Published online Nov 27, 2012. doi: 10.4254/wjh.v4.i11.291
Revised: August 17, 2012
Accepted: October 22, 2012
Published online: November 27, 2012
AIM: To incorporate estimated glomerular filtration rate (eGFR) into the model for end-stage liver disease (MELD) score to evaluate the predictive value.
METHODS: From January 2004 to October 2008, the records of 4127 admitted cirrhotic patients were reviewed. Patients who survived and were followed up as outpatients were defined as survivors and their most recent available laboratory data were collected. Patients whose records indicated death at any time during the hospital stay were defined as non-survivors (in-hospital mortality). Patients with incomplete data or with cirrhosis due to a congenital abnormality such as primary biliary cirrhosis were excluded; thus, a total of 3857 patients were enrolled in the present study. The eGFR, which was calculated by using either the modification of diet in renal disease (MDRD) equation or the chronic kidney disease epidemiology collaboration (CKD-EPI) equation, was incorporated into the MELD score after adjustment with the original MELD equation by logistic regression analysis [bilirubin and international normalized ratio (INR) were set at 1.0 for values less than 1.0].
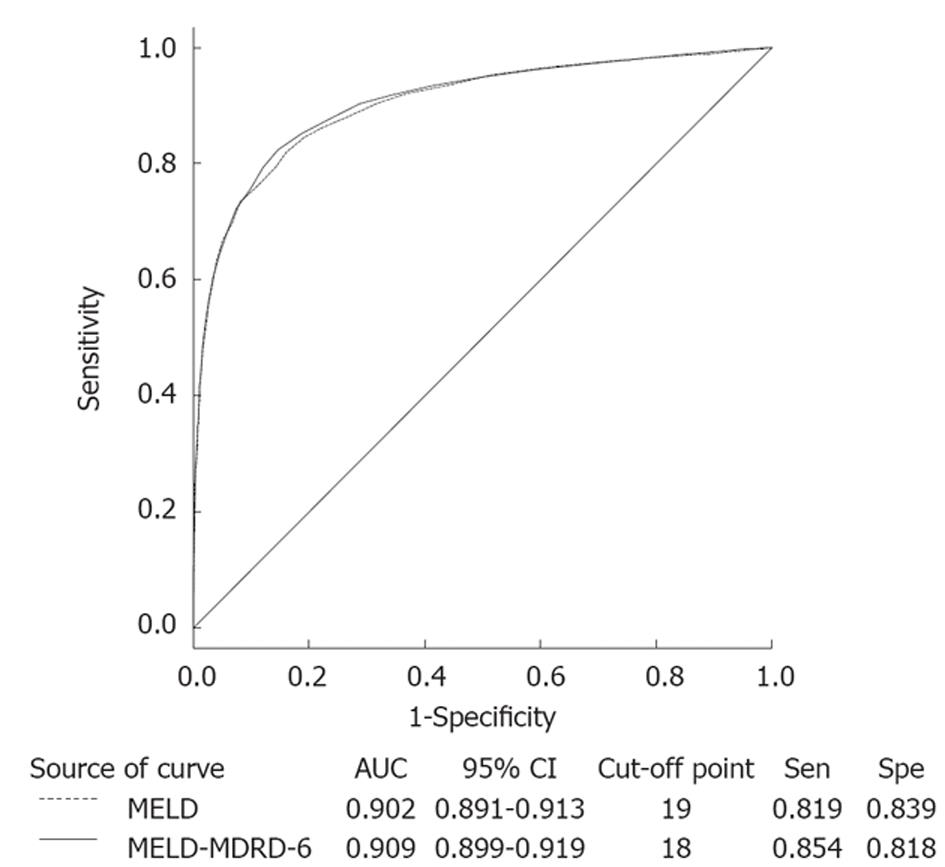
RESULTS: Patients defined as survivors were significantly younger, had a lower incidence of hepatoma, lower Child-Pugh and MELD scores, and better renal function. The underlying causes of cirrhosis were very different from those in Western countries. In Taiwan, most cirrhotic patients were associated with the hepatitis virus, especially hepatitis B. There were 16 parameters included in univariate logistic regression analysis to predict in-hospital mortality and those with significant predicting values were included in further multivariate analysis. Both 4-variable MDRD eGFR and 6-variable MDRD eGFR, rather than creatinine, were significant predictors of in-hospital mortality. Three new equations were constructed (MELD-MDRD-4, MELD-MDRD-6, MELD-CKD-EPI). As expected, original MELD score was a significant predictor of in-hospital mortality (odds ratio = 1.25, P < 0.001). MELD-MDRD-4 excluded serum creatinine, with the coefficients refit among the remaining 3 variables, i.e., total bilirubin, INR and 4-variable MDRD eGFR. This model represented an exacerbated outcome over MELD score, as suggested by a decrease in chi-square (2161.45 vs 2198.32) and an increase in -2 log (likelihood) (2810.77 vs 2773.90). MELD-MDRD-6 included 6-variable MDRD eGFR as one of the variables and showed an improvement over MELD score, as suggested by an increase in chi-square (2293.82 vs 2198.32) and a decrease in -2 log (likelihood) (2810.77 vs 2664.79). Finally, when serum creatinine was replaced by CKD-EPI eGFR, it showed a slight improvement compared to the original MELD score (chi-square: 2199.16, -2 log (likelihood): 2773.07). In the receiver-operating characteristic curve, the MELD-MDRD-6 score showed a marginal improvement in area under the curve (0.909 vs 0.902), sensitivity (0.854 vs 0.819) and specificity (0.818 vs 0.839) compared to the original MELD equation. In patients with a different eGFR, the MELD-MDRD-6 equation showed a better predictive value in patients with eGFR ≥ 90, 60-89, 30-59 and 15-29.
CONCLUSION: Incorporating eGFR obtained by the 6-variable MDRD equation into the MELD score showed an equal predictive performance in in-hospital mortality compared to a creatinine-based MELD score.
- Citation: Chen YW, Chang CW, Chang CW, Wang TE, Wu CJ, Chen HH. Is an estimated glomerular filtration rate better than creatinine to be incorporated into the end-stage liver disease score? World J Hepatol 2012; 4(11): 291-298
- URL: https://www.wjgnet.com/1948-5182/full/v4/i11/291.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i11.291
For over 30 years, the Child-Pugh score, which is based on 5 variables (ascites, encephalopathy, serum total bilirubin, serum albumin and prothrombin time), has been the main prognostic tool and has proved to be a robust prognostic predictor in different situations[1]. However, the value of this score is limited due to subjective interpretation of ascites and encephalopathy and an inappropriate classification of serum bilirubin. Increasing evidence in the literature suggests that the development of acute kidney injury is an ominous and common event in cirrhotic patients[2]. Therefore, routine serum creatinine tests have been found to significantly improve the prognostic accuracy of the Child-Pugh score and serum creatinine is an independent predictor of survival in cirrhotic patients[3]. In fact, renal function is 1 of the 3 variables [serum bilirubin, international normalized ratio (INR) and serum creatinine] in the model for end-stage liver disease (MELD) score, which is a good predictor for assessing 3 mo mortality and is currently used to determine priority for orthotopic liver transplantation[1,4,5].
Unlike the Child-Pugh score, the 3 variables of the MELD score are selected on the basis of statistical analysis and not empirical analysis. Even although serum creatinine has a strong prognostic value in cirrhotic patients, it is considered an insensitive predictor in such patients because of the patient’s reduced muscle mass; this may lead to an overestimation of creatinine clearance compared to inulin clearance[1,6,7]. Thus, serum creatinine is not a very accurate gauge, especially in detecting early loss of renal function in cirrhotic patients[1,6,8], and there are approximately 15% to 20% of patients whose survival cannot be accurately predicted by the MELD score[6,9].
Recently, Lim et al[10] suggested that there was a significant association between measured glomerular filtration rate (GFR) and survival after adjustment for MELD; however, estimated GFR (eGFR) calculated by the modification of diet in renal disease (MDRD) equation was only moderately correlated with measured GFR in cirrhotic patients[10]. The creatinine-based MDRD equation is widely used in the general population for calculating GFR and is considered a gold standard in nephrology[8,11]. It is also the best formula for the detection of moderate renal dysfunction in advanced liver disease[12,13]. Nowadays, most publications that mention the eGFR of cirrhotic patients have been using databases from liver transplant registries. The aim of the present study was to evaluate the difference between eGFR obtained either by MDRD or by the new creatinine-based equation, known as the chronic kidney disease epidemiology collaboration (CKD-EPI) formula[14], when eGFR was incorporated into the MELD score to predict in-hospital mortality in a broad population of cirrhotic patients.
This work was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association and the institutional review board.
We performed a retrospective, cross-sectional study on Taiwanese cirrhotic patients in the Mackay Memorial Hospital. Mackay Memorial Hospital is a tertiary referral center for liver disease. This study is a single center investigation and all patients of the study were afferent, directly diagnosed and followed-up in Mackay Memorial Hospital.
The design of this single-center study was retrospective and cross-sectional and the protocol was approved by the local ethics committee. Patients diagnosed with cirrhosis were selected from those admitted to Mackay Memorial Hospital between January 2004 and October 2008.
The records of 4127 cirrhotic patients from a total of 228 345 admitted patients were reviewed. Patients who survived and were followed up as outpatients were defined as survivors and their most recent available laboratory data were collected. Patients whose records indicated death at any time during the hospital stay were defined as non-survivors (cases of in-hospital mortality) and laboratory data for these patients comprised the data collected during their admission. In the case of patients with multiple admissions, the records before those of the last admission were excluded. Demographic data, Child-Pugh scores and information regarding underlying comorbidities were obtained from the most recent laboratory examinations. Patients with incomplete data or with cirrhosis due to congenital abnormality such as primary biliary cirrhosis were excluded; thus, a total of 3857 patients were enrolled in the present study. None of these patients had received liver transplants.
The eGFR was calculated according to the formula below:
MDRD-4[11] = 175 × (Scr)-1.154× (age)-0.203× (0.742 if female) × (1.178 if black),
MDRD-6[11] = 170 × (Scr)-0.999× (age)-0.176× (0.762 if female) × (1.180 if black) × (SUN)-0.170× (albumin)0.318,
CKD-EPI[14] = 141 × min (Scr/κ, 1)α× max (Scr/κ, 1)-1.209× 0.993age× (1.018 if female) × (1.159 if black),
where MDRD-4 is 4-variable MDRD, MDRD-6 is 6-variable MDRD, age is given in years, albumin in g/dL, Scr is serum creatinine (mg/dL), SUN is serum urea nitrogen concentration (mg/dL), κ is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of serum creatinine/κ or 1.
Continuous variables were summarized as mean ± SD unless otherwise stated. We initially compared the demographic data and laboratory variables of survivors and non-survivors using Student’s t test and χ2 test. To formally examine the relationship among different means of eGFR and MELD as predictors of in-hospital mortality, several multivariate models were constructed. MELD score was calculated according to the original description: MELD = 11.2 LN (INR) + 3.78 LN (bilirubin) + 9.57 LN (creatinine) + 6.43[15].
After adjustment with the original MELD equation by logistic regression analysis (bilirubin and INR were set at 1.0 for values less than 1.0), new MELD equations which incorporate eGFR to replace serum creatinine were constructed and listed below:
MELD-MDRD-4 = 8.82 LN (INR) + 4.07 LN (bilirubin) + (-5.13) LN [eGFR (MDRD-4)] + 30.57,
MELD-MDRD-6 = 8.78 LN (INR) + 383 LN (bilirubin) + (-5.14) LN [eGFR (MDRD-6)] + 30.05,
MELD-CKD-EPI = 8.80 LN (INR) + 4.01 LN (bilirubin) + (-5.37) LN [eGFR (CKD-EPI)] + 31.93.
The new MELD equations were rounded to the nearest integer for easy use. Unlike the original MELD equation, there was no preinstall upper limit in these new equations.
Logistic regression analysis were conducted for investigating the odds ratios (OR) of predicting in-hospital mortality by different models, different new MELD equations, and MELD equations in patients with different eGFR levels. The difference in different MELD equations in predicting in-hospital mortality was investigated by logistic regression analysis. The results of these analyses were used to construct a receiver-operating characteristic (ROC) curve from which we sought the optimum cutoff point for predicting successful sites. The optimum cutoff point was defined as the point on the ROC curve closest to the point (0, 1), where the false-positive rate was zero and the sensitivity was 100%. The area under the curve and 95% CI were calculated. A P value of less than 0.05 was considered statistically significant. All statistical analysis were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL, United States).
Table 1 presents the clinical characteristics, demographic data and laboratory data of the study subjects. Patients defined as survivors were significantly younger, had a lower incidence of hepatoma, lower Child-Pugh and MELD scores, and better renal function. The underlying causes of cirrhosis were very different from those in Western countries. In Taiwan, most cirrhotic patients were associated with the hepatitis virus, especially hepatitis B. Diagnoses such as non-alcoholic steatohepatitis or cholestatic liver disease were seldom confirmed and were classified as unknown.
| Parameter | All patients (n = 3857) | Survivors (n = 2375) | Non-survivors (n = 1482) | P value |
| Age, yr | 60.73 ± 14.05 | 59.02 ± 14.02 | 63.48 ± 13.65 | < 0.001 |
| Male, n (%) | 2665 (69.1) | 1651 (69.52) | 1014 (68.42) | NS (0.474) |
| Hepatoma, n (%) | 1385 (35.9) | 653 (27.5) | 732 (49.4) | < 0.001 |
| Cause of liver cirrhosis, n (%) | < 0.001 | |||
| Hepatitis C | 930 (24.1) | 568 (23.9) | 362 (24.4) | - |
| Hepatitis B | 1090 (28.3) | 631 (26.6) | 459 (31) | - |
| Alcoholic | 813 (21.1) | 580 (24.4) | 233 (15.7) | - |
| Hepatitis C + hepatitis B | 106 (2.7) | 70 (2.9) | 36 (2.4) | - |
| Hepatitis C + alcohol | 60 (1.6) | 39 (1.6) | 21 (1.4) | - |
| Hepatitis B + alcohol | 191 (5) | 127 (5.3) | 64 (4.3) | - |
| Hepatitis C + hepatitis B + alcohol | 33 (0.9) | 19 (0.8) | 14 (0.9) | - |
| Not hepatitis C, hepatitis B or alcohol | 634 (16.4) | 341 (14.4) | 293 (19.8) | - |
| Ascites, n (%) | 1861 (48.2) | 825 (34.7) | 1036 (69.9) | < 0.001 |
| Hepatic encephalopathy, n (%) | 1097 (28.4) | 434 (18.3) | 663 (44.7) | < 0.001 |
| Child–Pugh points | 8.36 ± 2.57 | 7.11 ± 1.97 | 10.37 ± 2.1 | < 0.001 |
| MELD score | 18.9 ± 10.26 | 13.15 ± 5.57 | 27.98 ± 9.36 | < 0.001 |
| Albumin, 3.5-5 g/dL | 2.95 ± 0.73 | 3.24 ± 0.68 | 2.49 ± 0.55 | < 0.001 |
| Total bilirubin, 0.3-1.2 mg/dL | 5.18 ± 8.16 | 2.24 ± 3.57 | 9.89 ± 10.81 | < 0.001 |
| INR | 1.89 ± 1.75 | 1.43 ± 0.43 | 2.72 ± 2.55 | < 0.001 |
| BUN, 8-12 mg/dL | 34.47 ± 35.55 | 17.63 ± 15.52 | 61.44 ± 41.49 | < 0.001 |
| Creatinine, 0.4-1.2 mg/dL | 1.94 ± 1.91 | 1.27 ± 1.35 | 3.01 ± 2.17 | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (MDRD-4) | 63.17 ± 46.12 | 79.14 ± 37.4 | 37.57 ± 47.24 | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (MDRD-6) | 54.87 ± 38.25 | 70.85 ± 33.29 | 29.32 ± 31.16 | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (CKD-EPI) | 65.39 ± 37.49 | 82.11 ± 30.28 | 38.59 ± 31.99 | < 0.001 |
There were 16 parameters included in univariate logistic regression analysis to predict in-hospital mortality. Those with a significant predicting value are listed in Table 2 and were further evaluated by multivariate logistic regression analysis. Both eGFR (MDRD-4) and eGFR (MDRD-6), rather than creatinine, were significant predictors of in-hospital mortality.
| Parameter | Beta coefficient | Standard error | Odds ratios (95% CI) | P value |
| Univariate logistic regression analysis | ||||
| Age, yr | 0.02 | 0.00 | 1.02 (1.02-1.03) | < 0.001 |
| Hepatoma | 0.95 | 0.07 | 2.57 (2.25-2.95) | < 0.001 |
| Ascites | 1.47 | 0.07 | 4.36 (3.80-5.02) | < 0.001 |
| Hepatic encephalopathy | 1.29 | 0.07 | 3.62 (3.13-4.19) | < 0.001 |
| Cause of liver cirrhosis (reference group: NBNCNA) | ||||
| Hepatitis C | -0.30 | 0.10 | 0.74 (0.61-0.91) | 0.004 |
| Hepatitis B | -0.17 | 0.10 | 0.85 (0.70-1.03) | NS (0.098) |
| Alcoholic | -0.76 | 0.11 | 0.47 (0.38-0.58) | < 0.001 |
| Hepatitis C + hepatitis B | -0.51 | 0.22 | 0.60 (0.39-0.92) | 0.020 |
| Hepatitis C + alcoholic | -0.53 | 0.17 | 0.59 (0.42-0.82) | 0.002 |
| Hepatitis B + alcoholic | -0.15 | 0.36 | 0.86 (0.42-1.74) | NS (0.670) |
| Hepatitis C + hepatitis B + alcoholic | -0.47 | 0.28 | 0.63 (0.36-1.09) | NS (0.098) |
| Child–Pugh points | 0.67 | 0.02 | 1.95 (1.87-2.03) | < 0.001 |
| MELD score | 0.22 | 0.01 | 1.25 (1.23-1.27) | < 0.001 |
| BUN, mg/dL | 0.07 | 0.00 | 1.07 (1.06-1.07) | < 0.001 |
| Creatinine, mg/dL | 0.72 | 0.03 | 2.05 (1.93-2.18) | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (MDRD-4) | -0.04 | 0.00 | 0.97 (0.96-0.97) | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (MDRD-6) | -0.05 | 0.00 | 0.95 (0.95-0.96) | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (CKD-EPI) | -0.04 | 0.00 | 0.97 (0.96-0.96) | < 0.001 |
| Albumin, g/dL | -1.97 | 0.07 | 0.14 (0.12-0.16) | < 0.001 |
| Total bilirubin, mg/dL | 0.20 | 0.01 | 1.22 (1.20-1.25) | < 0.001 |
| INR | 1.74 | 0.08 | 5.70 (4.87-6.67) | < 0.001 |
| Multivariate logistic regression analysis | ||||
| Age, yr | 0.03 | 0.01 | 1.03 (1.01-1.04) | < 0.001 |
| Hepatoma | 0.95 | 0.13 | 2.60 (2.02-3.34) | < 0.001 |
| Cause of liver cirrhosis (reference group: NBNCNA) | ||||
| Hepatitis C | -0.19 | 0.18 | 0.83 (0.58-1.18) | NS (0.299) |
| Hepatitis B | -0.28 | 0.19 | 0.76 (0.52-1.10) | NS (0.139) |
| Alcoholic | -0.21 | 0.22 | 0.81 (0.52-1.25) | NS (0.342) |
| Hepatitis C + hepatitis B | -1.03 | 0.40 | 0.36 (0.16-0.78) | 0.010 |
| Hepatitis C + alcoholic | -0.79 | 0.35 | 0.46 (0.23-0.90) | 0.023 |
| Hepatitis B + alcoholic | 0.32 | 0.57 | 1.38 (0.45-4.22) | NS (0.576) |
| Hepatitis C + hepatitis B + alcoholic | 0.54 | 0.47 | 1.71 (0.68-4.29) | NS (0.354) |
| MELD score | 0.14 | 0.03 | 1.14 (1.09-1.20) | < 0.001 |
| BUN, mg/dL | 0.04 | 0.00 | 1.05 (1.04-1.05) | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (MDRD-4) | 0.03 | 0.01 | 1.03 (1.02-1.04) | < 0.001 |
| eGFR, mL/(min∙1.73 m-2) (MDRD-6) | -0.03 | 0.01 | 0.97 (0.95-0.99) | < 0.001 |
| Albumin, g/dL | -1.24 | 0.13 | 0.29 (0.22-0.38) | < 0.001 |
| INR | 0.24 | 0.10 | 1.27 (1.04-1.54) | 0.019 |
Table 3 shows several multivariate models for the prediction of in-hospital mortality. As expected, model 1, containing the MELD score only, was a significant predictor of in-hospital mortality (OR = 1.25, P < 0.001). Model 2 excluded serum creatinine, with the coefficients refit among the remaining 3 variables, i.e., total bilirubin, INR and eGFR (MDRD-4). This model represented an exacerbated outcome over model 1, as suggested by a decrease in χ2 (2161.45 vs 2198.32) and an increase in -2 log (likelihood) (2810.77 vs 2773.90). Model 3 included eGFR (MDRD-6) as one of the variables and showed an improvement over model l, as suggested by an increase in chi-square (2293.82 vs 2198.32) and a decrease in -2 log (likelihood) (2810.77 vs 2664.79). Finally, when serum creatinine was replaced by eGFR (CKD-EPI), it showed a slight improvement compared to model 1 [χ2: 2199.16, -2 log (likelihood): 2773.07].
| Model | Variable | Odds ratio (95% CI) | P value | χ2 | -2 Log Likelihood |
| Model 1 | MELD | 1.25 (1.23-1.27) | < 0.001 | 2198.32 | 2773.90 |
| Model 2 | Total bilirubin1 | 2.25 (2.05-2.48) | < 0.001 | 2161.45 | 2810.77 |
| INR1 | 6.14 (4.56-8.27) | < 0.001 | |||
| eGFR (MDRD-4)1 | 0.22 (0.20-0.25) | < 0.001 | |||
| Model 3 | Total bilirubin1 | 2.17 (1.97-2.40) | < 0.001 | 2293.82 | 2664.79 |
| INR1 | 5.95 (4.39- 8.06) | < 0.001 | |||
| eGFR (MDRD-6)1 | 0.19 (0.17-0.22) | < 0.001 | |||
| Model 4 | Total bilirubin1 | 2.26 (2.05-2.48) | < 0.001 | 2199.16 | 2773.07 |
| INR1 | 6.28 (4.65-8.49) | < 0.001 | |||
| eGFR (CKD-EPI)1 | 0.20 (0.18-0.23) | < 0.001 |
The efficacy of new MELD equations for the prediction of in-hospital mortality is listed in Table 4. Compared to the original MELD equation, only MELD-MDRD-6 showed a better predictive value, as suggested by an increase in chi-square (2254.88 vs 2198.32) and a decrease in -2 log (likelihood) (2703.72 vs 2773.90). In the ROC curve (Figure 1), the MELD-MDRD-6 score showed a marginal improvement in area under the curve (0.909 vs 0.902), sensitivity (0.854 vs 0.819) and specificity (0.818 vs 0.839) compared to the original MELD equation. Table 5 compares the MELD and MELD-MDRD-6 equations in patients with different eGFR. The MELD-MDRD-6 equation showed a better predictive value in patients with eGFR ≥ 90, 60-89, 30-59 and 15-29.
| Equations | Odds ratio (95% CI) | P value | χ2 | -2 Log Likelihood |
| MELD | 1.25 (1.23-1.27) | < 0.001 | 2198.32 | 2773.90 |
| MELD-MDRD-4 | 1.27 (1.26-1.29) | < 0.001 | 2147.93 | 2824.30 |
| MELD-MDRD-6 | 1.29 (1.27-1.31) | < 0.001 | 2254.88 | 2703.72 |
| MELD-CKD-EPI | 1.28 (1.26-1.30) | < 0.001 | 2185.01 | 2787.21 |
| eGFR | Equations | Odds ratio (95% CI) | P value | χ2 | -2 Log Likelihood |
| ≥ 90 | MELD | 1.17 (1.13-1.21) | < 0.001 | 99.45 | 609.51 |
| MELD-MDRD-6 | 1.20 (1.16-1.24) | < 0.001 | 114.55 | 588.67 | |
| 60-89 | MELD | 1.22 (1.18-1.26) | < 0.001 | 185.77 | 636.17 |
| MELD-MDRD-6 | 1.27 (1.22 -1.32) | < 0.001 | 214.60 | 606.45 | |
| 30-59 | MELD | 1.21 (1.17-1.24) | < 0.001 | 283.21 | 755.92 |
| MELD-MDRD-6 | 1.26 (1.22-1.30) | < 0.001 | 312.75 | 725.22 | |
| 15-29 | MELD | 1.25 (1.19-1.30) | < 0.001 | 163.72 | 369.37 |
| MELD-MDRD-6 | 1.31 (1.24-1.39) | < 0.001 | 182.49 | 350.59 | |
| < 15 | MELD | 1.36 (1.27-1.45) | < 0.001 | 190.62 | 311.89 |
| MELD-MDRD-6 | 1.30 (1.23-1.39) | < 0.001 | 164.66 | 337.42 |
This retrospective, cross-sectional study involved a broader population of cirrhotic patients than only data from liver transplant registries. We attempted to incorporate eGFR obtained by different creatinine-based equations into the MELD equation to replace serum creatinine and predict in-hospital mortality. The new equation “MELD-MDRD-6”, which incorporates eGFR obtained by the 6-variable MDRD equation, only marginally improves the predictive value compared to the original MELD score.
The MELD score was initially created to predict survival following the elective transjugular intrahepatic portosystemic shunts procedure[4]. This model was subsequently validated as a predictor of survival in several cohort studies for various severities of liver disease. It is also used to determine the prioritization of transplant recipients in the United States[1,4,5]. The existing MELD equation contains 3 variables, each of which was selected on the basis of statistical analysis: INR and total bilirubin, both markers of liver function, and serum creatinine as the third variable, a marker of renal function. This highlights the prognostic value of renal function in cirrhotic patients. In the existing MELD equation, however, the values of bilirubin, INR and creatinine < 1.0 mg/dL are set to 1.0 mg/dL in order to avoid a negative value after natural logarithmic transformation[16]. Additionally, serum creatinine values > 4.0 mg/dL are capped at 4.0 mg/dL. Setting bilirubin, INR and creatinine levels < 1.0 mg/dL to 1.0 mg/dL implicitly assumes that mortality at 1.0 mg/dL is the same as at levels < 1.0 mg/dL. This assumption is problematic since the increase in serum creatinine from 0.3 mg/dL to 0.6 mg/dL usually reflects a 50% decrease in the eGFR, which could be defined as acute kidney injury. On the other hand, 45.81% of all patients (1767 patients) and 15.52% of all non-survivors (230 patients) had a creatinine value of < 1.0 mg/dL in this study and it is therefore unreasonable to neglect this group.
When incorporating eGFR into the MELD equation to replace serum creatinine, we set bilirubin and INR to 1.0 mg/dL when the value was < 1.0 mg/dL for the purpose of comparison with the original MELD equation. However, there was no adjustment of the eGFR value when using it for reconstructing new formulas. Compared to the original MELD equation, the new MELD equations preserve the “non-negative property” that MELD-MDRD-4 ranged from 1 to 60, MELD-MDRD-6 ranged from 2 to 60, and MELD-CKD-EPI ranged from 1 to 61. Furthermore, the original MELD score classified patients from 6 to 40; we did not preinstall the upper limit when using them to predict in-hospital mortality. Nonetheless, it might be more accurate or easier to preinstall the upper limit in these new MELD equations.
Serum creatinine, a marker of renal function, is a well-recognized predictor of survival in patients with liver disease and outcome after liver transplantation[6,17,18]. It has been suggested that renal function should be routinely monitored in all patients with advanced cirrhosis, especially those with ascites[17]. Although serum creatinine is the most useful and widely accepted indicator for estimating renal function in cirrhotic patients[19], it is less sensitive because of the associated reduced muscle mass, severe hyperbilirubinemia and diminished hepatic biosynthesis of creatinine, as well as the low-protein diet given to such patients[1,6,17]. In addition, the original MELD equation regards serum creatinine of < 1.0 mg/dL as 1.0 mg/dL, which leaves approximately 15% to 20% of patients whose survival cannot be accurately predicted by this score[6,9]. For that reason, we replaced serum creatinine by eGFR in the MELD equation. Cystatin C, in contrast to serum creatinine, is a more accurate surrogate marker of renal function since its serum concentration is independent of muscle mass or gender and can be reliably determined in patients with hyperbilirubinemia[20-22]. Theoretically, including cystatin C in a modified MELD score should increase the predictive performance. However, a clinical study in 429 cirrhotic patients showed that a cystatin C-based MELD score has an equal predictive performance compared to the creatinine-based model[23]. In the view of the high cost of cystatin C, more than 10-fold higher than enzymatic creatinine measurement, eGFR probably is more suitable than cystatin C to be incorporated into the MELD equation clinically.
To evaluate the predictive value of the new MELD equation in cirrhotic patients with normal renal function, we grouped patients into 5 groups according to their eGFR (Table 5). MELD-MDRD-6 was more accurate than the original MELD when eGFR was > 15 mL/(min∙1.73 m2) but not when it was < 15 mL/(min∙1.73 m2). There might be 3 reasons for this. Firstly, the MDRD equation tends to overestimate the GFR, especially when GFR was < 40 mL/(min∙1.73 m2)[24]. Secondly, patients receiving renal replacement therapy, whose eGFR is usually < 15 mL/(min∙1.73 m2), were not excluded in the present study. Thirdly, we did not preinstall the upper limit of these new equations which may make a difference for the predictive value.
How about incorporating the measured GFR into the MELD equation? Direct measurement of GFR using exogenous markers remains the major method to assess renal function in cirrhotic patients[1]. In these patients, inulin clearance has been considered the “gold standard” for measuring GFR. Although one study has shown that measured GFR is superior to both serum creatinine and eGFR at predicting outcome in cirrhotic patients[10], this technique requires a continuous intravenous infusion, takes more time for urine collections, is costly and potentially invasive. It is therefore impractical for the repeated assessments of renal function[1,7,17].
Theoretically, estimated GFR calculated by the creatinine-based equations should show a similar prognostic value to serum creatinine. However, both the Cockcroft-Gault and MDRD equations tend to overestimate GFR in patients with cirrhosis; a series has shown that only 66% of estimates were within 30% of the measured GFR[12,24-26]. The Cockcroft-Gault equation is thought to be less accurate than the MDRD equation since it incorporates body weight, which is markedly biased in patients with edema and/or ascites[25]. The MDRD-4 (simplified MDRD) equation is most often used to calculate GFR because it is considered to be as accurate as the original MDRD-6 equation[27]. However, its usefulness has not been proved in healthy individuals and its accuracy may be low in specific clinical settings[26,28]. Therefore, the MDRD-6 equation is considered the best, possibly because it incorporates blood urea nitrogen (BUN) and albumin levels, 2 variables which are abnormal in cirrhotic patients[28]. The CKD-EPI equation, a newly developed equation for estimating GFR, has been proposed as more accurate than the MDRD equation, especially when GFR is high. It shows less bias, improved precision and greater accuracy[14]. However, it also has not been used in patients with cirrhosis. In the present study, despite the fact that MELD-CKD-EPI showed a better prognostic value than MELD-MDRD-4, it was not better than the original MELD equation or MELD-MDRD-6. Our data showed that MELD-MDRD-6 has the better predictive value for in-hospital mortality compared to other equations. We suppose that it may be associated with the insertion of BUN and albumin as variables; in particular, serum albumin is an excellent predictor of mortality.
Findings about incorporated eGFR into the MELD equation to predict in-hospital mortality, however, need to be interpreted with caution. On the one hand, although statistically significant, the value added from MDRD-6 was limited (increase in the ROC from 0.902 to 0.909). This limited value may not add much to a treatment or decision algorithm or in predicting events. On the other hand, we did not further classify in-hospital mortality according to the causes since the predicted value might be different in different outcomes. Furthermore, the results here might not suitable for patients on the liver transplant waiting list or who are followed up long-term.
It was suggested the presence of diabetes increases the 5 year mortality rate up to 2.52-fold in cirrhotic patients[29]. The previous reports have reported that up to 96% of cirrhotic patients may have glucose intolerance and 30% could be clinically diagnosed as diabetes[30-32]. The etiology of cirrhosis is frequently associated with the prevalence of diabetes, such as non-alcoholic fatty liver disease, alcoholic hepatitis, hepatitis C virus infection and hemochromatosis[33]. Hepatitis C infection could down-regulate insulin receptors and enhance insulin resistance. Although hepatitis C related cirrhosis showed significant impact on in-hospital mortality in our series (Table 2), it lost its significance after entering multivariate analysis. Further prospective study is warranted to confirm the impact of hepatitis C related insulin resistance in cirrhotic patients.
The present study has several limitations. Firstly, the construction of new MELD equations was dependent on logistic regression analysis but not on a time-dependent Cox regression model, which is more appropriate for evaluating patients with continuously changing laboratory data. Secondly, the existing creatinine-based eGFR equations were not constructed for cirrhotic patients. Thus, a specific formula for incorporation into the MELD equation needs to be derived for calculating GFR in these patients in order to provide prognostic values with better accuracy. Thirdly, the study was retrospective and cross-sectional in nature and therefore a prospective cohort study is warranted to test and verify our conclusions.
In conclusion, renal function is an important prognostic factor for patients with cirrhosis and therefore the MELD score showed a good correlation with mortality risk in the patients included in the present study. However, the unreliability of serum creatinine in measuring renal function and the problematic assumption of serum creatinine in the MELD score makes it inaccurate when evaluating cirrhotic patients with early renal function impairment. Although incorporated estimated GFR obtained by the 6-variable MDRD equation into the MELD equation showed an improvement in predicting in-hospital mortality statistically, clinical superiority is negligible. Thus, the important issue is how to better assess true GFR when evaluating renal function in cirrhotic patients.
Serum creatinine is an unreliable marker for renal function in cirrhotic patients; therefore, the creatinine-based end-stage liver disease (MELD) score may be inaccurate for evaluating cirrhotic patients with normal or mild impaired renal function.
A specific formula derived for calculating glomerular filtration rate (GFR) in cirrhotic patients is warranted.
Incorporated estimated GFR (eGFR) which is obtained from the 6-variable diet in renal disease [modification of diet in renal disease (MDRD)] equation into the MELD formula has an equal predictive performance to the original creatinine-based MELD formula.
eGFR which is obtained by the 6-variable MDRD equation could replace serum creatinine in the MELD score.
MELD score: A scoring system for assessing the severity of chronic liver disease, useful in determining prognosis and prioritizing for liver transplant; MDRD: The most widely used equation to calculate GFR.
This is a unique paper that investigated whether bone marrow derived cells can contribute to liver fibrosis. The results are easy to understand and very persuasive, although number of mice using the analysis was limited.
Peer reviewer: Takumi Kawaguchi, MD, PhD, Assistant Professor, Department of Digestive Disease Information and Research, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan
S- Editor Song XX L- Editor Roemmele A E- Editor Li JY
| 1. | Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol. 2010;52:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Chen YW, Wu CJ, Wang TE, Chang CW, Chang CW, Chen HH. The mortality survey of older patients with cirrhosis in Taiwan--a single-center experience. J Am Geriatr Soc. 2010;58:2230-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Abad-Lacruz A, Cabré E, González-Huix F, Fernández-Bañares F, Esteve M, Planas R, Llovet JM, Quer JC, Gassull MA. Routine tests of renal function, alcoholism, and nutrition improve the prognostic accuracy of Child-Pugh score in nonbleeding advanced cirrhotics. Am J Gastroenterol. 1993;88:382-387. [PubMed] |
| 4. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 5. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1865] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 6. | Chen YW, Wu CJ, Chang CW, Lee SY, Sun FJ, Chen HH. Renal function in patients with liver cirrhosis. Nephron Clin Pract. 2011;118:c195-c203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Thomas L, Huber AR. Renal function--estimation of glomerular filtration rate. Clin Chem Lab Med. 2006;44:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2001] [Cited by in RCA: 2074] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 9. | Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1229] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 10. | Lim YS, Larson TS, Benson JT, Kamath PS, Kremers WK, Therneau TM, Kim WR. Serum sodium, renal function, and survival of patients with end-stage liver disease. J Hepatol. 2010;52:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [PubMed] |
| 12. | MacAulay J, Thompson K, Kiberd BA, Barnes DC, Peltekian KM. Serum creatinine in patients with advanced liver disease is of limited value for identification of moderate renal dysfunction: are the equations for estimating renal function better? Can J Gastroenterol. 2006;20:521-526. [PubMed] |
| 13. | Chen YW, Chen HH, Wang TE, Chang CW, Chang CW, Wu CJ. Difference between CKD-EPI and MDRD equations in calculating glomerular filtration rate in patients with cirrhosis. World J Gastroenterol. 2011;17:4532-4538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] |
| 15. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 16. | Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 542] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 18. | González E, Rimola A, Navasa M, Andreu H, Grande L, García-Valdecasas JC, Cirera I, Visa J, Rodés J. Liver transplantation in patients with non-biliary cirrhosis: prognostic value of preoperative factors. J Hepatol. 1998;28:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] |
| 20. | Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, Padrini R, Palatini P. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002;48:850-858. [PubMed] |
| 21. | Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 440] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Randers E, Kristensen JH, Erlandsen EJ, Danielsen H. Serum cystatin C as a marker of the renal function. Scand J Clin Lab Invest. 1998;58:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Finkenstedt A, Dorn L, Edlinger M, Prokop W, Risch L, Griesmacher A, Graziadei I, Vogel W, Zoller H. Cystatin C is a strong predictor of survival in patients with cirrhosis: is a cystatin C-based MELD better? Liver Int. 2012;32:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Cholongitas E, Shusang V, Marelli L, Nair D, Thomas M, Patch D, Burns A, Sweny P, Burroughs AK. Review article: renal function assessment in cirrhosis - difficulties and alternative measurements. Aliment Pharmacol Ther. 2007;26:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [PubMed] |
| 27. | Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 851] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 28. | Trombetta M, Spiazzi G, Zoppini G, Muggeo M. Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study. Aliment Pharmacol Ther. 2005;22 Suppl 2:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Kawaguchi T, Taniguchi E, Itou M, Sakata M, Sumie S, Sata M. Insulin resistance and chronic liver disease. World J Hepatol. 2011;3:99-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 30. | García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8:13-20. [PubMed] |
| 31. | Chen YW, Chen HH, Wang TE, Chang CW, Chang CW, Chen WC, Wu CJ. The dissociation between the diabetes and both Child-Pugh score and in-hospital mortality in cirrhotic patients due to hepatitis B, hepatitis C, or alcoholic. Hepatol Int. 2011;5:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 236] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (6)] |
| 33. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 432] [Article Influence: 20.6] [Reference Citation Analysis (0)] |