Revised: September 8, 2012
Accepted: November 14, 2012
Published online: January 27, 2013
AIM: To determine feasibility of liver transplantation in patients from the intensive care unit (ICU) by estimating graft and patient survival.
METHODS: This single center retrospective study included 39 patients who had their first liver transplant directly from the intensive care unit and 927 non-ICU patients who were transplanted from hospital ward or home between January 2005 and December 2010.
RESULTS: In comparison to non-ICU patients, ICU patients had a higher model for end-stage liver disease (MELD) at transplant (median: 37 vs 20, P < 0.001). Fourteen out of 39 patients (36%) required vasopressor support immediately prior to liver transplantation (LT) with 6 patients (15%) requiring both vasopressin and norepinephrine. Sixteen ICU patients (41%) were ventilator dependent immediately prior to LT with 9 patients undergoing percutaneous tracheostomy prior to transplantation. Twenty-five ICU patients (64%) required dialysis preoperatively. At 1, 3 and 5 years after LT, graft survival was 76%, 68% and 62% in ICU patients vs 90%, 81% and 75% in non-ICU patients. Patient survival at 1, 3 and 5 years after LT was 78%, 70% and 65% in ICU patients vs 94%, 85% and 79% in non-ICU patients. When formally comparing graft survival and patient survival between ICU and non-ICU patients using Cox proportional hazards regression models, both graft survival [relative risk (RR): 1.94, 95%CI: 1.09-3.48, P = 0.026] and patient survival (RR: 2.32, 95%CI: 1.26-4.27, P = 0.007) were lower in ICU patients vs non-ICU patients in single variable analysis. These findings were consistent in multivariable analysis. Although not statistically significant, graft survival was worse in both patients with cryptogenic cirrhosis (RR: 3.29, P = 0.056) and patients who received donor after cardiac death (DCD) grafts (RR: 3.38, P = 0.060). These findings reached statistical significance when considering patient survival, which was worse for patients with cryptogenic cirrhosis (RR: 3.97, P = 0.031) and patients who were transplanted with DCD livers (RR: 4.19, P = 0.033). Graft survival and patient survival were not significantly worse for patients on mechanical ventilation (RR: 0.91, P = 0.88 in graft loss; RR: 0.69, P = 0.56 in death) or patients on vasopressors (RR: 1.06, P = 0.93 in graft loss; RR: 1.24, P = 0.74 in death) immediately prior to LT. Trends toward lower graft survival and patient survival were observed for patients on dialysis immediately before LT, however these findings did not approach statistical significance (RR: 1.70, P = 0.43 in graft loss; RR: 1.46, P = 0.58 in death).
CONCLUSION: Although ICU patients when compared to non-ICU patients have lower survivals, outcomes are still acceptable. Pre-transplant ventilation, hemodialysis, and vasopressors were not associated with adverse outcomes.
- Citation: Sibulesky L, Heckman MG, Taner CB, Canabal JM, Diehl NN, Perry DK, Willingham DL, Pungpapong S, Rosser BG, Kramer DJ, Nguyen JH. Outcomes following liver transplantation in intensive care unit patients. World J Hepatol 2013; 5(1): 26-32
- URL: https://www.wjgnet.com/1948-5182/full/v5/i1/26.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i1.26
Liver transplantation (LT) is a life-saving procedure for patients with a wide range of end-stage liver diseases (ESLD). Significant improvement in surgical technique, medical management, and advances in immunosuppression therapy have all contributed to the success of LT. Recent studies have demonstrated that the overall outcome of LT depends on a combination of factors including recipient condition, donor organ quality, as well as the transplant center volume[1-4].
Liver disease is progressive in nature and the care for such patients is complex and challenging. As a result, transplant candidates may require intensive care unit admission while awaiting transplantation[5,6]. It is not uncommon for some patients to have multiorgan system failure (MOSF) requiring ventilatory support, hemodynamic support, and renal replacement therapy (RRT) in the course of their disease. Transplantation of such patients could lead to poor post-transplant outcomes[7,8]. Given the scarcity of organ donors, LT is currently offered to patients with the expected survival of at least 50% in 5 years after the transplantation[9]. As a result, controversy arises: from an individual stand point there is always a benefit to LT because the outcome of deteriorating ESLD is uniformly fatal. From a societal perspective futile outcomes are not acceptable in the time of donor organ shortage.
The current established absolute contraindications for LT include advanced cardiopulmonary disease, extrahepatic malignancy with metastasis, active substance abuse, sepsis, and inability to comply with medical treatment[10]. Despite multiple efforts, there is currently no agreed upon definition of “too sick to transplant”, nor there are standardized guidelines for when a critically ill patient should be removed from a transplant waiting list[11,12]. Criteria that are used to delist a sick patient are transplant program dependent. Many regard ventilatory support and vasopressor therapy in a cirrhotic patient as contraindications to proceeding with transplantation[6].
The aim of our study was to determine the feasibility of LT in patients from the ICU by estimating graft and patient survival in this patient group and also compare these outcomes with non-ICU patients. We also evaluated associations of pre-transplant donor and recipient characteristics with outcomes in ICU patients.
This single center retrospective study included 39 patients who underwent first LT directly from the ICU between January, 2005 and December, 2010 and 927 non-ICU patients, who underwent first LT over the same time period. Non-ICU patients were defined as patients transplanted from the hospital ward or home. This study was exempt from IRB review. Patients who underwent re-transplantation, multiple organ transplant, patients who underwent transplant for fulminant liver failure were excluded. For both ICU and non-ICU patients, information was collected regarding patient characteristics (age, gender, body mass index, etiology of ESLD, model for end-stage liver disease (MELD) score at transplant, previous abdominal operations), operative characteristics (operative time, blood transfusion), donor characteristics [age, gender, recipient-donor gender incompatibility, donation after cardiac death (DCD), donor risk index, cold ischemia time, warm ischemia time], and outcomes (date of graft loss, date of death, date of last follow-up). The following additional information was collected for ICU patients: pre-LT information (length of time from hospital admission to ICU admission, length of time from ICU admission to transplant, MELD at ICU admission, pre-transplant ambulatory status, mechanical ventilation, dialysis, vasopressor use and dose, tracheostomy, positive end-expiratory pressure (PEEP), FiO2, mean airway pressure, PaO2) at the time of transplant and post-LT information (tracheostomy, length of hospital stay, length of ICU stay, discharge status, readmission within 3 mo after LT).
Patient, operative, and donor characteristics were compared between ICU and non-ICU patients using a Wilcoxon rank sum test or Fisher’s exact test. The Kaplan-Meier method was used to estimate graft survival and patient survival after LT, censoring on the date of last follow-up for patients who did not experience graft loss or death (graft survival) or death (patient survival). Cox proportional hazards regression models were used to compare graft survival and patient survival between ICU and non-ICU patients. Single variable models (i.e., models with no adjustment for other variables) were utilized, as well as multivariable models adjusted for variables that differed between ICU and non-ICU patients with a P value of 0.10 or less, excluding variables that are known to differ between the two groups due to the nature of ICU patients (MELD at transplant), variables that are potentially on the causal pathway between ICU status and graft loss or death (operative time, blood transfusion), variables that did not occur in ICU patients (NASH diagnosis), or variables with any missing data in ICU patients. Relative risks (RRs) and 95%CIs were estimated. In ICU patients, associations of patient and donor characteristics with graft survival and patient survival were evaluated using Cox proportional hazards regression models. Only single variable analysis was performed; multivariable analysis was not attempted owing to the small number of ICU patients who experienced the endpoints of interest[13]. P≤ 0.05 was considered as statistically significant. All statistical analyses were performed using SAS (Version 9.2; SAS Institute, Inc., Cary, North Carolina) and R Statistical Software (Version 2.11.0; R Foundation for Statistical Computing, Vienna, Austria).
A comparison of patient, operative, and donor characteristics between ICU patients and non-ICU patients is displayed in Table 1. In comparison to non-ICU patients, ICU patients were less often male (54% vs 70%, P = 0.033), had a lower body mass index (BMI) (median: 25.7 vs 28.4, P = 0.091), and had a higher MELD at transplant (median: 37 vs 20, P < 0.001). Intraoperatively, ICU patients had a greater packed red blood cell transfusion requirement (median: 3850 mL vs 2800 mL, P = 0.002). When compared to non-ICU patients, the ICU patients received liver grafts from the younger median: 1.53 vs 1.66, P = 0.085).
| Variable | ICU patients(n = 39) | Non-ICU patients(n = 927) | P value |
| Patient characteristics | |||
| Age at transplant | 57 (33-74) | 57 (16-77) | 0.91 |
| Gender (male) | 21 (54%) | 653 (70%) | 0.033 |
| BMI | 25.7 (18.0-38.0) | 28.4 (16.4-61.1) | 0.091 |
| Diagnosis | |||
| Hepatitis C | 18 (46%) | 364 (41%) | 0.41 |
| ETOH | 10 (26%) | 138 (15%) | 0.11 |
| Cryptogenic cirrhosis | 8 (21%) | 147 (16%) | 0.5 |
| NASH | 0 (0%) | 76 (8%) | 0.066 |
| PSC | 0 (0%) | 61 (7%) | 0.17 |
| Other | 3 (8%) | 141 (15%) | 0.25 |
| MELD at transplant | 37 (24-50) | 20 (6-45) | < 0.001 |
| Previous operation | 13 (34%) | 402 (47%) | 0.13 |
| Operative characteristics | |||
| Operative time (min) | 230 (129-596) | 231 (100-745) | 0.69 |
| Blood transfusion (mL) | 3850 (1400-15 400) | 2800 (0-44 100) | 0.002 |
| Cold ischemia time (h) | 6.3 (3.4-10.4) | 6.3 (2.0-14.0) | 0.71 |
| Warm ischemia time (min) | 30 (18-84) | 31 (10-141) | 0.59 |
| Donor characteristics | |||
| Age | 42 (8-78) | 48 (7-88) | 0.016 |
| Gender (male) | 17 (46%) | 551 (59%) | 0.12 |
| Recipient-donor gender incompatibility | 15 (41%) | 348 (38%) | 0.73 |
| Donation after cardiac death | 6 (16%) | 146 (16%) | 1.00 |
| Donor risk index | 1.53 (0.88-2.60) | 1.66 (0.85-4.30) | 0.085 |
A summary of additional patient and post-operative characteristics for the 39 ICU patients is shown in Table 2. Fourteen out of 39 patients (36%) required vasopressor support immediately prior to LT with 6 patients (15%) requiring both vasopressin and norepinephrine. The range of the dose of vasopressin was 0.01 to 0.04 units/min, while norepinephrine dose ranged from 0.01 to 0.18 mcg/kg per minute. Sixteen ICU patients (41%) were ventilator dependent immediately prior to LT with 9 patients undergoing percutaneous tracheostomy prior to transplantation. The range of PEEP was 7 cm to 12 cm H2O and FiO2 ranged from 28% to 60%. Twenty-five ICU patients (64%) required dialysis preoperatively. 32 out of 39 patients (82%) required at least one out of three types of therapy. Median length of time from hospital admission to ICU admission was 3 d (range: 1-32 d). Median length of time from ICU admission to transplant was 12 d (range: 1-65 d). Median MELD at ICU admission was 32 (range: 15-52). Median length of hospital stay was 42 d (range: 15-516 d) and median length of ICU stay was 27 d (range: 7-327 d). Nineteen patients (49%) were discharged home, 14 patients (36%) were discharged to rehab. Six patients (15%) died on the same hospitalization, 2 of which died in the operating room.
| Variable | Summary(n = 39) |
| Patient characteristics | |
| Vasopressors | 14 (36%) |
| Vasopressin | 12 (31%) |
| Dose (units/min) | 0.04 (0.01-0.04) |
| Norepinephrine | 8 (21%) |
| Dose (mcg/kg per min) | 0.07 (0.01-0.18) |
| Vasopressin and norepinephrine | 6 (15%) |
| Length of time from hospital admission to ICU admission (d) | 3 (1-32) |
| Length of time from ICU admission to liver transplant (d) | 12 (1-65) |
| MELD at ICU admission | 32 (15-52) |
| MELD at transplant | 37 (24-50) |
| Pre-transplant ambulation | 14 (42%) |
| Dialysis | 25 (64%) |
| Tracheostomy | 9 (23%) |
| Mechanical ventilation | 16 (41%) |
| Positive end-expiratory pressure (cm H20) | 7 (7-12) |
| FiO2 (%) | 40 (28-60) |
| Mean airway pressure (cm H20) | 12 ( 9-18) |
| PaO2 (mmHg) | 103 (60-147) |
| Post-operative characteristics | |
| Post-operatively placed tracheostomy | 4 (10%) |
| Length of hospital stay | 42 (15-516) |
| Length of ICU stay | 27 (7-327) |
| Discharged status | |
| Home | 19 (49%) |
| Rehab | 14 (36%) |
| Death | 6 (15%) |
| 3-mo readmission | 16 (41%) |
The median length of follow up in the overall cohort of 966 patients was 3.5 years (range: 0 d-6.8 years). In the 39 ICU patients, median length of follow up was 1.8 years (range: 0 d-5.6 years). Graft survival and patient survival after LT in ICU patients and non-ICU patients are displayed (Figure 1 and Table 3). At 1, 3 and 5 years after LT, graft survival was 76%, 68% and 62% in ICU patients and 90%, 81% and 75% in non-ICU patients. Patient survival at 1, 3 and 5 years after LT was 78%, 70% and 65% in ICU patients compared to 94%, 85% and 79% in non-ICU patients. When formally comparing graft survival and patient survival between ICU and non-ICU patients using Cox proportional hazards regression models, both graft survival (RR: 1.94, 95%CI: 1.09-3.48, P = 0.026) and patient survival (RR: 2.32, 95%CI: 1.26-4.27, P = 0.007) were lower in ICU patients compared to non-ICU patients in single variable analysis. These findings were consistent in multivariable analysis, adjusting for the potentially confounding variables of patient gender and BMI, graft survival was significantly worse in ICU patients (RR: 2.03, 95%CI: 1.13-3.65, P = 0.018), as was patient survival (RR: 2.44, 95%CI: 1.32-4.50, P = 0.004).
| Estimate (95%CI) | ||
| Outcome/time since transplant | ICU patients(n = 39) | Non-ICU patients(n = 927) |
| Graft survival | ||
| 1 yr | 76% (62%-91%) | 90% (88%-92%) |
| 2 yr | 72% (58%-89%) | 84% (82%-87%) |
| 3 yr | 68% (52%-86%) | 81% (78%-83%) |
| 4 yr | 62% (44%-83%) | 78% (75%-81%) |
| 5 yr | 62% (41%-83%) | 75% (72%-78%) |
| Patient survival | ||
| 1 yr | 78% (65%-93%) | 94% (92%-95%) |
| 2 yr | 75% (61%-91%) | 89% (87%-91%) |
| 3 yr | 70% (55%-88%) | 85% (83%-87%) |
| 4 yr | 65% (47%-86%) | 82% (79%-84%) |
| 5 yr | 65% (43%-86%) | 79% (76%-82%) |
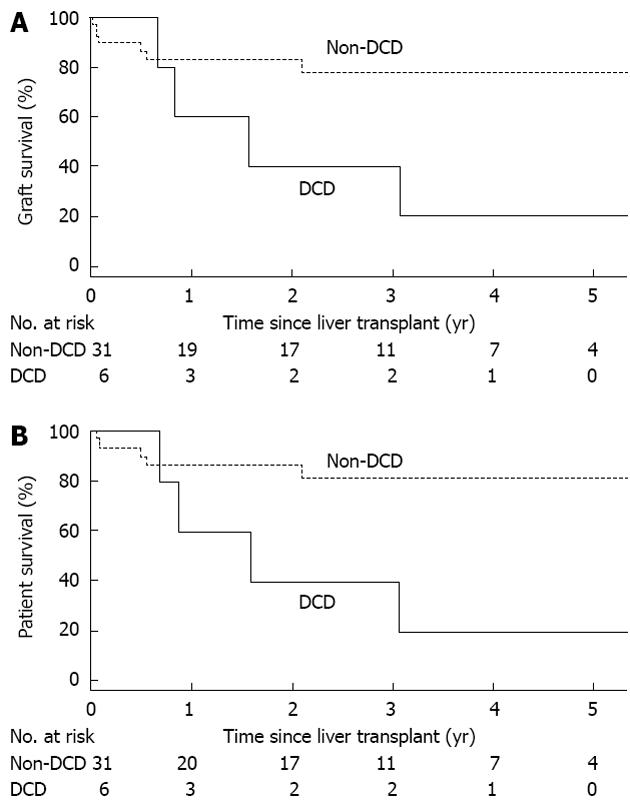
An evaluation of associations of patient and donor characteristics with graft survival and patient survival in ICU patients is provided in Table 4; a total of 12 ICU patients experienced graft loss or death, while 11 patients died. Although not statistically significant, graft survival was worse in both patients with cryptogenic cirrhosis (RR: 3.29, P = 0.056) and patients who received DCD grafts (RR: 3.38, P = 0.060). These findings reached statistical significance when considering patient survival, which was worse for patients with cryptogenic cirrhosis (RR: 3.97, P = 0.031) and patients who were transplanted with DCD livers (RR: 4.19, P = 0.033). The findings regarding DCD liver grafts and the outcomes of their recipients are further illustrated in Figure 2.
| Association with graft survival (graft loss or death endpoint) | Association with patient survival (death endpoint) | |||
| Variable | Relative risk (95%CI) | P value | Relative risk (95%CI) | P value |
| Patient characteristics | ||||
| Age at transplant (10 yr increase) | 0.98 (0.49-1.99) | 0.96 | 1.00 (0.47-2.11) | 0.99 |
| Gender (male) | 1.33 (0.42-4.19) | 0.63 | 1.10 (0.34-3.61) | 0.88 |
| BMI (10 unit increase) | 0.63 (0.24-1.66) | 0.35 | 0.57 (0.20-1.59) | 0.28 |
| Diagnosis | ||||
| Hepatitis C | 0.99 (0.32-3.08) | 0.99 | 0.79 (0.24-2.59) | 0.69 |
| ETOH | 0.57 (0.12-2.59) | 0.46 | 0.65 (0.14-3.02) | 0.58 |
| Cryptogenic cirrhosis | 3.29 (0.97-11.15) | 0.056 | 3.97 (1.14-13.87) | 0.031 |
| MELD at transplant (5 unit increase) | 0.99 (0.63-1.57) | 0.97 | 1.09 (0.68-1.75) | 0.72 |
| MELD at ICU admission (5 unit increase) | 0.85 (0.56-1.30) | 0.47 | 1.03 (0.66-1.61) | 0.89 |
| Previous operation | 1.07 (0.31-3.67) | 0.91 | 0.76 (0.20-2.95) | 0.69 |
| Vasopressors | 1.06 (0.32-3.51) | 0.93 | 1.24 (0.36-4.23) | 0.74 |
| Vasopressin | 0.78 (0.23-2.59) | 0.68 | 0.91 (0.27-3.11) | 0.88 |
| Norepinephrine | 0.29 (0.04-2.22) | 0.23 | 0.32 (0.04-2.51) | 0.28 |
| Vasopressin and norepinephrine | 0.44 (0.06-3.44) | 0.44 | 0.50 (0.06-3.90) | 0.51 |
| Length of time from hospital admission to ICU admission (doubling) | 0.91 (0.64-1.28) | 0.57 | 0.88 (0.61-1.27) | 0.49 |
| Ambulation | 1.30 (0.36-4.72) | 0.69 | 1.30 (0.36-4.72) | 0.69 |
| Dialysis | 1.70 (0.46-6.32) | 0.43 | 1.46 (0.38-5.54) | 0.58 |
| Tracheostomy | 1.00 (0.27-3.71) | 1.00 | 0.61 (0.13-2.83) | 0.53 |
| Mechanical ventilation | 0.91 (0.29-2.88) | 0.88 | 0.69 (0.20-2.37) | 0.56 |
| Donor characteristics | ||||
| Age (10 yr increase) | 0.97 (0.67-1.41) | 0.88 | 0.91 (0.61-1.36) | 0.65 |
| Gender (male) | 0.74 (0.21-2.63) | 0.64 | 0.52 (0.13-2.09) | 0.36 |
| Recipient-donor gender incompatibility | 1.33 (0.36-4.91) | 0.67 | 1.76 (0.45-6.87) | 0.41 |
| Donation after cardiac death | 3.38 (0.95-12.05) | 0.060 | 4.19 (1.12-15.70) | 0.033 |
| Donor risk index (1 unit increase) | 2.15 (0.54-8.62) | 0.28 | 1.61 (0.36-7.15) | 0.53 |
Given the aforementioned finding regarding DCD grafts and the consistently documented poorer outcomes of DCD grafts in the literature, we re-calculated graft survival and patient survival excluding 6 ICU patients with DCD donors. When excluding these 6 DCD patients from the ICU group, graft survival in the remaining 31 ICU patients at 1, 3 and 5 years was 78%, 73% and 73%, while patient survival at these time points was 81%, 76% and 76%. When comparing outcomes between this ICU patient subgroup with the overall cohort of 927 non-ICU patients in multivariable analysis, graft survival (RR: 1.59, 95%CI: 0.78-3.23, P = 0.20) and patient survival (RR: 1.80, 95%CI: 0.84-3.85, P = 0.13) were still lower in ICU patients, but these findings are no longer statistically significant.
Graft survival and patient survival were not significantly worse for patients on mechanical ventilation (RR: 0.91, P = 0.88 in graft loss; RR: 0.69, P = 0.56 in death) or patients on vasopressors (RR: 1.06, P = 0.93 in graft loss; RR: 1.24, P = 0.74 in death) immediately prior to LT. Trends toward lower graft survival and patient survival were observed for patients on dialysis immediately before LT, however these findings did not approach statistical significance (RR: 1.70, P = 0.43 in graft loss; RR: 1.46, P = 0.58, in death).
LT has evolved from an experimental procedure to a life-saving therapy for patients with end-stage liver disease. The complicated pathophysiology of end-stage liver disease, sophisticated surgery and challenging postoperative care requires center expertise and collaborative team of skilled, innovative clinicians, including surgeons, hepatologists, anesthesiologists, and transplant intensivists in order to achieve the best possible outcome.
Advanced liver disease frequently mandates ICU admission. The admission to ICU is associated with high mortality and LT becomes the only definitive therapeutic option for a decompensated cirrhotic patient. At this time, one of the most complex decisions the clinicians face is when an extremely ill candidate no longer becomes suitable for this procedure. Currently, there are no specific recommendations to define the individuals who are too sick to transplant and thus avoid futile therapy. It is left up to the center’s experience and subjective “eyeball test” to define criteria for delisting[12].
In our study, over a period of 6 years, critically ill patients who underwent LT directly from the ICU had an average MELD score of 37 at the time of transplantation, which was significantly higher than the average MELD of 20 in non-ICU patients. Post LT overall patient and graft survival rates in patients transplanted directly from the ICU were lower than in patients transplanted either from home or from the hospital ward. However, despite these poorer outcomes in ICU patients compared to non-ICU patients, they are still higher than what is considered acceptable by the transplant community[9].
One of the possible reasons for better outcomes in our patients is likely due to the high volume of LT operations at our center. Ozhathil et al[3] reported decreased risk of allograft failure and recipient death after LT in high volume centers defined as centers performing 78-215 cases per year. This has been demonstrated in retransplantation as well by Reese et al[14].
Further investigation of outcomes in ICU patients revealed that patients who received a DCD liver graft had more than 3-fold increased risk of losing a graft and more than a 4-fold increased risk of dying compared to the ICU patients who received a non-DCD graft. In fact, graft and patient survival between non-ICU patients and ICU patients excluding the patients who received DCD grafts were reasonably comparable, particularly at 5-year after LT where graft and patient survival were 73% and 76% in ICU patients and 75% and 79% in non-ICU patients. Our findings are consistent with the results reported previously in the literature[15]. DCD donors have recently been used to increase the number of deceased donors and bridge the gap between the number of available organs and the number of candidates on the waiting list. These organs are considered marginal because this type of graft is thought to be of inferior quality when compared to the liver grafts from DBD donors[16]. Analyzing the UNOS database, Mateo et al[15] have demonstrated that with DCD livers the graft survival at 1 year and 3 years was 71% and 60% respectively, which was significantly lower than 80% and 72% in patients who received DBD grafts. The graft survival significantly improved to 81% and 67% at 1 and 3 years respectively, if these organs were placed in low risk patients (i.e., patients without previous history of LT, non-ICU patients, patients not requiring life support, and patients not on dialysis), and became similar to that of DBD donors.
In our analysis of risk factors for graft loss and death in ICU patients, in addition to aforementioned DCD finding, we also observed that patients with cryptogenic cirrhosis had poorer patient and graft survival than patients with liver disease from other causes. However, this finding is of uncertain significance and should be further evaluated in larger series.
In the ICU, deteriorating patients with ESLD awaiting LT develop MOSF requiring mechanical ventilatory support, intermittent or continuous RRT, and pharmacologic hemodynamic support. Vasopressor requirement and intubation have been considered to be contraindications to transplantation and regarded as criteria for delisting[6]. We sought to investigate whether any of the above factors which have been traditionally linked to worse outcomes would be prognosticators of poor outcomes in our experience.
Mechanical ventilation is required for airway protection in a setting of hepatic encephalopathy, for respiratory failure due to ARDS, pulmonary edema, and infections. In previous reports it has been demonstrated that preoperative mechanical ventilation played a role in prolonged postoperative intubation[17]. Preoperative mechanical intubation has been identified as one of the independent risk factors for decreased patient and graft survival[4,18]. In our study 46% of patients transplanted from the ICU were on a ventilator at the time of LT. More than half of the intubated patients underwent percutaneous tracheostomy placement prior to LT. All the patients who were ventilator dependent prior to LT had PEEP of ≤ 12 mmHg and FiO2≤ 60%. In contrast to previous publications, in our sample of 39 patients transplanted from the ICU, ventilatory support prior to LT did not have a negative effect on patient or graft survival.
Patients with ESLD are in hyperdynamic state with low systemic arterial pressure sometimes requiring vasopressor support[6]. In our analysis, 36% of patients were on vasopressors with 15% of patients being on a combination of vasopressin and norepinephrine. The patients with active sepsis were not transplanted. Based on our analysis, the patients who required pharmacologic hemodynamic support at the time of LT did not experience inferior graft or patient survival as evidenced by RR of approximately one or less in magnitude.
Due to disturbances in renal function, renal failure develops in many patients with cirrhosis[19]. In most instances continuous RRT is the modality of choice due to patient hemodynamic instability. Multiple investigations have linked preoperative hemodialysis to poorer outcomes after LT[18,20-22]. In our cohort, 65% of patients were on dialysis, and while we did not observe a statistically significant association between dialysis and either graft or patient survival, the RR that we observed of 1.70 and 1.45, respectively, suggest a trend to lower outcomes.
Our study has several limitations. It has a retrospective design and a relatively small number of ICU patients who underwent LT. Related to the limited number of ICU patients, power to detect associations of recipient and donor characteristics with outcomes in ICU patient is limited, and the possibility of Type II error (i.e., a false-negative association) is important to consider. In addition, our results reflect the experience of a single high-volume center and thus might not be applicable to other centers. The criteria for ICU admission might vary from center to center.
In conclusion, to our knowledge, this is the largest study that directly examined the outcomes of the patients who have undergone LT directly from the ICU. We have demonstrated that patients who require mechanical ventilatory support, pharmacologic hemodynamic support, and RRT can have acceptable patient and graft outcomes after LT. A much larger group of ICU patients, likely from a multi-center study, is needed to better define criteria for a successful liver transplant.
Liver transplantation (LT) is a life-saving procedure for patients with end-stage liver diseases. Improvements in surgical technique, medical management, and advances in immunosuppression therapy have all contributed to the success of LT.
It is not uncommon for patients with significantly decompensated liver disease to require intensive care unit (ICU) admission for multiorgan system failure requiring ventilatory support, hemodynamic support, and renal replacement therapy (RRT) in the course of their disease. Transplantation of such patients could lead to poor post-transplant outcomes. Given the scarcity of organ donors transplantation of such patients could be futile and thus acceptable in the time of donor organ shortage.
This is the largest study that investigated the feasibility of LT in patients from the ICU, patients who require mechanical ventilatory support, pharmacologic hemodynamic support, and RRT by estimating graft and patient survival in this patient population. The authors have demonstrated that this group of patients can have acceptable patient and graft outcomes after LT.
The data can contribute to the widening of recipient criteria in LT.
The authors reported the outcomes following LT in ICU patients. The clinical result was very good for the new insight and encouragement of physicians dealing with this challenging filed.
P- Reviewers Selinger CP, Herceg Z S- Editor Xiong L L- Editor A E- Editor Li JY
| 1. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 2. | Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Ozhathil DK, Li YF, Smith JK, Tseng JF, Saidi RF, Bozorgzadeh A, Shah SA. Impact of center volume on outcomes of increased-risk liver transplants. Liver Transpl. 2011;17:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Desai NM, Mange KC, Crawford MD, Abt PL, Frank AM, Markmann JW, Velidedeoglu E, Chapman WC, Markmann JF. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Ginès P, Fernández J, Durand F, Saliba F. Management of critically-ill cirrhotic patients. J Hepatol. 2012;56 Suppl 1:S13-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Findlay JY, Fix OK, Paugam-Burtz C, Liu L, Sood P, Tomlanovich SJ, Emond J. Critical care of the end-stage liver disease patient awaiting liver transplantation. Liver Transpl. 2011;17:496-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Umgelter A, Lange K, Kornberg A, Büchler P, Friess H, Schmid RM. Orthotopic liver transplantation in critically ill cirrhotic patients with multi-organ failure: a single-center experience. Transplant Proc. 2011;43:3762-3768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Sobhonslidsuk A, Neff GW, Molina EG, Yamashiki N, Nishida S, Reddy KR, Tzakis AG, Schiff ER. Prediction of survival outcome of ICU patients awaiting orthotopic liver transplantation. Transplant Proc. 2002;34:1223-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Brown RS, Lake JR. The survival impact of liver transplantation in the MELD era, and the future for organ allocation and distribution. Am J Transplant. 2005;5:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Varma V, Mehta N, Kumaran V, Nundy S. Indications and contraindications for liver transplantation. Int J Hepatol. 2011;2011:121862. [PubMed] |
| 11. | Merion RM. When is a patient too well and when is a patient too sick for a liver transplant? Liver Transpl. 2004;10:S69-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Charpentier KP, Mavanur A. Removing patients from the liver transplant wait list: A survey of US liver transplant programs. Liver Transpl. 2008;14:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1490] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 14. | Reese PP, Yeh H, Thomasson AM, Shults J, Markmann JF. Transplant center volume and outcomes after liver retransplantation. Am J Transplant. 2009;9:309-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, Fong TL, Sher L, Jabbour N, Aswad S. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Huang CT, Lin HC, Chang SC, Lee WC. Pre-operative risk factors predict post-operative respiratory failure after liver transplantation. PLoS One. 2011;6:e22689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Markmann JF, Markmann JW, Markmann DA, Bacquerizo A, Singer J, Holt CD, Gornbein J, Yersiz H, Morrissey M, Lerner SM. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive primary liver transplants. Transplantation. 2001;72:1113-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 542] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 20. | Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Xu MQ, Wei YG. Predictors of patient survival following living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005;68:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. Renal replacement therapy and orthotopic liver transplantation: the role of continuous veno-venous hemodialysis. Transplantation. 2001;71:1424-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |