INTRODUCTION
Green tea obtained from the leaves of Camellia sinensis is thought to improve health due to antioxidant and anticarcinogenic effects[1-4]. Nonetheless, green tea has pro-oxidant effects, primarily due to epigallocatechin-3-gallate (EGCG)[2,3]. Lambert et al[5] reported dose-dependent hepatotoxicity in mice associated with pro-oxidant effects of high-dose EGCG. Hepatotoxicity associated with green tea extracts that contain high concentrations of EGCG is being reported[6-9]. Due to hepatotoxicity, Exolise® (Arkopharma, Carros, France), a weight-loss supplement was withdrawn from the market in France and Spain[8]. Exolise® contained EGCG[7,8]. The Drug-Induced Liver Injury Network indicated that in 6 of 28 cases of hepatotoxicity secondary to herbal and dietary supplements, green tea extract was the major component of the supplement[10]. Fong et al[6] reported 8 patients who developed drug-induced liver injury (DILI) due to Hydroxycut®, 3 of whom required liver transplantation. The authors concluded that green tea was likely the major ingredient in Hydroxycut® resulting in severe liver injury[6].
We report a young woman who developed severe hepatotoxicity while taking SlimQuick™ (Distributed by Wellnx Life Sciences, Wilmington, DE), an herbal weight-loss product containing green tea extract. This is important because we identified an often overlooked predisposition to DILI; the alpha-1 antitrypsin MZ phenotype[11].
CASE REPORT
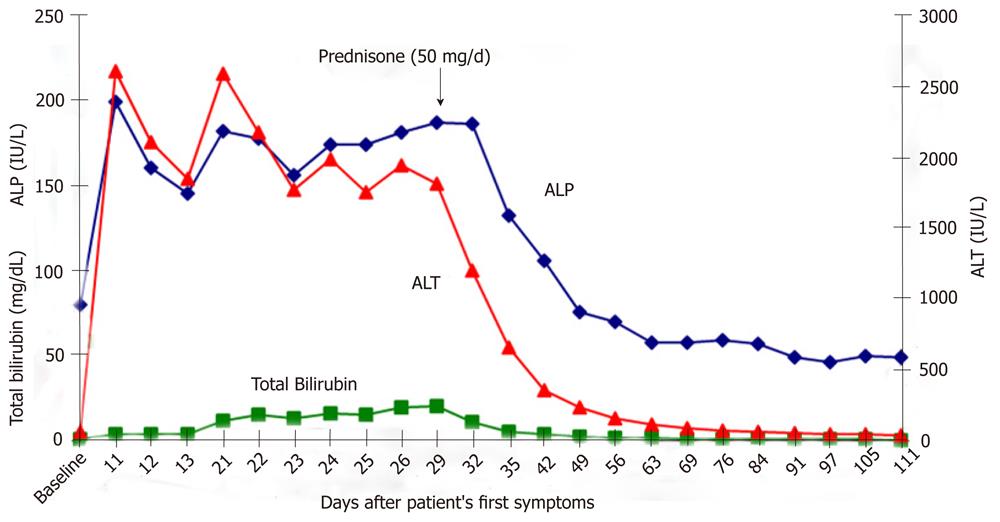
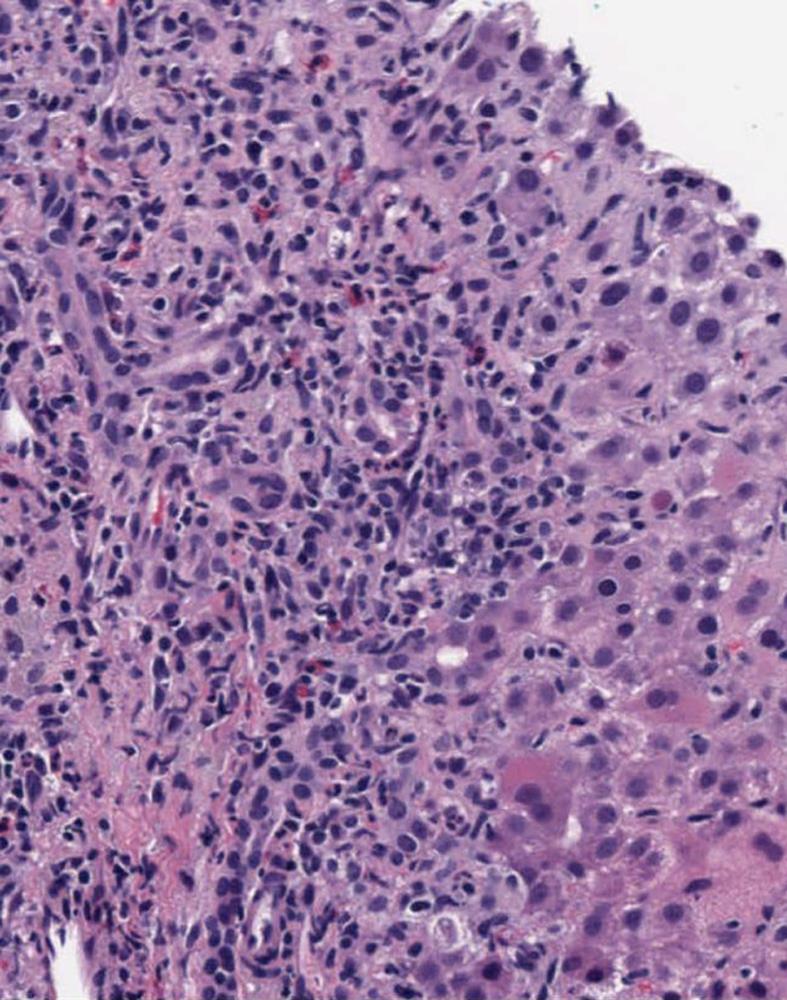
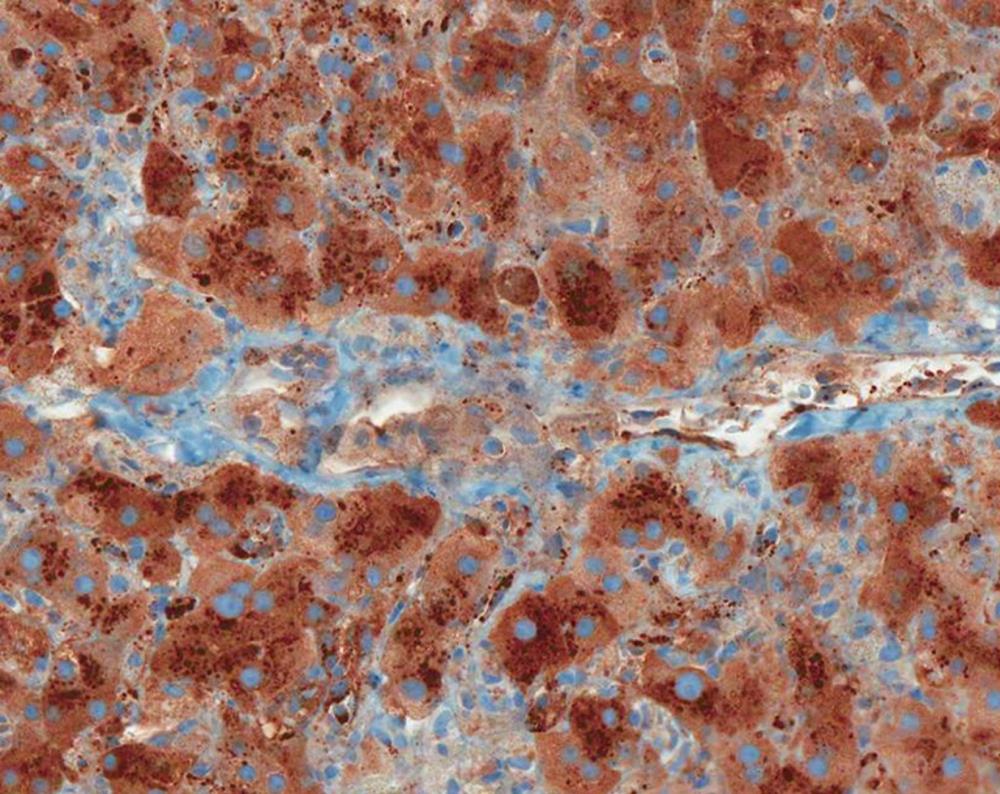
A 24-year-old white woman presented to her primary care physician with complaints of dark urine, acholic stools, right upper quadrant pain and progressive fatigue. She reported taking two caplets of SlimQuick™ orally on an empty stomach 6 h apart twice per day for three months to improve energy for marathon training. She took no other dietary supplements or medications except for oral tetracycline 500 mg/d orally for eleven months for acne. She stopped both drugs eight days after the onset of symptoms. Past medical, surgical and family histories were unremarkable. Two months as well as 3 years earlier, serum alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total bilirubin were normal (Figure 1). Eleven days after the symptoms began; ALT was 2615 IU/L, aspartate aminotransferase 2320 U/L, alkaline phosphatase 200 IU/L and total bilirubin level 4.0 mg/dL (Figure 1). A day later, the prothrombin time and international normalized ratio were 12 s and 1.2, respectively. Three weeks after her first symptoms, she was transferred to our institution for further evaluation. At presentation, vital signs were normal. The physical examination was normal except for icteric sclera and mild right upper quadrant abdominal tenderness. Serological tests for viral hepatitis, autoimmune hepatitis, Wilson disease and primary biliary cirrhosis were negative. The alpha-1 antitrypsin phenotype test was reported as MZ. Magnetic resonance imaging of the abdomen revealed hepatomegaly. Magnetic resonance cholangiopancreatography and abdominal ultrasound were normal. Transjugular liver biopsy revealed a hepatic venous pressure gradient of 3 mmHg. Histopathology demonstrated severe inflammatory infiltrate involving both the portal tracts and lobules (Figure 2). The infiltrate included plasma cells, lymphocytes, and neutrophils with prominent eosinophils (Figure 2). There was also focal necrosis with loss of hepatic parenchyma, with accompanying architectural collapse and proliferation of bile ductules (Figure 2). Alpha-1 antitrypsin staining with specific immunoperoxidase highlighted minute globules, predominantly within periportal hepatocytes, as well as diffuse background staining in hepatocytes (Figure 3). These globules were much smaller than those typically associated with alpha-1 antitrypsin disorder in homozygotes.
Figure 1 Two months earlier, our patient had normal serum alanine aminotransferase, alkaline phosphatase and total bilirubin (baseline).
The peak total bilirubin level lagged 19 d behind the peak ALT and ALP levels. There was striking improvement in serum aminotransferase and total bilirubin levels after initiation of prednisone (50 mg/d). Laboratory values remained normal after prednisone therapy was discontinued. ALT: Alanine aminotransferase; ALP: Alkaline phosphatase.
Figure 2 Portal tract and lobules with marked mixed inflammation and parenchymal loss (hematoxylin and eosin staining, X100).
Figure 3 Minute alpha-1 antitrypsin positive globules in periportal hepatocytes; diffuse background staining for alpha-1 antitrypsin is also presented (alpha-1 antitrypsin stain, X200).
Because the patient demonstrated no laboratory or clinical improvement three weeks after stopping SlimQuick™ and liver biopsy was consistent with marked inflammation with necrosis, treatment with Prednisone (50 mg/d) was initiated. All laboratory parameters rapidly improved within one week of therapy (Figure 1). Prednisone was tapered over 4 wk and stopped. On follow-up, the patient’s liver tests were normal and she was asymptomatic.
DISCUSSION
The major ingredient in SlimQuick™ caplets is green tea extract (Camellia sinensis leaf) containing 135 mg of EGCG. EGCG has pro-oxidant effects that can cause hepatotoxicity when administered at high doses[3,5,8]. SlimQuick™ caplets also contain other ingredients including rhodiola (Rhodiola rosea), chaste tree (Vitex agnus castus), Juniper (Juniperus communis), soy (Glycine max), Asian ginseng (Panax ginseng), Japanese knotweed (Polygonum cuspidatum) extracts, brown seaweed (Fucus vesiculosus), dandelion (Taraxacum officinale), yerba mate (Ilex Paraguariensis), uva-ursi (Arctostaphylos uva ursi), phytosterols (Glycine max), l-theanine, caffeine, vitamins D, K, B6 and B12, folate, and calcium. Boehm et al[1] reviewed 51 studies, including 27 case-control, 23 observational and 1 randomized controlled trial related to green tea consumption and concluded that drinking 3 to 5 cups of green tea per day provided at least 250 mg catechins per day and might be considered safe. We calculated that our patient was exposed to green tea extract that contained catechin in amounts higher than these suggested safe levels. Moreover, she was also directed to take it while fasting. This augments the likelihood of hepatotoxicity in animals and fasting humans achieve 5-fold higher catechin concentrations compared to fed controls[7]. Lambert et al[5] reported moderate to severe hepatic necrosis in 60% and mild necrosis in 40% of mice treated with two oral doses of 750 mg/kg EGCG per day.
We performed an Ovid Medline search that extended from 1948 until October 22, 2010 by combining key words “drug-induced liver injury” or “hepatotoxicity” and both common and scientific names of herbal ingredients of SlimQuick™. We also searched the Natural Medicines Comprehensive Database[12] for hepatotoxicity associated with any ingredients of SlimQuick™ other than green tea extract. Based on our search, we identified only two herbal ingredients in SlimQuick™ other than green tea extract associated with hepatotoxicity in animals or humans. Nowak et al[13] reported a radiographer exposed to hydroquinone fumes who developed toxic hepatitis. Hydroquinone is a component of uva ursi[14]. However, Nowak et al[13] associated toxic hepatitis with hydroquinone fumes, not an herbal medicine, uva ursi[13,15]. McGee et al[16] associated mate tea with veno-occlusive disease due to pyrroliziding alkaloids detected in small quantity in tea samples. However, they failed to detect pyrroliziding alkaloids in mate tea sold locally[16]. Our patient’s liver histopathology was inconsistent with veno-occlusive disease. In addition, the latency period of 12 wk, peak laboratory values and histopathological findings are more compatible with Exolise® and Hydroxycut® hepatotoxicity[6,7].
Besides taking a hepatotoxic weight-loss supplement, our patient was also heterozygous for alpha-1 antitrypsin deficiency. There remains considerable debate regarding the importance of the alpha-1 antitrypsin MZ phenotype [11,17-21]. Graziadei et al[17] suggested that the alpha-1 antitrypsin MZ phenotype might be a risk factor for chronic liver disease or liver failure. Rakela et al[22] showed that subjects with the alpha-1 antitrypsin MZ phenotype might develop liver disease later in life compared to subjects with the alpha-1 antitrypsin ZZ phenotype.
Given our patient’s baseline normal liver function, most likely the presence of alpha-1 antitrypsin MZ phenotype increased her vulnerability to severe hepatocellular injury. Tetracycline-induced liver injury was excluded as an offender based on the histopathology, leaving SlimQuick™ as the likely hepatotoxic agent. To our knowledge, this is the first report of herbal drug-associated DILI in the context of the alpha-1 antitrypsin MZ phenotype. This demonstrates the importance of seeking underlying susceptibility to hepatic injury in previously healthy subjects who develop DILI.