Published online Jul 27, 2012. doi: 10.4254/wjh.v4.i7.224
Revised: June 20, 2012
Accepted: July 21, 2012
Published online: July 27, 2012
AIM: To evaluate the effect of a 6 and 12 mo lifestyle modification intervention in nonalcoholic fatty liver diseases (NAFLD) in Chengyang District of Qingdao.
METHODS: Participants with NAFLD who had resided in Chengyang District for more than 5 years were enrolled in this study. After the 6 and 12 mo lifestyle modification intervention based on physical activity, nutrition and behavior therapy, parameters such as body weight, body mass index (BMI), waist circumference, serum alanine aminotransferase (ALT), aspartate aminotransferase values, serum cholesterol, triglycerides, fasting glucose, fasting insulin and visceral fat area (VFA), the liver-spleen ratio and the homeostasis model assessment of insulin resistance (HOMA-IR) were evaluated and compared between participants with and without the intervention.
RESULTS: Seven hundred and twenty-four participants were assigned to the lifestyle intervention group (LS) and 363 participants were assigned to the control group (CON). After the intervention, body weights in the LS group were significantly decreased compared to those in the CON group at 6 mo (11.59% ± 4.7% vs 0.4% ± 0.2%, P = 0.001) and at 12 mo (12.73% ± 5.6% vs 0.9% ± 0.3%, P = 0.001). Compared with the CON group, BMI was more decreased in the LS group after 6 and 12 mo (P = 0.043 and P = 0.032). Waist circumference was more reduced in the LS group than in CON (P = 0.031 and P = 0.017). After the 6 and 12 mo intervention, ALT decreased significantly in the LS group (P = 0.003 and P = 0.002). After 6 and 12 mo, the metabolic syndrome rate had decreased more in the LS group compared with the CON group (P = 0.026 and P = 0.017). After 12 mo, the HOMA-IR score decreased more obviously in the LS group (P = 0.041); this result also appeared in the VFA after 12 mo in the LS group (P = 0.035).
CONCLUSION: Lifestyle intervention was effective in improving NAFLD in both 6 and 12 mo interventions. This intervention offered a practical approach for treating a large number of NAFLD patients in the Chengyang District of Qingdao.
- Citation: Sun WH, Song MQ, Jiang CQ, Xin YN, Ma JL, Liu YX, Ma L, Lin ZH, Li CY, Liu L, Zhang M, Chu LL, Jiang XJ, Wan Q, Zhou L, Ren R, Meng LF. Lifestyle intervention in non-alcoholic fatty liver disease in Chengyang District, Qingdao, China. World J Hepatol 2012; 4(7): 224-230
- URL: https://www.wjgnet.com/1948-5182/full/v4/i7/224.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i7.224
In recent years, fuelled by rapid urbanization and abundant dietary and sedentary lifestyles, more chronic diseases have emerged with obesity in Chinese citizens. Nonalcoholic fatty liver (NAFLD) has appeared, accompanied with an obesity phenotype that is associated with several negative metabolic aberrations, including dyslipidemias, hypertension, insulin resistance and the metabolic syndrome[1]. NAFLD has been increasingly recognized as a condition strongly involved in the pathogenesis of the epidemically spreading metabolic diseases, type 2 diabetes and cardiovascular disease[2,3]. One study showed that women in the high liver fat group also had significantly higher fasting insulin, triglycerides, insulin resistance and blood pressure[4]. NAFLD could also lead to liver inflammation, fibrosis and even develop to cirrhosis. Recent studies indicated that in the general population in Shanghai and Guangdong, the prevalence of fatty liver were 17% and 15%, respectively[5]. Chengyang District is one of new developing downtown areas in Qingdao city. With the high calorie dietary as well as sedentary behaviors of Chengyang’s residents, obesity has become common in our daily clinical practice and the number of NAFLD is escalating in this area.
It is apparent that weight loss and lifestyle modification should be the primary target for treating NAFLD. Previous studies have reported a beneficial effect of a recommended diet combined with an increase in physical activity on the progression of NAFLD[6]. One study showed that a 7% to 10% weight reduction through intensive lifestyle intervention would lead to improvements in biochemical and histological features of nonalcoholic steatohepatitis (NASH)[7]. At present, there are still no established methods for intensive lifestyle modification in NAFLD. The aim of our study was to evaluate a 12 mo lifestyle modification intervention for NAFLD patients in Chengyang District and evaluate its effect of ameliorating NAFLD.
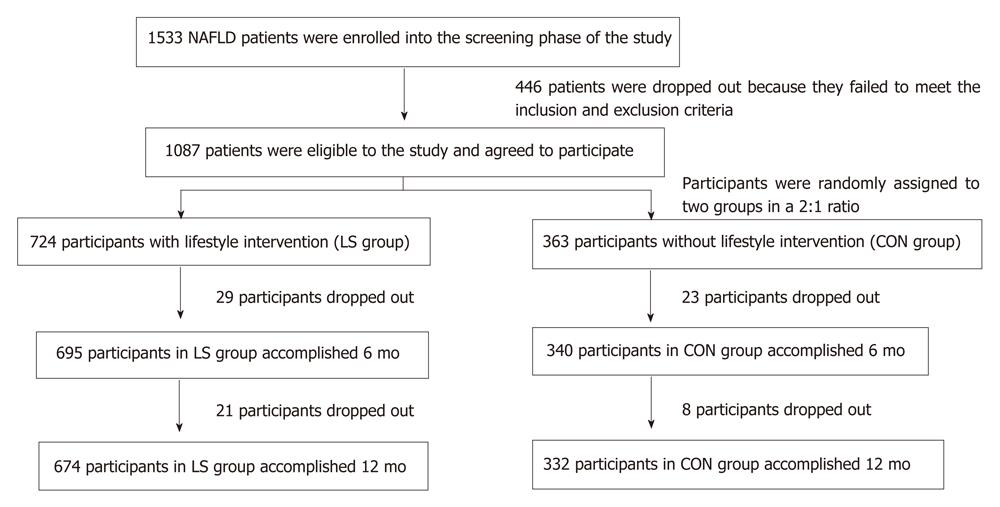
We recruited participants between January 2008 and October 2011 in this study. Participants were required to have elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) values (ALT > 41 or AST > 34 U/L) and body mass index (BMI) between 25 and 40. All participants had resided in Chengyang District for more than 5 years (Figure 1).
All participants underwent abdominal ultrasonography and NAFLD was diagnosed according to the following criteria: (1) slight diffuse increase in bright homogeneous echoes in the liver parenchyma with normal visualization of the diaphragm and portal and hepatic vein borders and normal hepatorenal echogenicity contrast; (2) diffuse increase in bright echoes in the liver parenchyma with slightly impaired visualization of the peripheral portal and hepatic vein borders; and (3) a marked increase in bright echoes at a shallow depth with deep attenuation, impaired visualization of the diaphragm and marked vascular blurring[8]. Exclusion criteria were: (1) hepatitis B virus (HBV) infection (HBV surface antigen and anti-HBc antibodies) or hepatitis C virus (HCV) infection (anti-HCV antibodies); (2) autoimmune liver disease or alcoholic liver disease (20 g/d alcohol); (3) use of medications associated with steatosis or steatohepatitis; and (4) use of insulin-sensitizing medications.
Participants were randomly assigned to a lifestyle intervention group or a control group in a 2:1 ratio. Randomization was performed using a random number generator developed by the project statistician. Data collection was obtained by trained staff not aware of the group assignment or sequence of measurement. The ultrasonography operator was blinded to the groups. Participants were allowed to start a new medication for management of hyperglycemia if medically necessary. Sulfonylureas, meglitinides, insulin and insulin-sensitizing agents (thiazolidinediones and metformin etc.) were available options.
A total of 1533 NAFLD patients were enrolled in the screening phase of the study; 1087 subjects completed the screening evaluation and underwent randomization. The baseline characteristics of the participants who underwent randomization are shown in Table 1.
| Variable | LS (n = 724) | CON (n = 363) | P value |
| Gender (M/F) | 464/260 | 229/134 | 0.632 |
| Age (yr) | 39.9 ± 13.1 | 37.4 ± 18.2 | 0.221 |
| Weight (kg) | 88.5 ± 18.4 | 84.3 ± 21.8 | 0.711 |
| Waist circumference (cm) | 106.3 ± 23.2 | 108.8 ± 15.7 | 0.954 |
| BMI | 37.7 ± 12.7 | 38.4 ± 17.1 | 0.842 |
| ALT (IU/L) | 59.3 ± 20.1 | 58.3 ± 18.8 | 0.513 |
| AST (IU/L) | 57.8 ± 26.6 | 59.3 ± 12.9 | 0.182 |
| GGT (IU/L) | 61.3 ± 11.2 | 59.8 ± 19.6 | 0.215 |
| Cholesterol (mmol/L) | 5.7 ± 0.9 | 5.8 ± 1.1 | 0.167 |
| Triglycerides (mmol/L) | 2.4 ± 0.4 | 2.2 ± 0.6 | 0.094 |
| Fasting glucose (mmol/L) | 7.5 ± 1.3 | 7.4 ± 1.6 | 0.332 |
| Fasting insulin (μIU/mL) | 17.7 ± 4.3 | 19.5 ± 5.7 | 0.185 |
| HOMA-IR | 2.4 ± 0.7 | 2.3 ± 0.9 | 0.231 |
| L/S ratio | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.423 |
| VFA, cm2 | 135.6 ± 38.7 | 138.9 ± 39.4 | 0.172 |
| Diabetes (%) | 66 (6.1) | 62 (5.7) | 0.876 |
| Metabolic syndrome (%) | 201 (18.5) | 186 (17.1) | 0.563 |
Participants in the control group were provided with basic education about NAFLD and principles of healthy eating, physical activity and weight control. The intervention group of lifestyle modification consisted of a diet tailored for the individual’s requirements and increased physical exercise. The nutritional course was based on a fat- and sugar- reduced diet compared with the common everyday nutrition of people in Qingdao. The diet contained 30% fat, 15% proteins and 55% carbohydrates, including 5% sugar. The nutritional course was based on the prevention concept of the optimized mixed diet; the scientific recommendations were translated into food-based dietary guidelines with consideration of the dietary habits and customs in Qingdao Chengyang. The target dietary energy intake was defined as standard ideal body weight of 25-30 kcal/kg. The exercise therapy consisted of walking, jogging, stair climbing and instructions in physical exercise as part of everyday life, especially a reduction in the amount of time spent watching television. Exercise therapy was performed to achieve a target of 23 metabolic equivalent tasks (METs)•h/week (physical activity) + 4 METs•h/week (exercise)[9-11].
Body weight (kg), height (m), BMI and waist circumference (cm2) were measured. Venous blood samples were taken from all patients at around 8am. After a 12 h overnight fast, hepatic function and total cholesterol, triglyceride, fasting plasma glucose (FPG) and plasma insulin were determined by automatic biochemistry analyzer (Hitachi, Japan). Homeostasis model assessment (HOMA) was used to detect the degree of insulin resistance by the formula: (HOMA) = [insulin (mU/L) × glucose (mmol/L)]/22.5[12].
The metabolic syndrome was diagnosed according to the International Diabetes Federation (IDF) consensus worldwide definition[13]. The IDF definition requires central obesity (measured as ethnic group-specific thresholds for waist circumference; for Chinese people: ≥ 80 cm for females and ≥ 90 cm for males) plus any two of the following four components: (1) serum triglycerides 1.70mmol/L or more, or specific treatment for this lipid abnormality; (2) high density lipoprotein-cholesterol 1.03 mmol/L or less in males and 1.29 mmol/L or less in females, or specific treatment for this lipid abnormality; (3) blood pressure 130/85 mmHg or more, or treatment for previously diagnosed hypertension; and (4) FPG 6.16 mmol/L or more, or previously diagnosed type 2 diabetes[14,15].
The abdominal computed tomography (CT) protocol was as follows. Before starting the intervention, the patient’s liver fat deposition was assessed by an abdominal CT scan to determine the liver-spleen ratio (L/S ratio) and visceral fat accumulation [visceral fat area (VFA), cm2]. Images were reconstructed at 10 mm increments. All patients underwent abdominal CT in the morning after a 12 h overnight fast. VFA was measured at the level of the umbilicus by FatScan software version 3.0. Radiological assessments were made every 6 mo after starting the treatment. All patients provided written informed consent and the study protocol was approved by the Ethics Committee of Qingdao Chengyang People’s Hospital.
At baseline, there were 724 patients in the lifestyle intervention group (LS) group and 363 patients in the control group (CON) group. After 6 mo, 29 patients in the LS group and 23 patients in the CON group were excluded from the results because they refused to continue the investigation, without any reasons. After 12 mo, a total of 50 patients in the LS group and 31 patients in the CON group were excluded, as shown in Figure 1.
Continuous variables are summarized as mean ± SD. Pearson χ2 was used to measure the enumeration data between two groups. Proportions and categorical variables were tested by the χ2 test. Analysis of variance (ANOVA) for repeated measurements was used to examine differences between two groups with and without lifestyle intervention. The overall within-subject effect of the intervention was estimated in a doubly-multivariate repeated-measures ANOVA (for repeated measurements). Post hoc tests compared means of different time points and were adjusted for multiple testing. All statistical analyses were conducted using SPSS 13.0. A two-tailed P < 0.05 was considered statistically significant.
For over 6 mo, 96.0% (695/724) of patients in the LS group and 93.7% (340/363) patients in the CON group were followed. There was no difference in the follow-up ratio between these two groups (P = 1.457). After 6 mo, there were no significant differences between these two groups for other factors of age, height, fasting glucose and fasting insulin (all P > 0.05).
After 12 mo, there were 93.1% (674/724) of patients in the LS group and 91.5% (332/363) patients in the CON group. There was no difference in the follow-up ratio between two groups (P = 1.754). After 12 mo, there were also no significant differences between these two groups for other factors of age, height, fasting glucose and fasting insulin (all P > 0.05).
The initial body weight between the LS and CON groups did not differ significantly. Body weight at the end of 6 mo obviously decreased from 88.5 ± 18.4 kg to 83.5 ±21.6 kg in the LS group, but in the CON group there was no difference in the body weight decrease (from 84.3 ± 21.8 kg to 85.7 ± 16.8 kg). There was obviously a difference between these two groups (P = 0.036) (Table 2). After 6 mo, percentage weight reduction of participants in the LS group was significantly greater than those in the CON group (11.59% ± 4.7% vs 0.4% ± 0.2%, P = 0.001). After 6 mo, compared with the CON group’s BMI change (from 38.4 ± 17.1 at baseline to 39.7 ± 17.3), the BMI change in the LS group at baseline to 6 mo (from 37.7 ± 12.7 to 28.2 ± 11.5) was a more obvious decrease than in the CON group (P = 0.043).
| Variable | Group | Baseline | 6 mo | P value |
| Gender (M/F) | LS | 442/253 | 442/253 | 1.457 |
| CON | 221/119 | 221/119 | ||
| Age (yr) | LS | 39.9 ± 13.1 | 38.7 ± 12.4 | 1.658 |
| CON | 37.4 ± 18.2 | 39.7 ± 14.6 | ||
| Weight (kg) | LS | 88.5 ± 18.4 | 83.5 ± 21.6 | 0.036 |
| CON | 84.3 ± 21.8 | 85.7 ± 16.8 | ||
| BMI | LS | 37.7 ± 12.7 | 28.2 ± 11.5 | 0.043 |
| CON | 38.4 ± 17.1 | 39.7 ± 17.3 | ||
| Waist circumference | LS | 106.3 ± 23.2 | 99.4 ± 33.7 | 0.031 |
| (cm) | CON | 108.8 ± 15.7 | 109.3 ± 21.8 | |
| ALT (IU/L) | LS | 59.3 ± 20.1 | 49.1 ± 18.3 | 0.003 |
| CON | 58.3 ± 18.8 | 59.3 ± 24.5 | ||
| AST(IU/L) | LS | 57.8 ± 26.6 | 54.2 ± 25.3 | 0.614 |
| CON | 59.3 ± 12.9 | 58.6 ± 14.5 | ||
| GGT (IU/L) | LS | 67.8 ± 19.6 | 61.8 ± 15.2 | 0.071 |
| CON | 68.3 ± 11.2 | 69.3 ± 14.1 | ||
| Cholesterol | LS | 5.7 ± 0.9 | 4.8 ± 1.4 | 0.046 |
| (mmol/L) | CON | 5.8 ± 1.1 | 5.8 ± 1.8 | |
| Triglycerides | LS | 2.4 ± 0.4 | 2.7 ± 0.7 | 0.785 |
| (mmol/L) | CON | 2.2 ± 0.6 | 2.5 ± 0.5 | |
| Fasting glucose | LS | 7.5 ± 1.3 | 6.2 ± 1.6 | 0.736 |
| (mmol/L) | CON | 7.4 ± 1.6 | 7.1 ± 2.3 | |
| Fasting insulin | LS | 16.7 ± 4.3 | 13.4 ± 5.1 | 0.347 |
| (μIU/mL) | CON | 17.5 ± 8.7 | 18.3 ± 5.6 | |
| HOMA-IR | LS | 2.4 ± 0.7 | 1.17 ± 0.6 | 0.232 |
| CON | 2.3 ± 0.9 | 2.74 ± 0.7 | ||
| L/S ratio | LS | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.894 |
| CON | 0.7 ± 0.2 | 0.8 ± 0.3 | ||
| VFA, cm2 | LS | 145.6 ± 38.7 | 132.0 ± 59.5 | 0.137 |
| CON | 148.9 ± 39.4 | 149.2 ± 41.6 | ||
| Metabolic syndrome | LS | 371 (53.4) | 142 (20.4) | 0.026 |
| (%) | CON | 182 (53.2) | 159 (46.8) |
In the LS group, the waist circumference reduced from the beginning 106.3 ± 23.2 cm to 99.4 ± 33.7 cm after 6 mo. Compared with the waist circumference in the CON group from 108.8 ± 15.7 cm to 109.3 ±21.8 cm, there was an obvious decrease in waist circumference in the LS group (P = 0.031).
After 12 mo, body weight in the LS group decreased from 88.7 ± 18.6 kg to 81.7 ± 19.3 kg, compared with the CON group’s weight from 84.8 ± 21.3 kg to 86.1 ± 14.9 kg. There was a greater decrease in the LS group (P = 0.036). After 12 mo, the percentage weight reduction of participants in the LS group was significantly greater than those in the CON group (12.73% ± 5.6% vs 0.9% ± 0.3%, P = 0.001). After 12 mo, compared with the CON group’s BMI change (from 38.8 ± 17.5 at baseline to 37.4 ± 14.8), BMI change in the LS group was from 37.6 ± 11.4 to 26.6 ± 9.3), more obvious than in the CON group (P = 0.032) (Table 3).
| Variable | Group | Baseline | 12 mo | P value1 |
| Gender (M/F) | LS | 431/243 | 431/243 | 1.754 |
| CON | 215/117 | 215/117 | ||
| Age (yr) | LS | 38.7 ± 14.2 | 37.9 ± 12.3 | 0.896 |
| CON | 37.5 ± 19.2 | 36.4 ± 17.2 | ||
| Weight (kg) | LS | 87.8 ± 17.8 | 81.7 ± 19.3 | 0.013 |
| CON | 84.8 ± 21.3 | 86.1 ± 14.9 | ||
| BMI (kg/m2) | LS | 37.3 ± 15.4 | 26.6 ± 9.3 | 0.032 |
| CON | 38.8 ± 17.5 | 37.4 ± 14.8 | ||
| Waist circumference | LS | 107.2 ± 22.6 | 98.7 ± 28.4 | 0.017 |
| (cm) | CON | 107.3 ± 15.6 | 112.6 ± 19.4 | |
| ALT (IU/L) | LS | 59.5 ± 20.4 | 36.6 ± 16.9 | 0.002 |
| CON | 58.6 ± 18.2 | 62.3 ± 20.3 | ||
| AST (IU/L) | LS | 57.9 ± 25.7 | 52.2 ± 13.5 | 0.726 |
| CON | 58.8 ± 14.7 | 58.3 ± 11.9 | ||
| GGT (IU/L) | LS | 68.7 ± 18.8 | 57.4 ± 10.3 | 0.059 |
| CON | 68.8 ± 11.7 | 69.1 ± 18.2 | ||
| Cholesterol | LS | 5.8 ± 0.8 | 4.3 ± 1.6 | 0.027 |
| (mmol/L) | CON | 5.9 ± 1.0 | 5.9 ± 2.1 | |
| Triglycerides | LS | 2.3 ± 0.5 | 2.1 ± 0.5 | 0.859 |
| (mmol/L) | CON | 2.3 ± 0.6 | 2.8 ± 0.7 | |
| Fasting glucose | LS | 7.4 ± 1.4 | 5.8 ± 1.4 | 0.615 |
| (mmol/L) | CON | 7.3 ± 1.7 | 7.1 ± 2.3 | |
| Fasting Insulin | LS | 16.9 ± 4.2 | 12.4 ± 8.6 | 0.072 |
| (μIU/mL) | CON | 17.8 ± 8.9 | 21.5 ± 5.7 | |
| HOMA-IR | LS | 2.5 ± 0.6 | 0.96 ± 0.5 | 0.041 |
| CON | 2.4 ± 0.7 | 2.87 ± 0.6 | ||
| L/S ratio | LS | 0.8 ± 0.4 | 1.0 ± 0.2 | 0.214 |
| CON | 0.7 ± 0.3 | 0.8 ± 0.3 | ||
| VFA, cm2 | LS | 145.9 ± 37.6 | 124.3 ± 37.3 | 0.035 |
| CON | 148.5 ± 39.2 | 164.5 ± 48.8 | ||
| Metabolic syndrome | LS | 366 (54.3) | 112 (16.6) | 0.017 |
| (%) | CON | 178 (53.6) | 142 (42.8) |
In the LS group, the waist circumference reduced from the beginning 107.2 ± 22.6 cm to 98.7 ± 28.4 cm after 12 mo. Compared with the CON group’s from 107.3 ± 15.6 cm to 112.6 ± 19.4 cm, there was an obvious decrease in waist circumference in the LS group (P = 0.017).
In the LS group, after 6 mo intervention, ALT decreased from 59.3 ± 20.1 U/L to 49.1 ± 18.3 U/L; however the change in ALT in the CON group was from 58.3 ± 18.8 U/L to 59.3 ± 24.5 U/L. ALT decreased significantly over time in the LS group with lifestyle intervention but not in the CON group after 6 mo (Table 2). The ALT decrease in the LS group was more obvious than in the CON group (P = 0.001). There was no difference of AST and GGT between two groups (both P > 0.05).
After 12 mo, ALT decreased significantly in the LS group from 59.5 ± 20.4 U/L to 36.6 ± 16.9U/L but not in the CON group from 58.6 ± 18.2 U/L to 62.3 ± 20.3U/L (P = 0.002). There was no obvious difference in AST and GGT in the two groups (both P >0.05).
In the LS group, after 6 mo the metabolic syndrome rate changed from 371 (53.4%) initially to 142 (20.4%); however, in the CON group, the metabolic syndrome rate changed from 182 (53.2%) to 159 (46.8%). There was a greater difference between the LS and CON groups (P = 0.026).
After 12 mo, the metabolic syndrome rate was initially 366 (54.3%) to 112 (16.6%) in the LS group and in the CON group, the rate was from 178 (53.6%) to 142 (42.8%). There was a greater difference between the LS and CON groups (P = 0.017).
After 6 and 12 mo, there was no obvious difference in cholesterol, triglycerides, fasting glucose, fasting insulin and L/S ratio in the LS and CON groups (all P > 0.05). However, after 12 mo, the HOMA-IR score decreased obviously (P = 0.041) although after 6 mo lifestyle intervention there was no obvious change of HOMA-IR. This result also appeared in the VFA after 6 and 12 mo interventions. After 12 mo, the VFA decreased obviously (P = 0.035), but after 6 mo lifestyle intervention, there was no obvious decrease of VFA.
Chengyang is a district of Qingdao in Shandong province. It has an area of 553.2 km² and had around 737 200 inhabitants in the 2011 6th national census. In the last ten years, Chengyang’s economy has developed by leaps and bounds; improved living standards and modern lives result in its residents having a sedentary lifestyle and being overweight. Pang et al performed a citywide nutrition survey in Qingdao and the result showed that fat energy accounted for 35.3% of the total and animal fat accounted for 35.0% of dietary fat[16]. Tian and her colleague investigated 4078 residents in Qingdao and found the prevalence rate of central obesity (with the increasing of waist circumference) was 57.2%[17].
Although the prevalence and manifestations of NAFLD in inhabitants of Chengyang-one major district of Qingdao have not been fully investigated, NAFLD accompanied with obesity is being observed in our clinic with increasing frequency. Research indicates that obesity may be the most significant single risk factor for the development of NAFLD in adults worldwide[7]. Some strategies to modulate this burden of NAFLD are likely to have benefits beyond attenuating liver disease to the broader realm of obesity-related cardiometabolic risk reduction. Tock et al[7,18] hypothesized that a 7% to 10% weight reduction through intensive lifestyle intervention would lead to improvements in biochemical and histological features of NAFLD. A multitude of weight loss methods have been performed, including low-calorie diets and medicine therapies, but a well-designed structured lifestyle intervention performs well for longer-term weight loss maintenance[18].
Because there is still no broadly approved pharmacological therapy for NAFLD now, we planned this study, the first large longitudinal study in obese NAFLD patients in Chengyang District of Qingdao. We found that intensive lifestyle intervention could improve biochemical features of NAFLD. Both the 6 and 12 mo lifestyle modification interventions in our study resulted in clinically relevant improvements in body weight, BMI, waist circumference, liver function (ALT levels) and metabolic syndrome incidence. However, to achieve alleviation such as insulin resistance (HOMA-IR) and visceral fat accumulation, our patients needed a 12 mo lifestyle intervention.
During the 6 and 12 mo period of study, the patients continued the regimen in collaboration with physicians, hygienists, dietitians and nurses. For physical activity, we emphasized limiting hours of television viewing every week. One slogan we encouraged our patients with was “You burn more calories sleeping than watching TV”[19]. For diet, we strictly limited the amount of carbohydrates according to local eating customs of Chengyang District. Our study also showed that lifestyle intervention after 6 mo had similar results as after 12 mo, except insulin resistance (HOMA-IR) and visceral fat accumulation. NAFLD patients could achieve an improvement in HOMA-IR and visceral fat accumulation after a 12 mo lifestyle intervention. According to the second hit hypothesis, insulin resistance plays a key role in a later stage of liver diseases: insulin is involved in switching from NAFLD to non-alcoholic steatohepatitis[20]. Early intervention in patients with fatty liver can revert the systemic phenotype associated with insulin resistance[21].
It seems that for patients with metabolic syndrome, a 6 mo lifestyle intervention was not enough and at least 12 mo should been applied; longer-term intervention perhaps could achieve a better therapeutic effect. It was very encouraging that a 6 mo lifestyle intervention had some effect but a 12 mo lifestyle intervention had a better effect for Chengyang NAFLD patients.
The Japanese Community Health Promotion and Nutrition Section of the Health Sciences Council has put forward a slogan: “Firstly, physical activity and exercise. Secondly, diet and complete smoking cessation. Lastly, medication”. More emphasis was placed on policies for physical activities and exercise[22-25]. Our study result is consistent with this view. More lifestyle intervention should be performed in obese NAFLD patients in Chengyang District. Lifestyle intervention should be the first method in the treatment of NAFLD patients in Chengyang District of Qingdao.
We thank Dr. Yang Zhen of Shanghai No. Tenth People’s Hospital for his advice and help in statistics.
Present modern lifestyle results in more obesity in Chinese citizens, Nonalcoholic fatty liver (NAFLD) being one of the most common. NAFLD often accompanies obesity, dyslipidemias, hypertension, insulin resistance and the metabolic syndrome. Lifestyle intervention would lead to improvements of obesity as well as NASH. It is helpful to guide people with NAFLD to make lifestyle modifications.
Recent studies showed that weight reduction achieved through lifestyle interventions could improve liver histology in NASH and a 7% to 10% weight reduction was recommended. Although there were some weight loss and lifestyle modification studies for NASH in China, quantified measures such as weight reduction and waist circumference change had not been put forward.
The 6 and 12 mo life modification intervention based on physical activity, nutrition and behavior therapy is effective in improving NAFLD in Chengyang District of Qingdao. This intervention offers a practical approach for treating a large number of NAFLD patients in this urbanizing district.
The study results suggest that suitable life modification intervention is a potential therapeutic way that could be used to improve and prevent a large number of NAFLD patients in Chengyang District of Qingdao.
Life modification intervention is based on physical activity, nutrition and behavior therapy. It includes a diet tailored to the individual’s requirements and increased physical exercise.
This is a good descriptive study in which the authors showed that lifestyle intervention is effective in improving NAFLD in both 6 and 12 mo. This intervention offers a practical approach for treating a large number of NAFLD patients in Chengyang District of Qingdao.
Peer reviewers: Claudio Chiesa, Professor, Neurobiology and Molecular Medicine, National Research Council, Via del Fosso del Cavaliere, 100, Rome 00133, Italy; Dr. Ignazio Grattagliano, Internal Medicine, P.zza G.Cesare 11, Bari 70124, Italy
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 4. | Spassiani NA, Kuk JL. Exercise and the fatty liver. Appl Physiol Nutr Metab. 2008;33:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, Hu PJ. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419-6424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Oza N, Eguchi Y, Mizuta T, Ishibashi E, Kitajima Y, Horie H, Ushirogawa M, Tsuzura T, Nakashita S, Takahashi H. A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol. 2009;44:1203-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 971] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 8. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1447] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 9. | Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498-S504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5211] [Cited by in RCA: 5598] [Article Influence: 223.9] [Reference Citation Analysis (0)] |
| 10. | Nakagaichi M, Tanaka K. Development of a 12-min treadmill walk test at a self-selected pace for the evaluation of cardiorespiratory fitness in adult men. Appl Human Sci. 1998;17:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Astrand PO, Rodahl K. Applied sports physiology. 3rd ed.Astrand PO, Rodahl K, Dahl HA, editors. Physiological bases of exercise. New York: McGraw-Hill; 1986; 646-682. |
| 12. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24499] [Article Influence: 612.5] [Reference Citation Analysis (0)] |
| 13. | International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. New York: McGraw-Hill 2009; Available from: http: //www.idf.org/webdata/docs/MetS_def_update2006.pdf. |
| 14. | Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5323] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 15. | Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child. 2009;94:437-442. [PubMed] |
| 16. | Pang ZH, Chen XR, Wang HJ, Shi XX, Yuan M. Evaluation of dietary pattern and nutritional status of residents in Qingdao city. Zhongguo Gonggong Weisheng. 2006;22:91-92. |
| 17. | Tian XC, Pang ZC, Wang SJ, Chen L, Ning F, Qiao Q. Relation of Waist Circumference with Blood Pressure, Lipid and Glucose Metabolism among Residents aged over 35 in Qingdao City. Yufang Yixue Luntan. 2011;17:199-121. |
| 18. | Tock L, Prado WL, Caranti DA, Cristofalo DM, Lederman H, Fisberg M, Siqueira KO, Stella SG, Antunes HK, Cintra IP. Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol. 2006;18:1241-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kubey R, Csikszentmihalyi M. Television addiction is no mere metaphor. Sci Am. 2002;286:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1510] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 21. | Sookoian S, Pirola CJ. DNA methylation and hepatic insulin resistance and steatosis. Curr Opin Clin Nutr Metab Care. 2012;15:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Gossard AA, Lindor KD. Current therapies for nonalcoholic fatty liver disease. Drugs Today (Barc). 2011;47:915-922. [PubMed] |
| 23. | Malavolti M, Battistini NC, Miglioli L, Bagni I, Borelli L, Marino M, Scaglioni F, Bellentani S. Influence of lifestyle habits, nutritional status and insulin resistance in NAFLD. Front Biosci (Elite Ed). 2012;4:1015-1023. [PubMed] |
| 24. | Nobili V, Sanyal AJ. Treatment of nonalcoholic fatty liver disease in adults and children: a closer look at the arsenal. J Gastroenterol. 2012;47:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 210] [Cited by in RCA: 224] [Article Influence: 16.0] [Reference Citation Analysis (1)] |