Published online Oct 27, 2012. doi: 10.4254/wjh.v4.i10.284
Revised: September 15, 2012
Accepted: October 26, 2012
Published online: October 27, 2012
Biliary atresia (BA) is one of the major hepatobiliary abnormalities in infants and one of the causes of hepatic osteodystrophy. Bone disease may be caused by the malabsorption of calcium and magnesium by vitamin D in hepatobiliary diseases in which bile flow into the intestines is deficient or absent. Bone fracture before Kasai hepatic portoenterostomy or within one month after the procedure in an infant with BA is very rare. We herein report two infants: one infant with BA who initially presented with a bone fracture before Kasai hepatic portoenterostomy, and the other at 4 wk after Kasai hepatic portoenterostomy, and also provide a review of the literature. Moreover, we conclude that clinicians should consider BA in infants with bone fracture during early infancy.
- Citation: Okada T, Honda S, Miyagi H, Minato M, Taketomi A. Hepatic osteodystrophy complicated with bone fracture in early infants with biliary atresia. World J Hepatol 2012; 4(10): 284-287
- URL: https://www.wjgnet.com/1948-5182/full/v4/i10/284.htm
- DOI: https://dx.doi.org/10.4254/wjh.v4.i10.284
Clinical findings in children with biliary atresia (BA) characteristically include jaundice and acholic stools at 1 or 2 mo after birth[1]. Osteodystrophy is a well-recognized complication of chronic liver disease. BA is one of the major hepatobiliary abnormalities in infants and one of the causes of hepatic osteodystrophy[1].
Vitamin D is hydroxylated at the carbon 25 position to form 25-hydroxy-vitamin D (25-OH-D)[2]. This occurs primarily in the liver[2]. Bile is important for the intestinal absorption of calcium and magnesium because it is necessary for the absorption of vitamin D[1].
In chronic liver disease, particularly where there is chronic cholestasis, generalized skeletal demineralization or rachitic change is seen[3]. Multiple spontaneous fractures of both the ribs and long bones have been reported in such infants. Furthermore, bone fractures are sometimes noted in patients with BA in the end-stage before liver transplantation[4]. However, bone fracture before Kasai hepatic portoenterostomy and within one month after the procedure in infants with BA is very rare.
We report two infants: firstly, a patient with BA who initially presented with bone fracture before Kasai hepatic portoenterostomy, and secondly, a patient with the onset of bone fracture within one month after Kasai hepatic portoenterostomy, and also provide a review of the literature.
A girl was born vaginally at 39 wk gestation, weighing 2522 g. She presented with neither jaundice nor acholic stools. The infant was fed human milk. She was well nourished but was observed to have jaundice at a medical check-up at 1 mo of age. Abdominal ultrasonography (US) and computed tomography showed a sufficiently large gallbladder. Total and direct bilirubin (DB) decreased gradually at the follow-up checks. The patient presented with acholic stools and increased jaundice at the age of 5 mo, and was subsequently admitted to our institution for further examinations. Laboratory studies upon admission revealed the following: asparate aminotransferase (AST) 337 IU/L (normal range), alanine aminotransferase (ALT) 241 IU/L (normal range), total bilirubin (TB) 11.3 mg/dL, DB 7.4 mg/dL, alkaline phosphatase (ALP) 5,547 IU/L (normal range), γ-glutamyl transpeptidase (γ-GTP) 457 IU/L (normal range), choline esterase 192 IU/L (normal range), and serum calcium 8.1 mg/dL (normal range). There was severe jaundice noted at the conjunctiva. The findings on abdominal US were unevenness on the liver surface and an atrophic gallbladder which did not contract after the feeding of milk. Magnetic resonance cholangio-pancreatography (MRCP) revealed dilatation of neither the common bile duct nor intrahepatic bile duct. Therefore, BA was suspected based on these findings, and the infant underwent an exploratory laparotomy at 182 d of age. The patient started oral vitamin D at 173 d of age.
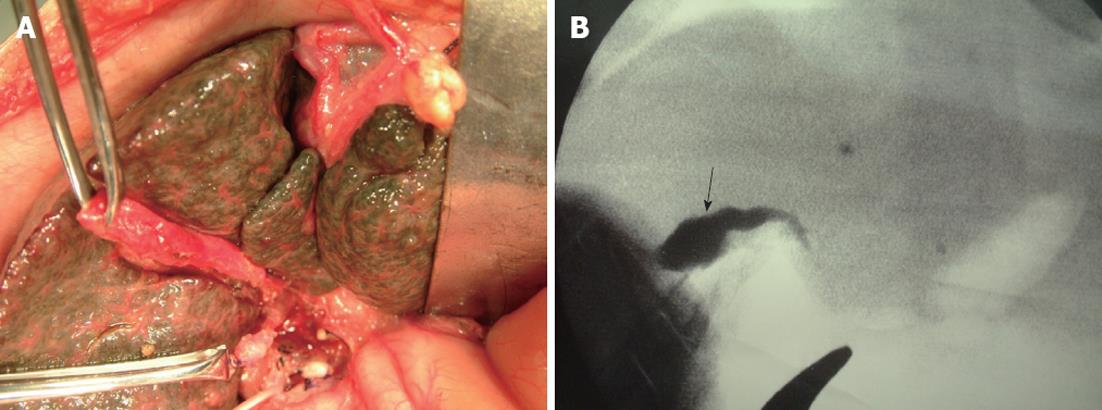
On laparotomy, the liver was brown and firm with a dull edge, suggesting cholestasis (Figure 1A). Intraoperative cholangiography revealed a patent gallbladder and no patency of the extrahepatic bile duct (Figure 1B). The macroscopic findings showed that the bilateral hepatic ducts and extrahepatic bile duct consisted of only remnants. The infant was diagnosed as BA (IIb1γ)[5] based on cholangiographic and macroscopic findings. The remnants were totally removed en block and a Roux-en-Y hepaticojejunostomy was performed with a Roux loop of 60 cm applied antecolically. Microscopic findings of the liver biopsy specimen were pre-cirrhotic.
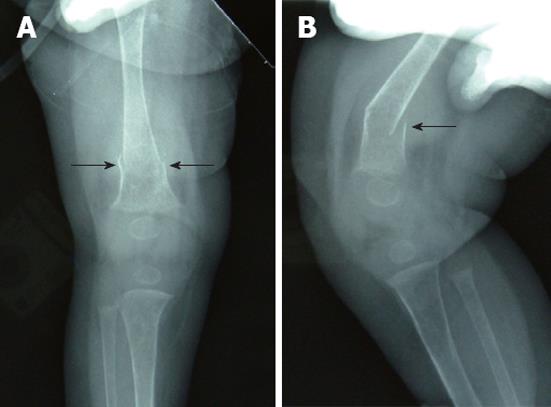
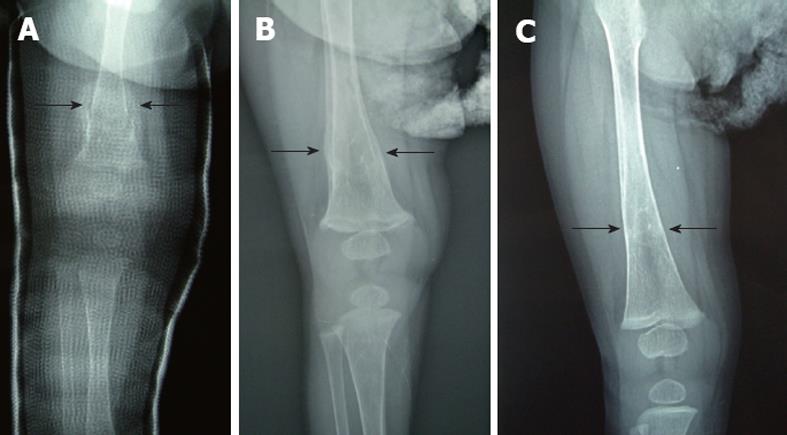
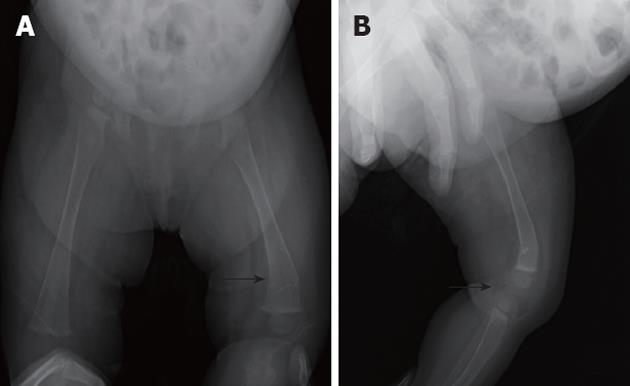
The patient could not move her right leg 1 d before the laparotomy, and a plain skeletal radiograph of the femur was performed 7 d after the HJ, when the general condition of the patient was stable. A displaced fracture of the right distal femur was shown by the plain radiograph (Figure 2A and B). Hepatic osteodystrophy was suspected based on the fact that there was no history of femur trauma and the patient suffered from chronic cholestasis. Child abuse by the family was not considered from the situation. Callus formation was seen 8 d after the application of an immobilizing plaster bandage (Figure 3A). The plaster bandage was removed after 20 d and the fracture of the right femur was cured at 6 mo post fracture (Figure 3B and C). The patient coughed up blood due to the perforation of esophageal varices and underwent a living-related liver transplantation at 10 mo of age. The postoperative course of living-related liver transplantation was uneventful and she is currently well at 4 years of age.
A girl was born vaginally at 36 wk gestation, weighing 2310 g. She presented with neither jaundice nor acholic stools. She was well nourished but was observed to have jaundice at a medical check-up at 1 mo of age. The patient presented with acholic stools and increased jaundice at the age of 3 mo at a medical check-up, and was consequently admitted to our institution for further examinations. Laboratory studies upon admission revealed the following: AST 573 IU/L, ALT 377 IU/L, TB 6.6 mg/dL, DB 4.4 mg/dL, ALP 2248 IU/L, γ-GTP 666 IU/L, choline esterase 181 IU/L, and serum calcium 9.2 mg/dL. The findings on abdominal US and MRCP were just as same as those of the case 1. Therefore, BA was suspected, and the infant underwent an exploratory laparotomy at 113 d of age. The patient started oral vitamin D at 3 mo of age.
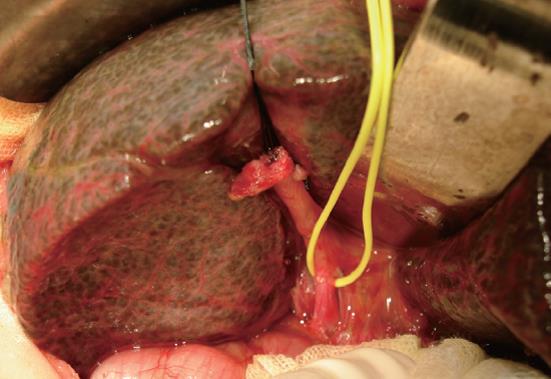
On laparotomy, the liver was brown and firm with a dull edge, suggesting cholestasis (Figure 4). Intraoperative cholangiography revealed a patent gallbladder and no patency of the extrahepatic bile duct. The infant was diagnosed as BA (IIb1γ)[5] based on cholangiographic and macroscopic findings. The remnants were totally removed en block and a Roux-en-Y hepaticojejunostomy was performed with a Roux loop of 60 cm applied antecolically. Microscopic findings of the liver biopsy specimen were cirrhotic.
The patient could not move her left leg at 28 d post-laparotomy. A displaced fracture of the left distal femur was shown by plain skeletal radiograph (Figure 5A and B). Hepatic osteodystrophy was suspected based on the fact that there was no history of femur trauma and the patient suffered from chronic cholestasis. Child abuse by the family was not considered from the situation. Callus formation was seen 14 d after the application of an immobilizing plaster bandage. The plaster bandage was removed after 20 d and the fracture of the left femur was cured at 6 mo after post-fracture. Jaundice has been resolved and she is currently well at 11 mo of age.
BA is a rare disease with an incidence of approximately 1:10 000 live births in Japan and the Far East[6]. The most frequent symptom is prolonged jaundice. Several reports have shown that osteodystrophy was associated with severe chronic liver disease despite the administration of vitamin and mineral supplements[1]. Argao et al[7] suggested that the bone mineral content of patients with hepatic osteodystrophy did not improve despite successful normalization of the serum 25-OH vitamin D concentration by enhancing vitamin D absorption from the gastrointestinal tract. Chongsrisawat et al[8] reported that osteoporosis was recognized in up to 80% of a group of jaundiced BA patients in comparison with only 13.6% in a non-jaundiced group.
In BA, metabolic disturbance results from impairment of the passage of bile salts into the alimentary canal. As a consequence, the inadequate emulsification of fat results in the incomplete absorption of vitamin D. Vitamin D is hydroxylated to 25-OH-D in the liver[2]. Additionally, over the course of the disease, liver cirrhosis develops and the hydroxylation of vitamin D is impaired. Vitamin D and hence calcium absorption are thus diminished. 25-OH-D is thought to be converted to more active forms, 125- or 2125-dehydro-OH-D. Rickets and osteoporosis were reported to be found in 23 of 39 patients (59%) with surgically unrepaired BA[1].
We herein report two infants: one infant with BA who initially presented with a bone fracture before Kasai hepatic portoenterostomy, and the other at 4 wk after Kasai hepatic portoenterostomy. There are a number of factors which may be important in the etiology of bone fractures in children, including trauma, metabolic bone disease, drugs, and immobilization[3]. However, the lack of significant trauma in the majority of cases (91%) is a notable feature in children with BA[3]. Hill et al[3] reported 12 (19%) children with fractures before and after transplantation out of 63 undergoing liver transplanatation. Eight of 12 children with fractures in BA had no identifiable trauma. The age at the time of fracture in BA ranged from 3 to 16 mo after birth, and the affected children suffered from osteopenia (generalized reduction in bone density). The fracture site was the ribs or long bones, and multiple fractures were seen in 2 children with BA (7 and 8 mo after birth). However, Hill et al[3] did not describe administering vitamin D supplements. BA patients with severe cholestasis have a risk of bone fracture despite the administration of essential vitamins and minerals such as our cases. In our cases, BA was diagnosed at 6 mo after birth in case 1 and at 3 mo after birth in case 2, with suspected severe cholestasis.
Conservative management such as immobilization using plaster bandages is generally effective for fractures in BA, and there were no complications related to fractures in our cases. In the literature, internal fixation was required in one case with oxalosis for a fractured neck of the femur[1]. The early diagnosis and treatment of BA before the occurrence of bone fracture is important. The measurement of reflected light from the surface of feces by near infrared reflectance spectroscopy was introduced by Akiyama et al[9] for the differential diagnosis of cholestatic diseases in infants. Another method, mass screening using color picture cards depicting normal and acholic stools, was carried out at 1 and 2 mo after birth in a Japanese prefecture[10]. Eight cases of BA were detected using this mass screening method during a 3-year period, with a specificity of 99.9% and a sensitivity of 80.0%. Such screening procedures could result in improved detection of BA in infants before bone disorders occur.
In summary, clinical awareness of BA should be maintained both in terms of careful handling to prevent possible bone fracturing and also in considering fractures as a possible diagnostic factor in children with reluctance to use a limb, even in the absence of previous trauma, before Kasai hepatic portoenterostomy. Radiological awareness is also important to avoid missing unsuspected fractures on radiographs.
Peer reviewers: Qiang Liu, PhD, Vaccine and Infectious Disease Organization, University of Saskatchewan, 120 Veterinary Road, Saskatoon, Saskatchewan, S7N 5E3, Canada; Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, via A. Manzoni 113, 20089 Rozzano, Milan, Italy
S- Editor Song XX L- Editor Webster JR E- Editor Yan JL
| 1. | Kobayashi A, Kawai S, Utsunomiya T, Obe Y. Bone disease in infants and children with hepatobiliary disease. Arch Dis Child. 1974;49:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Toki A, Todani T, Watanabe Y, Sato Y, Ogura K, Yoshikawa M, Yamamoto S, Wang ZQ. Bone mineral analysis in patients with biliary atresia after successful Kasai procedure. Tohoku J Exp Med. 1997;181:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Hill SA, Kelly DA, John PR. Bone fractures in children undergoing orthotopic liver transplantation. Pediatr Radiol. 1995;25 Suppl 1:S112-S117. [PubMed] |
| 4. | Katsura S, Ogita K, Taguchi T, Suita S, Yoshizumi T, Soejima Y, Shimada M, Maehara Y. Effect of liver transplantation on multiple bone fractures in an infant with end-stage biliary atresia: a case report. Pediatr Surg Int. 2005;21:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Kasai M, Sawaguchi S, Akiyama T, Saito J, Suruga K, Kira J, Ueta T, Okamoto E, Kimura S, Ikeda K. A proposal of new classification of biliary atresia. J Jpn Soc Pediatr Surg. 1976;12:327-331. |
| 6. | Hashizume K, Nakajo T, Naito H, Naito T, Aso S, Aso K, Omiya T, Kamamorita K. Hemorrhagic disease of the infant accompanied with biliary atresia. J Jpn Soc Pediatr Surg. 1980;16:561-568. |
| 7. | Argao EA, Specker BL, Heubi JE. Bone mineral content in infants and children with chronic cholestatic liver disease. Pediatrics. 1993;91:1151-1154. [PubMed] |
| 8. | Chongsrisawat V, Ruttanamongkol P, Chaiwatanarat T, Chandrakamol B, Poovorawan Y. Bone density and 25-hydroxyvitamin D level in extrahepatic biliary atresia. Pediatr Surg Int. 2001;17:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Akiyama T, Yamauchi Y. Use of near infrared reflectance spectroscopy in the screening for biliary atresia. J Pediatr Surg. 1994;29:645-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Maki T, Sumasaki R, Matsui . A mass screening for biliary atresia. Jpn J Pediatr Surg. 1999;31:242-246. |