Published online May 27, 2012. doi: 10.4254/wjh.v4.i5.176
Revised: September 19, 2011
Accepted: April 25, 2012
Published online: May 27, 2012
AIM: To optimize a xeno-free cryopreservation protocol for primary human hepatocytes.
METHODS: The demand for cryopreserved hepatocytes is increasing for both clinical and research purposes. Despite several hepatocyte cryopreservation protocols being available, improvements are urgently needed. We first compared controlled rate freezing to polystyrene box freezing and did not find any significant change between the groups. Using the polystyrene box freezing, we compared two xeno-free freezing solutions for freezing of primary human hepatocytes: a new medium (STEM-CELLBANKER, CB), which contains dimethylsulphoxide (DMSO) and anhydrous dextrose, both permeating and non-permeating cryoprotectants, and the frequently used DMSO - University of Wisconsin (DMSO-UW) medium. The viability of the hepatocytes was assessed by the trypan blue exclusion method as well as a calcein-esterase based live-dead assay before and after cryopreservation. The function of the hepatocytes was evaluated before and after cryopreservation by assessing enzymatic activity of 6 major cytochrome P450 isoforms (CYPs): CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A7.
RESULTS: The new cryoprotectant combination preserved hepatocyte viability significantly better than the standard DMSO-UW protocol (P < 0.01). There was no significant difference in viability estimation between both the trypan blue (TB) and the Live-Dead Assay methods. There was a correlation between viability of fresh hepatocytes and the difference in cell viability between CB and DMSO protocols (r2 = 0.69) using the TB method. However, due to high within-group variability in the activities of the major CYPs, any statistical between-group differences were precluded. Cryopreservation of human hepatocytes using the cryoprotectant combination was a simple and xeno-free procedure yielding better hepatocyte viability. Thus, it may be a better alternative to the standard DMSO-UW protocol. Estimating CYP activities did not seem to be a relevant way to compare hepatocyte function between different groups due to high normal variability between different liver samples.
CONCLUSION: The cryoprotectant combination may be a better alternative to the standard DMSO-UW protocol in primary human hepatocyte cryopreservation.
- Citation: Saliem M, Holm F, Tengzelius RB, Jorns C, Nilsson LM, Ericzon BG, Ellis E, Hovatta O. Improved cryopreservation of human hepatocytes using a new xeno free cryoprotectant solution. World J Hepatol 2012; 4(5): 176-183
- URL: https://www.wjgnet.com/1948-5182/full/v4/i5/176.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i5.176
Liver disease is a major health problem worldwide. Liver transplantation is still the golden standard treatment for acute liver failure and end-stage liver disease. Lack of donor organs, among others, is still a major obstacle[1]. Hepatocyte transplantation is gaining more attention as an alternative today[2,3]. Hepatocyte transplantation may function as a bridge to liver transplantation when donors are not available, especially in hepatic emergencies such as acute liver failure. Hepatocytes are also needed for drug metabolizing enzyme induction studies in vitro. Hepatocytes can be successfully isolated from resected livers and from livers not suitable for transplantation. In many situations, cryopreservation is desired to ship hepatocytes between laboratories and hospitals. Furthermore, hepatocytes isolated from liver samples are produced acutely when a tissue is available and often in larger amounts than immediately needed. In addition, hepatocyte cryopreservation might also be an advantage in research related to stem cell differentiation to hepatocytes[4-6]. Hence, an efficient cryopreservation method for human hepatocytes is essential.
The first fully investigated hepatocyte cryopreservation protocol was published in the 1980s[7,8]. Since then, many groups have put efforts into optimizing the cryopreservation method[9-14]. In spite of such efforts, significant loss of viability and function of hepatocytes after thawing is still a major problem. Quality of the starting liver tissue, warm and cold ischemia times, and hepatocyte isolation protocols may also influence the outcome of the cryopreservation. The cryopreservation process itself also has several components that still need to be fine-tuned in order to get a fully optimized protocol. Pre-incubation of hepatocytes with anti-oxidants, cryoprotectants included in freezing medium, addition and dilution of freezing medium, cell density in freezing medium, and medium cooling and warming rates are considered to be the most important steps to be adjusted.
Dimethylsulfoxide in the University-of-Wisconsin solution (DMSO-UW) is one of the most widely used cryoprotectant combinations for hepatocyte cryopreservation in many laboratories[15]. Although the theoretical arguments behind using a controlled rate freezer are convincing, many laboratories still use common laboratory polystyrene boxes placed into a low temperature freezer. As a first step, we compared the two methods, controlled-rate freezer (CRF) vs a polystyrene box (PSB) in an ordinary -70 °C freezer, using only DMSO-UW in hepatocyte preparations from 4 patients.
In a recently published study from our group, we evaluated the use of a new xeno-free cryopreservation solution (STEM-CELLBANKERTM, CB) containing DMSO and anhydrous dextrose in cryopreservation of human embryonic and induced pluripotent stem cells[16].
In this study, we compared STEM-CELLBANKERTM, CB and standard DMSO-UW medium using the PSB method. The viability of hepatocytes was assessed by two different methods, trypan blue exclusion and live-dead assay.
Isolated hepatocytes from thirteen adult liver samples were used in this study. Liver tissue was obtained after partial hepatectomy because of primary or secondary tumors (Table 1). Ethical approval for the study was granted by the Regional Ethical Review Board in Stockholm, Sweden. Hepatocytes were isolated using a three-step collagenase perfusion procedure as described before[17]. In brief, the liver sample was perfused using the following warm (37 °C) solutions: Hank’s Buffered Salt Solution (Cambrex, in vitro, Stockholm, Sweden) containing Ethylene Glycol Tetraacetic Acid (Sigma, Stockholm, Sweden); Hank’s Buffered Salt Solution only; and finally Eagle’s Minimum Essential Medium with Earle’s salts (Cambrex, in vitro) containing Collagenase XI (Sigma). Digested tissue was then transferred to cold (4 °C) Eagle’s Minimum Essential Medium in a sterile beaker, chopped with scissors, and filtered through sterile gauze. The hepatocytes were collected by centrifugation and the Collagenase removed. Pellets were resuspended and washed twice in cold Eagle’s Minimum Essential Medium by centrifugation at 50 g for 5 min at 4 °C to obtain hepatocytes.
| Liver | Gender | Age(yr) | Viability(%) | Diagnosis |
| L1 | F | 43 | 75 | Deceased donor, head trauma |
| L2 | F | 16 | 74 | Deceased donor, head trauma |
| L3 | M | 60 | 83 | Deceased donor, anoxia |
| L4 | M | 55 | 73 | Deceased donor, head trauma |
| L5 | M | 46 | 84 | PSC, CCC |
| L6 | M | 72 | 76 | Colorectal metastasis |
| L7 | M | 36 | 80 | PSC, CCC |
| L8 | M | 49 | 78 | CCC |
| L9 | M | 69 | 68 | Gallbladder cancer |
| L10 | F | 60 | 70 | Gallbladder cancer |
| L11 | F | 65 | 75 | CCC |
| L12 | F | 73 | 83 | Colorectal metastasis |
| L13 | F | 62 | 76 | Colorectal metastasis |
Viability of freshly isolated hepatocytes was compared to thawed hepatocytes cryopreserved using either the CB protocol or the standard DMSO-UW protocol. The viability of the hepatocytes was first estimated using the trypan blue exclusion method[18]. Hepatocytes were diluted in trypan blue (TB) (Sigma) and TB negative and positive cells were immediately counted under light microscopy in triplicates using a hemocytometer. Viability was also estimated using calcein-esterase based Live-Dead Assay (LDA) using LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes, Eugene, United States) according to the manufacturer’s instructions. Hepatocytes were washed in phosphate buffered saline and incubated on a cover slip at 37 °C for 15 min. The live-dead reagent was prepared freshly each time with a volume of 200 µL added to hepatocytes on cover slips. Hepatocytes were then incubated for 45 min in the dark at room temperature. Thereafter, hepatocytes were carefully mounted on glass slides and examined under the fluorescence microscope (Olympus, IX71, Japan). Three different high power fields were pictured for each sample.
The activity of the major cytochrome P450 enzymes (CYPs) CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A7 were assessed for freshly isolated hepatocytes and for thawed hepatocytes cryopreserved using either the CB protocol or the standard DMSO-UW protocol. This was done using luminescence-based assays utilizing specific P450-Glo substrates and their specific luciferin detection reagents (Promega, Madison, WI, United States) according to the manufacturer’s instructions. In brief, 4 × 104 hepatocytes suspended in William’s E Medium (Lonza, Denmark) were incubated with their specific luminogenic substrates on a white opaque 96-well plate (Corning, Costar, United States). Substrate-specific Luciferin Detection Reagents were then added to detect the amount of free luciferin as an indication for different CYPs activity in a luminescence plate reader (BMG LABTECH, FLUstar OPTIMA, Germany). CYP activities were normalized to the amount of double-stranded DNA per well. Samples were transferred to a black opaque 96-well plate (Corning) and freshly prepared PicoGreen Reagent (Quant-iT PicoGreen dsDNA Reagent and Kit) (Molecular Probes) was then added directly to the wells according to the manufacturer’s instructions. The plate was incubated in the dark at room temperature and PicoGreen fluorescence was measured at 480 nmEx/520 nmEm using fluorescence plate reader (TECAN, infinite F500, Austria).
Initially, isolated hepatocytes from four different patients were used to test two different methods of freezing down the hepatocytes. The controlled rate freezer Planer Kryo 10 series III model K10/16 using the program described by Diener et al[19] was compared to placing the tubes in a closed polystyrene box in -70 °C. Hepatocytes in UW + 12% DMSO were transferred to 3 mL cryopreservation tubes on ice. Half of the tubes were frozen in a controlled rate freezer and transferred to the vapor phase of a liquid nitrogen tank when the cycle was completed. The other half of the tubes were wrapped in tissue paper and put into a common laboratory PSB. The box was sealed shut with tape and quickly placed into a -70 °C freezer. After 2 d, the frozen tubes were transferred to the vapor phase of a liquid nitrogen tank for storage.
Primary human hepatocytes from nine different preparations were cryopreserved using either the CB protocol or the standard DMSO-UW protocol. CB is a new xeno-free, chemically defined cryopreservation solution, containing a mixture of both permeating as well as non-permeating cryoprotectants (ZENOAQ, 1-1 Tairanoue, Sasagawa, Asaka-machi, Koriyama, Fukushima 963-0196, Japan). It contains 10% DMSO, glucose and the high polymer anhydrous dextrose described in the Japanese Pharmacopeia as cryoprotectants. For cryopreservation of hepatocytes using the standard method, a cryoprotection solution composed of 12% DMSO in UW was prepared. Ice-cold freezing solution was then added to the cell pellet in a concentration of 7 × 106 cells/mL. Hepatocytes were brought into suspension by gently inverting the tubes. Cell suspension was distributed to 3.5 mL cryotubes. Cryotubes were transferred to a polystyrene box and kept in a -70 °C freezer overnight. Cryotubes were then transferred to liquid nitrogen and kept in the vapor phase. For freezing hepatocytes using the CB protocol, the same procedure, cold CB, was added directly to the cell pellet in a concentration of 2 × 106 cells/mL.
For thawing of frozen hepatocytes, the cryotubes were incubated in a 37 °C water bath for 1-2 min until ice crystals started to melt. Hepatocytes were reconstituted in two different ways according to the protocol used. For hepatocytes cryopreserved in DMSO-UW, the contents of the cryotubes were transferred to a 50 mL tube. An equal volume of cold William’s E Medium was then added gradually to the hepatocytes on ice. This was repeated 3 times, 5 min apart. For hepatocytes cryopreserved in CB, the contents of the cryotubes were similarly transferred to a 50 mL tube. An equal volume of a cold washing solution, a thawing buffer containing NaCl (CELLOTION; ZENOAQ), was directly added to the hepatocytes once on ice. Hepatocytes were then washed twice in cold William’s E Medium by centrifugation at 50 g for 5 min at 4 °C.
Viability and function of thawed hepatocytes from both methods were then assessed as described above for the freshly isolated hepatocytes.
Analysis of variance and the non-parametric Kruskal-Wallis tests were carried out using the PASW statistics 18 software. Test results were considered statistically significant when P values were < 0.05.
Hepatocyte viability for the four samples used to compare CRF to PSB in an ordinary -70 °C freezer is presented in Table 2. Preserving hepatocytes in a PSB gives viability (35.5 ± 9.2) not significantly different from the freshly isolated. However the use of CRF gives a significantly lower viability (2.8 ± 12.8) (P < 0.01) compared to fresh (76.3 ± 4.6). Therefore, we only used the PSB method when comparing the two cryoprotectants.
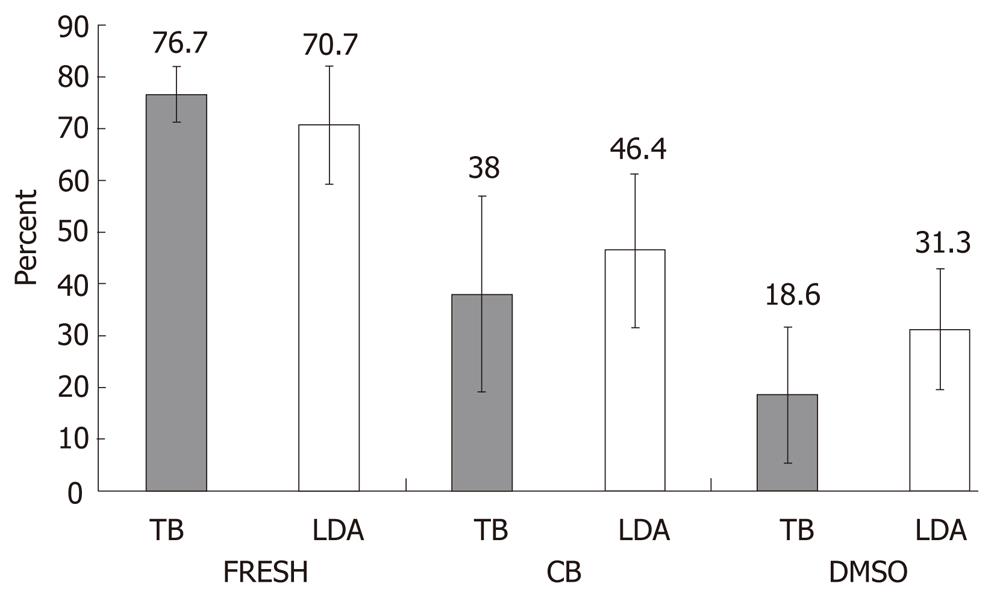
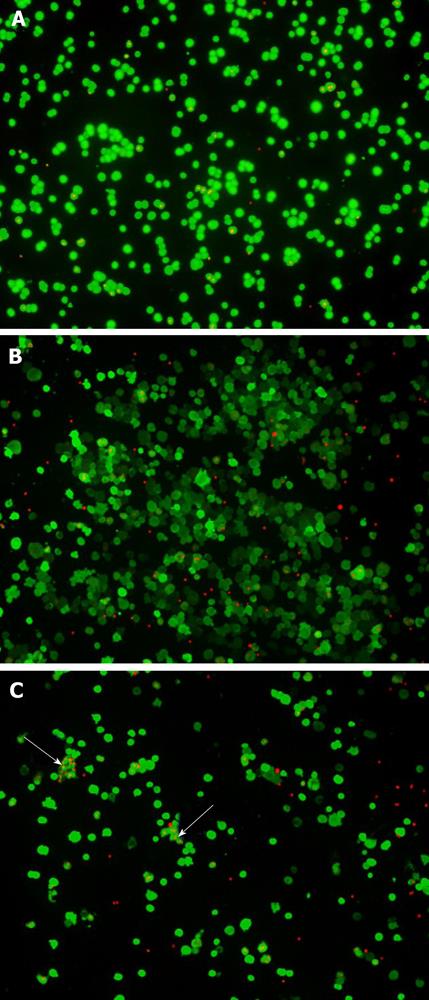
Viability of hepatocytes from nine liver samples cryopreserved by CB or DMSO-UW protocols was estimated using the two different methods TB and LDA (Figure 1). In the LDA method, live hepatocytes showed ‘‘green’’ fluorescence in their cytoplasm upon active uptake and conversion of calcein AM to the more fluorescent Calcein. Ethidium-1 entered dead hepatocytes through their damaged cell membranes and bound to nucleic acids showing ‘‘red’’ fluorescence in the nuclei. Upon thawing, hepatocytes cryopreserved in DMSO-UW were sticking together in clumps, which were difficult to dissolve in contrast to hepatocytes cryopreserved in CB (Figure 2).
A two-way analysis of variance was performed to investigate the influence of the two different cryopreservation protocols on hepatocyte viability and if this was influenced by the method used for viability estimation. The change in hepatocyte condition (fresh, cryopreserved in CB, or cryopreserved in DMSO-UW) did have a significant effect on viability; F (2, 48) = 62.9, P < 0.001, η2 = 0.724. In a pairwise comparison, hepatocytes cryopreserved in CB did have better viability after thawing than hepatocytes cryopreserved in DMSO-UW (P < 0.05). There was no significant difference in viability estimation between both the TB and the LDA methods; F (1, 48) = 1.99, P > 0.05, η2 = 0.040. There was no significant interaction between hepatocyte condition and the viability estimation method; F (2, 48) = 2.50, P > 0.05, η2 = 0.094, indicating that the difference in hepatocyte viability was mainly due to changes in their condition and not due to the method used.
The higher the viability of fresh hepatocytes, the better the survival was after cryopreservation using the CB protocol. This was indicated by a positive correlation between viability of fresh hepatocytes and the difference in cell viability between CB and DMSO protocols with R2 = 0.69 using the TB method. This might indicate that hepatocytes with good viability preserve better in CB than in DMSO-UW.
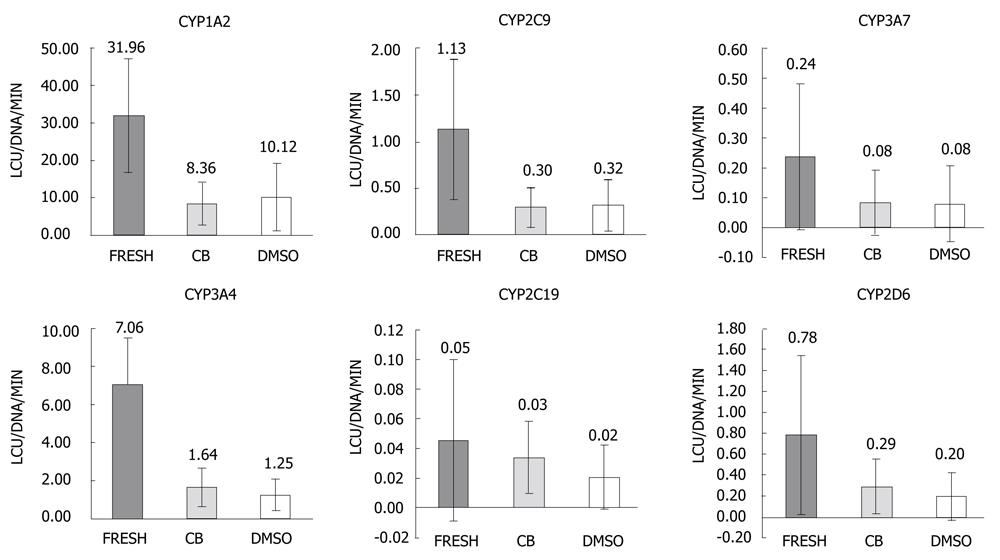
Function of thawed hepatocytes cryopreserved using either CB protocol or DMSO-UW protocol was compared to freshly isolated primary human hepatocytes. The activities of the following CYPs were estimated in three groups; CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A7. CYP activities were normalized to the amount of double-stranded DNA. The within-groups variability was high as manifested by the high standard deviation values that exceeded the mean values in many cases (Figure 3).
One-way analysis of variance was carried out to compare activity of each CYP in the three groups. Changes in hepatocyte condition between the three groups had significant effect on CYP1A2 activity; F (2, 24) = 12.21, P < 0.001, η2 = 0.504. This effect was mainly due to differences between the fresh group and both the cryopreserved groups as there was no statistically significant difference between CB and DMSO-UW groups in pairwise comparisons. For CYP3A4 activity, there was also significant difference between the three groups; F (2, 24) = 11.90, P < 0.001, η2 = 0.498, which was mainly due to differences between the fresh group and both the cryopreserved groups as there was no significant difference between the CB and DMSO-UW groups. Similarly, for CYP2C9 activity; F (2, 24) = 13.97, P < 0.001, η2 = 0.538, and there was no significant difference between the CB and DMSO-UW groups.
There was no significant difference between the three groups regarding activities of the following CYPs; CYP3A7 [F (2, 24) = 1.38, P > 0.05, η2 = 0.103], CYP2C19 [F (2, 24) = 1.08, P > 0.05, η2 = 0.082], and CYP2D6 [F (2, 24) = 3.35, P > 0.05, η2 = 0.218]. There were no statistically significant differences between the CB and DMSO-UW groups regarding the activities of these CYPs.
The potentially high demand of primary human hepatocytes necessitates the need for a fully optimized cryopreservation protocol. Presently, there is no fully optimized cryopreservation protocol for hepatocytes. This is despite many efforts with varying degrees of success[9-14]. Much effort is still needed to be put in testing, for instance hepatocyte pre-incubation with anti-oxidants prior to cryopreservation or including non-permeating cryoprotectants in the freezing solution[20]. In this study, we introduced a new experimentally optimized xeno-free cryoprotectant medium (CB), for the first time for cryopreservation of hepatocytes. In comparison to DMSO-UW, CB is also a xeno-free freezing solution but it further contains both permeating and non-permeating cryoprotectants at carefully tested concentrations[16].
The corner stone in evaluating the success of any hepatocyte cryopreservation protocol is to compare viability of hepatocytes, their function and their plating efficiency before and after freezing[20]. Here, we evaluated viability using two methods, TB and LDA. We also evaluated function of hepatocytes by testing the enzymatic activity of major CYPs isoforms, CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A7, before and after freezing.
During hepatocyte cryopreservation, the density at which hepatocytes are frozen may affect their viability on thawing. A cell density between 3 and 10 × 106 cells/mL is usually recommended[13]. Lower cooling and higher warming rates usually have a lower incidence of intracellular ice crystal formation that dramatically affects hepatocyte viability after cryopreservation. The rate at which the cryopreservation solution is diluted may affect viability as well[14]. Controlled rate freezing is gaining more interest as a better alternative to using a polystyrene box in -70 °C freezer[13,21]. However, in our small pilot study where we compared both freezers, we did not find using the CRF better than the ordinary -70 °C freezer. These findings were supported in reports by others[20,22,23] where no difference was shown between CRF, the Nalgene propan-2-ol device or simply using -20 °C and -70 °C freezers. The aim of our study was to compare the efficacy of two complete cryopreservation protocols, CB protocol and DMSO-UW protocol. There were few differences between the two protocols. The CB protocol had lower freezing cell density compared to the DMSO-UW protocol, while the latter had gradual dilution of the freezing medium upon thawing compared to the CB protocol.
Upon evaluating the viability of hepatocytes before and after cryopreservation using the two protocols, we could conclude that the CB protocol, in addition to being simpler and faster, yielded a better cell survival of the cells in comparison to the DMSO-UW protocol. Using the TB or LDA method in assessing viability of hepatocytes, the results were similar. Hence, it is possible to use only the LDA method in the future because the LDA method had some advantages over the TB method. For example, the active uptake of calcein AM by the live hepatocytes indirectly tests their transport function at the same time. However, one drawback with the LDA is the long time it takes to perform, in contrast to the rapid TB method.
In general, assessing hepatocyte function is not an easy task. Hepatocytes perform a vast number of different functions ranging from energy metabolism, synthesis of proteins and hormones to metabolism of xenobiotics and bile production. Choosing one or a few functions to represent the overall vitality of the cell is therefore difficult. Moreover, the high variability between one liver to another usually makes it difficult to define “the normal liver”. There are many reasons for variability: genetic polymorphism, gene expression modulation, the tissue quality, and tissue handling before and during hepatocyte isolation are potential reasons[24]. Gene expression modulation can occur due to various environmental factors e.g. food and xenobiotics. In this study, we could see a high within-group variability depicted by the high standard deviation values that exceeded the mean values in many of the cases.
There was an obvious tendency for hepatocyte function to be higher in fresh in comparison to cryopreserved hepatocytes. The same tendency was seen in the CB group, as well as the DMSO-UW group. This hierarchy was seen in 33 out of 54 comparisons. However, there was no significant difference between fresh and cryopreserved groups in the case of CYP2C19, CYP2D6 and CYP3A7 activities in contrast to the activities of CYP1A2, CYP2C19 and CYP3A4. This might be due to the high within-group variability. In some cases, CYPs activity was higher in cryopreserved hepatocytes compared to fresh hepatocytes or in the DMSO-UW group compared to the CB group. This is in line with what was found by Li et al[11] where there was no significant difference between the fresh and the cryopreserved hepatocytes regarding their drug-metabolizing enzyme activities or their bile acid conjugation and secretion[25]. Results from those two previous studies suggested that the functions of the hepatocytes were equally good before and after cryopreservation. In other words, cryopreservation had no impact on hepatocyte function when it comes to their drug-metabolizing enzyme activities[11].
In this study, we conclude that CYP activity might not be the best choice in choosing between different hepatocyte cryopreservation protocols and more stringent measurements of function might be needed when evaluating advanced functions of liver cells. Both protocols tested yielded hepatocytes with good P450 function; however, the CB protocol gave a higher viability than the widely-used hepatocyte cryoprotectant DMSO-UW. CB is also xeno-free and might be useful in cryopreservation of clinical-grade primary human hepatocytes. In conclusion, in this study we show that CB is a good freezing solution for hepatocytes.
We thank Dr. Roberto Gramignoli (Department of Pathology, University of Pittsburgh, PA, United States) for excellent technical support and Associate Professor Paolo Parini (Division of Clinical Chemistry, Department of Laboratory Medicine, Karolinska Institute, Stockholm, Sweden) for helpful discussion regarding the statistical analysis of the data.
Efficient preservation of primary human hepatocytes by freezing is very important both for research and clinical purposes. An efficient preservation method is also needed for hepatocytes obtained from stem cells in the future. There are many hepatocyte freezing protocols available with varying degrees of success. Protocols which do not include any animal substances are mandatory for clinical use of human hepatocytes. The different protocols tested different freezing methods and different freezing solutions. Up to now, there is no ideal protocol available for preserving hepatocytes. The new freezing solution the authors are using contains chemicals which protect inside and outside cells during freezing.
Research in the field of human hepatocyte freezing focuses, among others, on testing several freezing solutions. The new freezing solution they are using was tested before with other cell types with great preservation capacity.
The new freezing solution we tested here has not been tested before with human hepatocytes. It was tested with embryonic stem cells previously in the group and proved to be much more effective than the standard protocol. The results suggest that this new solution can be a better alternative to the standard freezing solution. Being xeno-free makes it suitable for freezing of hepatocytes in clinical settings. The results also argue against using liver cytochrome P450 enzymes in evaluating a given hepatocyte freezing protocol because of the huge between-samples variability and since even dying hepatocytes can still express them.
The study results suggest that the new freezing solution can be a better alternative to the standard solution in freezing primary human hepatocytes and hepatocytes derived from stem cells.
Dimethylsulphoxide (DMSO) is an organosulfur compound used as a solvent and constitutes a very important part in many freezing solutions. University of Wisconsin solution is an intracellular-like preservation medium typically used during organ transplantation. (STEM-CELLBANKER, CB) is the new freezing medium and contains mainly DMSO and anhydrous dextrose. Freezing solutions can protect cells, either by penetrating through their cell membrane (permeating cryoprotectant) or by stabilizing the outside of the cells (non-permeating cryoprotectant). Cytochrome P450 isoforms (CYPs) are drug-metabolizing enzymes present mainly in the liver.
Overall, the paper demonstrates very little difference between preservation methods. Viability is low with both storage and freezing methods. Similarly, there is much variability in CYP activities in fresh hepatocytes and frozen hepatocytes. Based on this, it is not clear that this study rises to the level of a full research paper. The authors have miscalculated the viability of controlled-rate freezer hepatocytes in Table 2-the viability is 20.8%, not 2.8%. Additional tests of viability and functionality could be included - MTT, mitochondrial function.
Peer reviewer: Dr. Shannon M Bailey, University of Alabama at Birmingham, 1665 University Blvd, Ryals 623, Birmingham, AL 35294, United States
S- Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | 1 Najimi M, Sokal E. Liver cell transplantation. Minerva Pediatr. 2005;57:243-257. [PubMed] |
| 2. | Stéphenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317-1323. [PubMed] |
| 3. | Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559-569. [PubMed] |
| 4. | Stock P, Brückner S, Ebensing S, Hempel M, Dollinger MM, Christ B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc. 2010;5:617-627. [PubMed] |
| 5. | Lavon N. Generation of hepatocytes from human embryonic stem cells. Methods Mol Biol. 2010;640:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 951] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 7. | Fuller BJ, Morris GJ, Nutt LH, Attenburrow VD. Functional recovery of isolated rat hepatocytes upon thawing from –196 degrees C. Cryo-Lett. 1980;1:139-146. |
| 8. | Loretz LJ, Li AP, Flye MW, Wilson AG. Optimization of cryopreservation procedures for rat and human hepatocytes. Xenobiotica. 1989;19:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Rijntjes PJ, Moshage HJ, Van Gemert PJ, De Waal R, Yap SH. Cryopreservation of adult human hepatocytes. The influence of deep freezing storage on the viability, cell seeding, survival, fine structures and albumin synthesis in primary cultures. J Hepatol. 1986;3:7-18. [PubMed] |
| 10. | Dou M, de Sousa G, Lacarelle B, Placidi M, Lechene de la Porte P, Domingo M, Lafont H, Rahmani R. Thawed human hepatocytes in primary culture. Cryobiology. 1992;29:454-469. [PubMed] |
| 11. | Li AP, Lu C, Brent JA, Pham C, Fackett A, Ruegg CE, Silber PM. Cryopreserved human hepatocytes: characterization of drug-metabolizing enzyme activities and applications in higher throughput screening assays for hepatotoxicity, metabolic stability, and drug-drug interaction potential. Chem Biol Interact. 1999;121:17-35. [PubMed] |
| 12. | Alexandre E, Viollon-Abadie C, David P, Gandillet A, Coassolo P, Heyd B, Mantion G, Wolf P, Bachellier P, Jaeck D. Cryopreservation of adult human hepatocytes obtained from resected liver biopsies. Cryobiology. 2002;44:103-113. [PubMed] |
| 13. | Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl. 2010;16:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Hewitt NJ. Optimisation of the cryopreservation of primary hepatocytes. Methods Mol Biol. 2010;640:83-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Illouz S, Nakamura T, Webb M, Thava B, Bikchandani J, Robertson G, Lloyd D, Berry D, Wada H, Dennison A. Comparison of University of Wisconsin and ET-Kyoto preservation solutions for the cryopreservation of primary human hepatocytes. Transplant Proc. 2008;40:1706-1709. [PubMed] |
| 16. | Holm F, Ström S, Inzunza J, Baker D, Strömberg AM, Rozell B, Feki A, Bergström R, Hovatta O. An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells. Hum Reprod. 2010;25:1271-1279. [PubMed] |
| 17. | Strom SC, Dorko K, Thompson MT, Pisarov LA, and , Nussler AK. Large scale isolation and culture of human hepatocytes. (Franco, D, Boudjema, K, and Varet, B eds) Îlots de Langerhans et hépatocytes: vers une utilisation therapeutique. Paris: Les Editions INSERM 1998; 195-205. |
| 18. | Ahlberg J, Einarsson K, Westberg G. Spontaneous dissolution of gallstones. A case report. Acta Chir Scand Suppl. 1980;500:3-5. [PubMed] |
| 19. | Diener B, Utesch D, Beer N, Dürk H, Oesch F. A method for the cryopreservation of liver parenchymal cells for studies of xenobiotics. Cryobiology. 1993;30:116-127. [PubMed] |
| 20. | Stéphenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: is it time to change the strategy? World J Gastroenterol. 2010;16:1-14. [PubMed] |
| 21. | Mitry RR, Lehec SC, Hughes RD. Cryopreservation of human hepatocytes for clinical use. Methods Mol Biol. 2010;640:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lloyd TD, Orr S, Berry DP, Dennison AR. Development of a protocol for cryopreservation of hepatocytes for use in bioartificial liver systems. Ann Clin Lab Sci. 2004;34:165-174. [PubMed] |
| 23. | Stéphenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16:409-419. [PubMed] |
| 24. | Klieber S, Torreilles F, Guillou F, Fabre G. The use of human hepatocytes to investigate drug metabolism and CYP enzyme induction. Methods Mol Biol. 2010;640:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Shitara Y, Li AP, Kato Y, Lu C, Ito K, Itoh T, Sugiyama Y. Function of uptake transporters for taurocholate and estradiol 17beta-D-glucuronide in cryopreserved human hepatocytes. Drug Metab Pharmacokinet. 2003;18:33-41. [PubMed] |