs8099917 TT genotype strongly correlates with treatment response in HCV genotype 1-infected Asian patients. It has also been reported that linkage disequilibrium between the two IL-28B SNPs, rs8099917 and rs12979860, is strong in Japanese HCV patients[30]. In the present study, IL-28B SNP rs8099917 but not IL-28B SNP rs12979860 was evaluated.
Peginterferon-alpha plus ribavirin treatment led to an SVR rate of approximately 50% in patients infected with HCV genotype 1[4]. The efficacy and safety of peginterferon and ribavirin combination therapy in patients with HCV and PNALT are similar to those in patients with abnormal ALT[9]. Dual peginterferon/ribavirin therapy is no longer the standard therapy for chronic HCV infection. Combination therapy with telaprevir or boceprevir led to higher SVR rates in patients infected with HCV genotype 1[31]; however, severe adverse events are often observed with the use of these drugs[32,33]. In daily clinical practice, it must be decided whether patients with HCV and PNALT should receive treatment based the balance between disease progression and treatment efficacy. Until new interferon-sparing regimens are introduced[31], our findings suggest that IL-28B SNP rs8099917 could be helpful in selecting patients with HCV and PNALT who should receive treatment.
Baseline plasma interferon-gamma inducible protein-10 (IP-10 or CXCL10) levels are strongly associated with IL-28B genotypes[18]. Honda et al[17] reported that hepatic interferon-stimulated genes (ISGs) are associated with IL-28B genotypes. We have also reported that concomitant assessment of lower-hepatic STAT1-nuclear translocation and IL-28B genotypes is useful for the prediction of SVR in HCV-infected patients[19]. Additionally, we have recently demonstrated that IL-28B induces ISGs that are reportedly associated with the progression of HCV-related pathogenesis and antiviral activities against HCV[34]. Further studies will be needed.
In a previous study, the IL-28B minor genotype was associated with lower inflammatory activity in the liver[22]. In contrast, the proportion of IL-28B genotypes did not differ between patients with PNALT and abnormal ALT in the present study. Further studies will be needed to clarify the association between IL-28B SNP rs8099917 and serum ALT levels[35]. In conclusion, IL-28B rs8099917 TT was associated with SVR in HCV genotype 1-infected Asian patients with PNALT. This finding sheds new light on the treatment options for HCV genotype 1-infected patients with PNALT.
We would like to thank the medical staff at Chiba University Hospital for their assistance and support during the study.
Although the progression of hepatic fibrosis appears to be slow in chronic hepatitis C patients with persistently normal alanine aminotransferase (PNALT), differing opinions about the natural history of hepatitis C virus (HCV) carriers with PNALT exist, suggesting that it is most likely not always benign and that a more severe evolution of liver disease can occur. It is difficult to determine whether chronic hepatitis C patients with PNALT should be treated. Interleukin 28B (IL-28B) genotypes have been reported to be predictive of the treatment response to peginterferon plus ribavirin in chronic hepatitis C patients.
Whether there is an association between the IL-28B rs8099917 genotype and treatment response in HCV-infected Asian patients with PNALT is unknown. In this study, the authors demonstrated an association between the IL-28B rs8099917 genotype and treatment response in HCV-infected Asian patients with PNALT.
Recent reports have highlighted the importance of the IL-28B genotype in the treatment of HCV genotype 1-infected Asian patients with PNALT. This is the first study to report an association between the IL-28B rs8099917 genotype and treatment response in HCV-infected Asian patients with PNALT.
IL-28B rs8099917 appears to be useful for identifying chronic hepatitis C patients with PNALT who will benefit from treatment.
IL-28B SNP rs8099917 is located approximately 8 kb upstream of IL-28B, which is in linkage disequilibrium with rs12979860 (located approximately 3 kb upstream of IL-28B). These SNPs are strongly associated with the natural and treatment-induced eradication of HCV.
The authors examined the association between the IL-28B genotype and treatment response in HCV-infected patients with PNALT. Their study revealed that the proportion of IL-28B genotypes did not differ between patients with PNALT and patients with abnormal ALT. The authors also demonstrated an association between the IL-28B rs8099917 genotype and treatment response in HCV-infected Asian patients with PNALT. The results are interesting and the IL-28B genotype may be very helpful in the treatment of patients with chronic hepatitis C with PNALT.
P- Reviewers: Genesca J, Marin JJG, Tischendorf JJW S- Editor Ma YJ L- Editor A E- Editor Yan JL
| 1. | Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Semin Liver Dis. 1995;15:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [PubMed] |
| 3. | Tan A, Yeh SH, Liu CJ, Cheung C, Chen PJ. Viral hepatocarcinogenesis: from infection to cancer. Liver Int. 2008;28:175-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Lagging M, Rembeck K, Rauning Buhl M, Christensen P, Dalgard O, Färkkilä M, Hellstrand K, Langeland N, Lindh M, Westin J. Retreatment with peg-interferon and ribavirin in patients with chronic hepatitis C virus genotype 2 or 3 infection with prior relapse. Scand J Gastroenterol. 2013;48:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Yu ML, Huang CF, Huang JF, Chang NC, Yang JF, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology. 2011;53:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Nunnari G, Pinzone MR, Cacopardo B. Lack of clinical and histological progression of chronic hepatitis C in individuals with true persistently normal ALT: the result of a 17-year follow-up. J Viral Hepat. 2013;20:e131-e137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Omata M, Kanda T, Yu ML, Yokosuka O, Lim SG, Jafri W, Tateishi R, S . Hamid S, Chuang WL, Chutaputti A, Wei L, Sollano J, Sarin SK, Kao JH, W. McCaughan G. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409-435. [RCA] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Zeuzem S, Diago M, Gane E, Reddy KR, Pockros P, Prati D, Shiffman M, Farci P, Gitlin N, O’Brien CB. Peginterferon alfa-2a (40 kilodaltons) and ribavirin in patients with chronic hepatitis C and normal aminotransferase levels. Gastroenterology. 2004;127:1724-1732. [PubMed] |
| 10. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2240] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 11. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 12. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2721] [Article Influence: 170.1] [Reference Citation Analysis (0)] |
| 13. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1502] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 14. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1773] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 15. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1686] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 16. | Tillmann HL, Thompson AJ, Patel K, Wiese M, Tenckhoff H, Nischalke HD, Lokhnygina Y, Kullig U, Göbel U, Capka E. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586-1592, 1592.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shirasaki T, Horimoto K. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 18. | Lagging M, Askarieh G, Negro F, Bibert S, Söderholm J, Westin J, Lindh M, Romero A, Missale G, Ferrari C. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6:e17232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Miyamura T, Kanda T, Nakamoto S, Wu S, Fujiwara K, Imazeki F, Yokosuka O. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 759] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 21. | Bibert S, Roger T, Calandra T, Bochud M, Cerny A, Semmo N, Duong FH, Gerlach T, Malinverni R, Moradpour D. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J Exp Med. 2013;210:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Sato M, Kato N, Tateishi R, Muroyama R, Kowatari N, Li W, Goto K, Otsuka M, Shiina S, Yoshida H. IL28B minor allele is associated with a younger age of onset of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J Gastroenterol. 2013;May 22; Epub ahead of print. [PubMed] |
| 23. | Hodo Y, Honda M, Tanaka A, Nomura Y, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Sakai A. Association of interleukin-28B genotype and hepatocellular carcinoma recurrence in patients with chronic hepatitis C. Clin Cancer Res. 2013;19:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Agúndez JA, García-Martin E, Maestro ML, Cuenca F, Martínez C, Ortega L, Carballo M, Vidaurreta M, Agreda M, Díaz-Zelaya G. Relation of IL28B gene polymorphism with biochemical and histological features in hepatitis C virus-induced liver disease. PLoS One. 2012;7:e37998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;46:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Miyamura T, Kanda T, Nakamoto S, Wu S, Jiang X, Arai M, Fujiwara K, Imazeki F, Yokosuka O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses. 2012;4:1264-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Tsukiyama-Kohara K, Yamaguchi K, Maki N, Ohta Y, Miki K, Mizokami M, Ohba K, Tanaka S, Hattori N, Nomoto A. Antigenicities of Group I and II hepatitis C virus polypeptides--molecular basis of diagnosis. Virology. 1993;192:430-437. [PubMed] |
| 28. | Tanaka T, Tsukiyama-Kohara K, Yamaguchi K, Yagi S, Tanaka S, Hasegawa A, Ohta Y, Hattori N, Kohara M. Significance of specific antibody assay for genotyping of hepatitis C virus. Hepatology. 1994;19:1347-1353. [PubMed] |
| 30. | Kobayashi M, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Hosaka T, Kawamura Y, Kobayashi M, Saitoh S, Arase Y. Association of two polymorphisms of the IL28B gene with viral factors and treatment response in 1,518 patients infected with hepatitis C virus. J Gastroenterol. 2012;47:596-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Kanda T, Yokosuka O, Omata M. Treatment of hepatitis C virus infection in the future. Clin Transl Med. 2013;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | Hynicka LM, Heil EL. Anemia management in patients with chronic viral hepatitis C. Ann Pharmacother. 2013;47:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Kanda T, Jiang X, Nakamoto S, Nakamura M, Miyamura T, Wu S, Yokosuka O. Different effects of three interferons L on Toll-like receptor-related gene expression in HepG2 cells. Cytokine. 2013;64:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Nakamura M, Kanda T, Miyamura T, Wu S, Nakamoto S, Yokosuka O. Alanine aminotransferase elevation during peginterferon alpha-2a or alpha-2b plus ribavirin treatment. Int J Med Sci. 2013;10:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |