Published online Jul 27, 2011. doi: 10.4254/wjh.v3.i7.175
Revised: May 6, 2011
Accepted: May 13, 2011
Published online: July 27, 2011
In this review we outline the different mechanisms mediating hepatocarcinogenesis. We also discuss possible targets of bioactive herbal agents at different stages of hepatocarcinogenesis and highlight their role at each individual stage. We gathered information on the most common herbal prescriptions and extracts thought to be useful in prevention or sensitization for chemotherapy in management of hepatocellular carcinoma (HCC). The value of this topic may seem questionable compared to the promise offered for HCC management by chemotherapy and radiation. However, we would recommend the use of herbal preparations not as alternatives to common chemo /and or radiotherapy, but rather for prevention among at-risk individuals, given that drug/herb interactions are still in need of extensive clarification. The bioactive constituents of various herbs seem to be promising targets for isolation, cancer activity screening and clinical evaluation. Finally, herbal preparations may offer a cost effective protective alternative to individuals known to have a high risk for HCC and possibly other cancers, through maintaining cell integrity, reversing oxidative stress and modulating different molecular pathways in preventing carcinogenesis.
- Citation: Abdel-Hamid NM, Nazmy MH, Mahmoud AW, Fawzy MA, Youssof M. A survey on herbal management of hepatocellular carcinoma. World J Hepatol 2011; 3(7): 175-183
- URL: https://www.wjgnet.com/1948-5182/full/v3/i7/175.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i7.175
Hepatocellular carcinoma (HCC) is the third deadliest and fifth most common malignancy worldwide[1-3]. It is a highly malignant tumor having high morbidity and motality. HCC has a poor prognosis due to its rapid infiltrating power which leads to complicating liver cirrhosis[4]. The rate of HCC is increasing worldwide between 3% and 9% annually[5,6]. The incidence ranges from less than 10 cases per 100 000 in North America and Western Europe to 50-150 cases per 100 000 in parts of Africa and Asia[7]. Hepatocarcinogenesis is associated with a background of chronic and persistent infection of hepatitis B virus (HBV) and hepatitis C virus (HCV)[8]. These infections along with alcohol and aflatoxin B1 exposure are widely recognized etiological agents in HCC[9].
In Egypt, epidemiology of HCC is characterized by marked demographic and geographic variations[10,11]. Over the last decade, a remarkable increase, from 4.0% to 7.2%, was observed in the proportion of chronic liver disease (CLD) patients with HCC. The predominant age group (40-59 years) showed a slight increase compared with older groups (> 60 years). A significant increase, from 82.5% to 87.6%, was observed in the proportion of HCC among males. The calculated risk of HCC development is nearly three times higher in men than in women[12]. A unique invisible risk factor for development of HCC in Egypt could be Schistosomal infection and its injection therapy. Schistosomiasis induces immune suppression, which could result in increased persistence of viremia following acute infection of both hepatitis B and C[13].
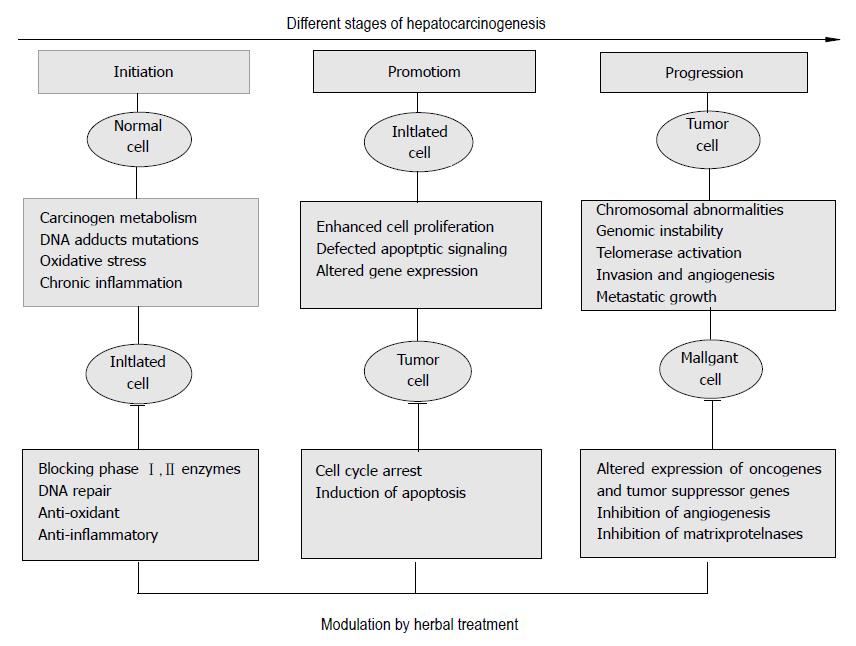
HCCs are phenotypically (morphology and microscopy) and genetically heterogenous tumors, possibly reflecting the heterogeneity of etiological factors implicated in HCC development, the complexity of hepatocyte functions and the late stage at which HCCs usually become clinically symptomatic and detectable[14,15]. Hepatocarcinogenesis is a multi-factor, multi-step and complex process[8]. It involves three distinguishable but closely connected stages: initiation (normal cell → transformed or initiated cell), promotion (initiated cell → preneoplastic cell), and progression (preneoplastic cell → neoplastic cell)[16]. Malignant transformation of hepatocytes may occur, regardless of the etiological agent, through a pathway of increased liver cell turnover, induced by chronic liver injury and regeneration in a context of inflammation, immune response, and oxidative DNA damage[17-19].
Since ancient times, natural products, herbs and spices have been used as remedies for various diseases, incluing cancer (Table 1). The term chemoprevention was coined in the late 1970s and referred to a pharmacological intervention aimed to arrest or reverse the process of carcinogenesis[20]. Previous attempts were made to identify agents or combinations which could exhibit any of the following characteristics: (1) prevention of tumor initiation; (2) delay or arrest of the development of tumors; (3) extention of cancer latency periods; (4) reduction in cancer metastasis and mortality; and (5) prevention of recurrence of secondary tumors[21]. Recently, the focus has been directed towards molecular targeting of herbal compounds to identify the mechanism(s) of action of these newly discovered bioactive compounds. Moreover, it has been recognized that single agents may not always be sufficient to provide chemopreventive efficacy and therefore the new concept of combination chemoprevention by multiple agents or by the consumption of “whole foods” has become an increasingly attractive area of study[22]. Steps in the development of cancer at cellular level are described below.
| Compound | Ref. | Composition | Effect |
| Herbs with cancer chemotherapeutic effect | |||
| Geiji-Bokryung-Hwan | [78,79] | It is composed of five different herbs of Cinnamomi Ramulus, Poria Cocos Hoelen (Pachymae Fungus), Moutan Cortex Radicis, Paeoniae Radix, and Persicae Semen. The active constituents are antioxidative phenolic compounds, trans-cinnamic acid, taxifolin, protocatechuic acid, trans-o-hydroxy cinnamic acid, protocatechuic aldehyde, benzoic acid, trans-o-methoxy cinnamic acid, cis-o-methoxy cinnamic acid, 4-hydroxybenzoic acid, coumarin, daucosterol, Paeoniflorin, albiflorin and benzoylalbiflorin, paeonol and paeoniflorin. | The inhibitory effects of Geiji-Bokryung-Hwan (GBH) on the growth of cancer cell lines (HepG2 and Hep3B) and cancer chemopreventive activity were investigated. Tumor inhibition was found to be mediated via the inhibition of COX-1 activity. |
| Ganfujian granules | [80] | Ganfujian granules are an oral preparation consisting of dietary and medicinal Chinese herbs including Chinese yam (Rhizoma Dioscoreae), hawthorn fruit (Fructus Crataegi) and Chinese date (FructusZiziphi Jujubae). The active constituents are flavonoids including oligomeric procyanidins (OPCs), vitexin, vitexin 4'-O-rhamnoside, quercetin, and hyperoside | The herb was found to reduce and delay the incidence of diethylnitrosamine-induced hepatocarcinoma by exerting direct or indirect effects on the cell cycle and inhibiting uncontrolled proliferation of rat hepatocytes. |
| Maharishi amrit kalash | [81] | Maharishi Amrit Kalash (MAK) is composed of a mixture of two herbal mixtures, MAK-4 and MAK-5. The active constituents are multiple antioxidants including alpha-tocopherol, beta-carotene, ascorbate, bioflavonoid, catechin, polyphenols, riboflavin and tannic acid. | MAK was found to inhibit liver carcinogenesis when given as supplement to diet. The authors of this study suggested that the mechanism of this inhibition involved the prevention of excessive oxidative damage. |
| Scutellaria baicalensis and Bupleurum scorzonerifolfium willd | [43] | Chinese medicinal herbs. The active constituents are antioxidant flavonoids, baicalein, wogonin, neobaicalein, and skullcapflavone. | The these herbs were found to enhance the chemopreventive effect of selenium on N-nitosobis (2-oxopropyl) amine-induced liver cancers in Syrian hamsters. |
| Huqi san (Qi-protecting powder) | [28,82] | Huqi san is composed of eight medicinal herbs including (Ramulus Visci, Radix Astragali seu Hedysari, Radix Curcumae, Radix Salviae Miltiorrhizae). The active constituents are polysaccharides, flavonoids, alkaloids and tanshinones. | The inhibitory effect of Huqi san on rat prehepatocarcinoma, which was induced via diethylinitrosamine (DEN), was investigated. It was found to inhibit the over-expression of c-jun, c-fos, and c-myc oncogenes, which were shown to play an important role in the pathogenesis of hepatocellular carcinoma. Huqi san was also reported to inhibit DEN induced oxyradical formation in cultured hepatocytes, leading to suppression of oxidative DNA damage. |
| Milk thistle | [83,84] | Milk thistle, commonly known as silymarin, is extracted from Silybum marianum. The active constituents are flavonoids from which silibinin and silymarin are the biologically most active compound. | It has been shown that a topical application of silymarin on mice results in complete inhibition of an epidermal carcinogen and prevents the formation of pyrimidine dimers, which are considered to be potential skin cancer agents. |
| Herbs with cancer chemotherapeutic effect | |||
| Songyou Yin | [85] | This herbal extract is composed of a mixture of 5 Chinese medicinal herbs (Salvia miltiorrhiza, Astragalus membranaceus, lycium borbarum crataegus pinnatifida and trionyx sinensis). The active constituents are diterpenoid tanshinones, flavonoids and saponins. | "Songyou Yin" attenuates tumor proliferation and prolongs survival of nude mice bearing hepatocellular tumors without distinct toxicity. These findings suggest that "Songyou Yin" has some potential in the treatment of hepatocellular carcinoma. |
| Millettia reticulata benth | [86] | Millettia reticulata Benth is one of the oldest tonic herbs in traditional Chinese medicine. The active constituents are flavonoid derivatives: (-)-epicatechin, naringenin, 5,7,3',5'tetrahydroxyflavanone, formononetin, isoliquiritigenin, and genistein. | It was demonstrated that Millettia reticulata Benth flavonoid derivatives have a positive inhibitory effect on the viability of human cancer cells (including HepG2, SK-Hep-1, Huh7, PLC5, COLO 205, HT-29, and SW 872 cells). This Chinese herb also induces apoptosis in hepatocellular carcinoma cells via both Fas- and mitochondria-mediated pathways. |
| Bushen huayu jiedu recipe | [87] | "bushen huayu jiedu recipe" (BSHYJDR) is a mixture of several herbs including Chinese Cassia Bark, Psoralea, Zedoary, Rhubarb. The active constituents are alkaloids, flavonoid, arsenic trioxide, cinnamic acid, rhubarb and rhubarb substance. | BSHYJDR was found to inhibit transplanted hepatocarcinoma in mice. This effect is improved in combination with chemotherapy (cisplatin (DDP)). |
| Star 99 | [88] | Chinese herbal compound | Human hepatocellular carcinoma was transplanted in nude mice and treated with Star 99 (intratumoral injection 10 days following to cancer transplantation). The herbal compound was shown to inhibit and destruct liver cancer cells, in particular the membrane, cytoplasm and nucleus of the caner hepatocyte. |
| Daesungki-Tang | [89] | This is a preparation consisting of four herbs: Rhei radix et rhizoma (the roots of Rheum coreanum Nakai), Aurantiii frutus immaturus (immature fruits of Poncirus trifolita Rafin), Magnoliae cortex (the stem bark of Magnolia officinalis Rehd. Et Wils), and Mirabilite (Matrii sulfas). The active constituents are magnolol, honokiol, physcion, chrysophanol, emodin, rhein, and aloe-emodin, naringenin glucuronide and hesperetin glucuronide. | This herb is widely used in the treatment of cancer metastasis. DST extracts were shown to inhib the invasion of the human hepatocellular carcinoma cell line, Hep 3B. On this basis, DST may be a promising antitumor agent. |
| Lycium barbarum and rehmannia glutinosa | [90] | Lycium barbarum (LBE) and Rehmannia glutinosa (RGE) are traditionally used as chinese medicines and herbal foods in China. The active constituents are beta-carotene, vitamin C, vitamins B1 and B2, beta-sitosterol, linoleic acid, immunologically active polysaccharides, sesquiterpenoids (cyperone, solavetivone), tetraterpenoids (zeaxanthin, physalin), and betaine . | Hot water-extracted Lycium barbarum (LBE) and Rehmannia glutinosa (RGE) were found to inhibit cell proliferation and induce p53 mediated apoptosis in hepatocellular carcinoma and inhibit oxidative DNA cleavage induced by various DNA damage chemicals. It also has immunological functions which lead to suppression of malignant cell growth. |
| Semen coicis | [91] | Semen Coicis is a traditional chinese herbal medicine which yields the extract Kang-Lai-Te (KLT). The active constituents are protein, fat, carbohydrate, vitamin B1, amino acids (leucine, lysine, arginine, tyrosine), Coix factors, Coix esters, triterpenoids. | KLT was found to inhibit HepG2 cell growth via a mechanism involving induction of apoptosis through activation of the Fas/FasL pathway. |
| Paeoniae radix | [58] | This crude drug from the root of Paeonia lactiflora Pallas is used in many traditional prescriptions in China and Japan. The active constituents are Paeoniflorin, albiflorin and benzoylalbiflorin. | Paeoniae Radix was found to inhibit the growth of hepatoma cell lines HepG2 and Hep3B via induction of apoptosis in a p53 independent pathway. |
| Qingrejiedu, huoxuehuayu, and fuzhengguben | [92] | Qingrejiedu, Huoxuehuayu, and Fuzhengguben (QHF) medicinal herbs. The active constituents are chlorogenic acid, geniposide, baicalin, forsythin, indirubin. ligustrazine chuanxiong, saponins, and isoflavonoids. | The QHF mixture was found to be more efficient in combating cancer than its separate ingredients. It was also reported to relieve symptoms that appear in patients with hepatocellular carcinoma and to decrease tumor growth by increasing the antitumor effect of cisplatin (DDP). |
| Delisheng | [93] | Delisheng is a n atural medicinal compound composed of ginseng, milk vetch root, secretion bufonis and cantharidium. | The activity of Delishng on the human hepatocellular carcinoma cell line HepG2 was investigated using the MTT assay, and compared to that of the chemotherapeutic drugs 5-fluorouracil and adriamycin. Delisheng was proved to have a positive anti-tumor activity, comparable to that of the chemotherapeutic drugs used. |
| Astragalus membranaceus | [94] | This herb, also known as aka huang chi, is one of the fundamental herbs used in traditional Chinese medicine. The active constituents are polysaccharides, saponins, flavonoids, amino acids. | The herb was found to improve the function of T lymphocytes in cancer patients compared with untreated cells. |
| Morarah and khaltita | [95] | Medicinal herbs. The active constituents is Kahalalide F. | Morarah and Khaltita were found to induce cell death in a heptoma (Huh-7) cell line, suggesting that these herbs could have a promising anti-cancer effect. |
Initiation involves gene mutation, carcinogen metabolism and aberrant DNA repair. In this initial stage, environmental carcinogens (e.g. dietary, tobacco, pollution) induce one or more simple mutations, including transitions or small deletions in genes which control the process of carcinogenesis. Activated carcinogens exert their effects by forming covalent adducts with individual molecules of DNA or RNA, causing deletions of genetic material or mistranslation of the DNA sequence which may produce mutations in critical genes, such as tumor suppressors and oncogenes[23]. Reactive oxygen species (ROS) are generated normally as part of the normal oxidative metabolism or may be end-products of the breakdown of xenobiotic compounds (Figure 1). Oxidative stress can result in extensive DNA damage. Antioxidant herbs which scavenge activated oxygen species are able to stimulate DNA repair pathways to prevent or overcome oxidative DNA damage. Vitamin C, genistein and compounds originating from cruciferous vegetables are among the most well-studied for their scavenger properties[24]. In addition, chronic inflammation may predispose individuals to certain cancers. Most precancerous and cancerous tissues show signs of inflammation involving the movement of innate immune cells into the tissue, the presence of specific inflammatory signaling molecules (i.e. cytokines and chemokines), changes in tissue structure (remodeling) and the formation of new blood vessels (angiogenesis). Further studies have found that cancer-associated inflammation actually promotes tumor growth and progression[25]. Several pro-inflammatory gene products (i.e. TNF-α , IL-6) have a critical role in regulation of apoptosis, proliferation, angiogenesis, invasion and metastasis. Their expression is mainly regulated by the transcription factor NF-κB, which is constitutively active in most tumors and is induced by carcinogens and chemotherapeutic agents. TNF-alpha can initiate signaling pathways which lead to the activation of NF-κB, the initiation of MAPK cascades, and cell death[26]. These observations imply that anti-inflammatory agents that suppress NF-κB or NF-κB-regulated products should have a potential in both the prevention and treatment of cancer[27].
Recently, diallyl sulphide (DAS) obtained from garlic and vitamin C were reported to decrease the levels of circulatory TNF-α and IL-6 in DENA-induced hepatocarcinogenesis[28]. Previous reports showed that vitamin C can inactivate nuclear factor kappa B in endothelial cells during the inflammation process, independently of its atioxidant activity. Therefore, the anti-inflammatory activity of ascorbic acid (AA) may be mediated by multifactorial mechanisms, which are not necessarily associated with its intrinsic antioxidant activity[29]. DAS also was found to promote an anti inflammatory environment by cytokine modulation, leading to an overall inhibition of NF-kB activity in the surrounding tissue[30]. In addition, DAS may enhance antioxidants and suppresses inflammatory cytokines through the activation of Nrf2 transcription factor[31].
This stage is characterized by dysregulation of signaling pathways which normally control cell proliferation and apoptosis (Figure 1). Apoptotic signaling within the cell is transduced mainly via two molecular pathways: the death receptor pathway (also called the extrinsic pathway) and the mitochondrial pathway (also called the intrinsic pathway)[32]. Both pathways activate a variety of proteases, mainly caspases (cysteinyl aspartate-specific proteases), and endonucleases, which finally degrade cellular components. Caspases are constitutively expressed as inactive proenzy-mes, generally require proteolytic processing for their acti-vation, and are capable of self-activation as well as activa-ting each other in a cascade-like process[33]. The extrinsic and the intrinsic pathways are not mutually exclusive and hepatocytes require mitochondrial involvement to amplify the apoptotic signal initiated by death receptors. The intrinsic pathway is triggered by various extra- or intracellular signals that induce mitochondrial dysfunction, resulting in altered membrane permeability and release into the cytosol of mitochondrial proteins, including proapoptogenic factors such as cytochrome c[34]. The Bcl-2 family is the best characterized protein family involved in the regulation of apoptotic cell death. The anti-apoptotic members of this family, such as Bcl-2, prevent apoptosis either by sequestering proforms of death-driving cysteine proteases called caspases (a complex called the apoptosome) or by preventing the release of mitochondrial apoptogenic factors such as cytochrome c and apoptosis-inducing factor into the cytoplasm. After entering the cytoplasm, cytochrome c and apoptosis inducing factor directly activate caspases that cleave a set of cellular proteins to cause apoptotic changes[35,36]. In contrast, pro-apoptotic members of this family, such as Bax, trigger the release of caspases from death antagonists via heterodimerization and also by inducing the release of mitochondrial apoptogenic factors into the cytoplasm via acting on mitochondrial permeability transition pores, thereby leading to caspase activation. Thus, the Bcl-2 family of proteins is crucial in critical life-death decisions within the common pathway of apoptosis[37].
Many of the molecular events altered in HCC progression are compromise the balance between survival and apoptotic signals in preneoplastic hepatocytes. Some physiological proapoptotic molecules (e.g. Bax) are down-regulated or inactivated in HCC, but the balance between death and survival is mainly disrupted by over activation of anti apoptotic signals (e.g. Bcl-2). Cancer cells show stronger requirements for these intracellular pathways to survive[38] and many cancer cells resist apoptosis through the upregulation of Bcl-2 gene[39,40]. This resistance allows damaged and mutated cells to survive, and ultimately proliferate. It also prolongs the lifespan of cells and makes them more likely to develop mutations. Cells also become resistant to the cytotoxic action of various agents, such as chemotherapy[38,41-43]. Thus, induction of apoptosis in tumor cells as well as the inhibition of increased cell proliferation are vital therapeutic goals for herbal treatment of malignancies. Many herbal agents appear to target signaling intermediates in apoptosis-inducing pathways. Thus, targeting apoptosis pathways in premalignant cells, where these pathways are still relatively intact, may be an effective mechanism for chemoprevention[40].
Previous studies have shown that treatment with DAS significantly modulates DNA levels in DENA-initiated hepatocarcinogenesis, suggesting interference with mitotic pathways and enhancement of apoptosis of cancer cells[44,45]. This effect may be related to the ability of DAS to induce direct perturbation of mitochondria, resulting in apoptotic damage to the cancer cells[46,47]. Other studies have reported that ascorbate induces cell cycle arrest and apoptosis in various tumor cells such as lymphoma, leukemia[48], melanoma[49], brain tumor[50], prostate cancer[51] and stomach cancer cells[52]. It is possible that AA exerts this effect by inhibiting either gene expression and/or activity of mutant p53, vascular endothelial growth factor (VEGF), phosphotyrosine kinase, and protein kinase C or by enhancing gene expression and/or activity of p53 wild-type, transforming growth factor beta (TGFb), mitogenactivated protein (MAP) kinase, caspase, cyclin A and D and their kinases[53,54]. These anti-promotional agents can also target specific signaling pathways for hormone receptors, cell cycle check-point markers, transcription factors, mitogen-activated protein kinases, rate-limiting enzymes (e.g. cyclooxygenases), cell junctions and tumor suppressor genes (e.g. p53). Promotion, unlike initiation, is reversible and so identifying agents which can stop or reverse the process of promotion is of a great importance[55].
This stage is characterized by invasion, angiogenesis, metastatic growth, and genetic alterations within the karyotype of the cells due to accumulation of mutated genes, resulting in chromosomal abnormalities (see Figure 1). Angiogenesis, the development of new blood vessels from endothelial cells, is a crucial process which allows the malignant cells to get the nutrients and oxygen, which are essential for cancer progression[56]. Tumors that outgrow their oxygen supply cannot form masses greater than 1-2 mm in diameter without developing central necrosis. Neoplasms are genetically plastic and often adapt by switching on genes that increase their ability to invade and metastasize. Tumours do not grow progressively unless they induce a blood supply from the surrounding stroma. The tumour angiogenic switch seems to be activated when the balance shifts from angiogenic inhibitors to angiogenic stimulators[57]. During angiogenesis, endothelial cells are stimulated by various growth factors, including vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). Thus, blocking the growth of new blood vessels, and thereby reducing nutrients and oxygen supply to tumour cells seems to be a successful strategy to prevent cancer metastasis[58].
The process of cancer metastasis consists of a series of interrelated sequential steps, each of which is rate-limiting and may be a target for therapy. The outcome of the process depends on both the intrinsic properties of the tumour cells and the responses of the host. These steps are summarized as follows: (1) Transformation of normal cells into tumour cells; (2) Extensive vascularization (angiogenesis) involving production and secretion of pro-angiogenic factors by tumour cells and host cells to establish a capillary network from the surrounding host tissue; (3) Local invasion to the host stroma via thin-walled venules, fragmented arterioles, and lymphatic channels which offer little resistance to penetration and entry of tumour cells into the circulation; (4) Detachment and embolization, in which most circulating tumour cells are rapidly destroyed, but those that survive arrest in the capillary beds of distant organs by adhering either to capillary endothelial cells or to the exposed subendothelial basement membrane; (5) Extravasation into a new host organ or tissue; and (6) Proliferation within the new host organ or tissue with the micrometastasis developing a vascular network and evading destruction by host defenses. The cells can then continue to invade blood vessels, enter the circulation, and produce additional metastases[59-61].
Recently, there has been significant interest in developing agents which can delay cancer cell progression to metastasis. Many anti-angiogenic herbs, such as curcumin[62], grape seed extract[63,64], and green tea, have been identified[65,66]. These phytochemicals interact at multiple levels to suppress the inflammatory, hyperproliferative and transformative processes that promote angiogenesis. They inhibit aminopeptidase-N (CD13), a member of the matrix metalloproteinase family that is implicated in the angiogenic switch process. They can also interfere with the expression of VEGF by suppressing a series of angiogenic pathways including production of transforming growth factor beta (TGF-Β), amplification of cyclooxygenase-2 (COX-2) and epidermal growth factor receptor (EGFR), and amplification of nuclear factor kappa-B (NF-κB) signaling. They may also interfere with endothelial cell function by inhibiting the engagement of specific integrins. Other anti-angiogenic herbs include Chinese wormwood, Chinese skullcap, resveratrol and Chinese magnolia tree, ginkgo biloba, quercetin, ginger, panax ginseng[67,68].
Most anti-cancer herbs can exert both chemopreventive and chemotherapeutic actions. Taking into consideration the sequence of events in carcinogenesis (i.e. initiation, promotion and progression), the boundary between the two actions of herbal agents during progression of cancer is unclear. In other words, the same herbal agent can both act as a chemopreventive agent for healthy or high risk patients, and can be used as a therapeutic agent or chemotherapy adjuvant to increase efficacy, decrease side effects of conventional cytotoxic drugs, and prevent tumour metastasis and recurrence in cancer patients. This dual action of herbal medicines combined with their ability to target multiple biochemical and physiologic pathways involved in tumour development and to minimize normal-tissue toxicity emphasize their importance as an attractive alternative means of controlling malignancy[19].
Although herbal medicine has become a popular complementary and alternative strategy for cancer, doubts concerning interference with the action of conventional chemotherapeutic drugs have been raised recently. Considering the narrow therapeutic borders of oncolytic drugs, the usef of herbs could increase the risk of clinically relevant herb-anticancer drug interactions. In addition, the lack of sufficient information about possible mechanisms for such interactions makes it very difficult to accurately evaluate their possible adverse effects[69]. We have tried to highlight the negative side of random use of herbal treatments without medical supervision and the extent to which they can affect the safety and efficacy of chemotherapy in cancer patients.
Herb-drug interactions can occur at different levels (pharmaceutical, pharmacodynamic or pharmacokinetic), but pharmacokinetic interactions are the most likely to occur and can result in changes in absorption, distribution, metabolism, or excretion of chemotherapeutic drugs[70]. Drug-metabolizing systems are among the main targets for such interactions. Phase I enzymes, mainly cytochrome P450, detoxify a variety of endogenous and exogenous chemicals and activate many carcinogens[71]. PhaseIIenzyme systems, which include glutathione S-transferase (GST), 3-quinone reductase, sulfotransferases, and UDP-glucuronosyl-transferase, catalyze the reduction or conjugation of phase I metabolites to various watersoluble molecules and accelerate the rate of metabolite excretion[72,73]. Herbs can either inhibit or induce these systems, thus modulating the action of oncolytic drugs. Inhibition occurs when a herbal agent reduces the normal activity level of a certain metabolic enzyme or drug transporter involved in the disposition of the chemotherapeutic agent via a competitive or noncompetitive mechanism, thereby leading to higher plasma levels of the cytotoxic drug[74,75]. On the other hand, induction is a much slower process, in which herbs increase the mRNA and protein levels of the relevant metabolizing enzyme or drug transporter, resulting in lower plasma levels of chemotherapeutic agent. In either case, significant clinical interactions can occur which may cause greater toxicity or therapeutic failure[70,76,77].
Peer reviewers: Takuji Tanaka, MD, PhD, The Tohkai Cyto-pathology Institute, Cancer Research and Prevention (TCI-CaRP), 4-33 Minami-Uzura, Gifu 500-8285, Japan
S- Editor Zhang HN L- Editor Hughes D E- Editor Zhang L
| 1. | el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87-107, vi. [PubMed] |
| 2. | El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 467] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Srivatanakul P, Sriplung H, Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev. 2004;5:118-125. [PubMed] |
| 4. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 5. | Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 8. | Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8-24. [PubMed] |
| 9. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 10. | Rahman El-Zayadi A, Abaza H, Shawky S, Mohamed MK, Selim OE, Badran HM. Prevalence and epidemiological features of hepatocellular carcinoma in Egypt-a single center experience. Hepatol Res. 2001;19:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | el-Zayadi AR, Badran HM, Barakat EM, Attia Mel-D, Shawky S, Mohamed MK, Selim O, Saeid A. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005;11:5193-5198. [PubMed] |
| 12. | Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Ghaffar YA, Fattah SA, Kamel M, Badr RM, Mahomed FF, Strickland GT. The impact of endemic schistosomiasis on acute viral hepatitis. Am J Trop Med Hyg. 1991;45:743-750. [PubMed] |
| 14. | Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 374] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 15. | Suriawinata A, Xu R. An update on the molecular genetics of hepatocellular carcinoma. Semin Liver Dis. 2004;24:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Kinghorn AD, Su BN, Jang DS, Chang LC, Lee D, Gu JQ, Carcache-Blanco EJ, Pawlus AD, Lee SK, Park EJ. Natural inhibitors of carcinogenesis. Planta Med. 2004;70:691-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127:S56-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 329] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models. Acta Pharmacol Sin. 2007;28:1409-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Kapadia GJ, Azuine MA, Takayasu J, Konoshima T, Takasaki M, Nishino H, Tokuda H. Inhibition of epstein-barr virus early antigen activation promoted by 12-O-tetradecanoylphorbol-13-acetate by the non-steroidal anti-inflammatory drugs. Cancer Lett. 2000;161:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 178] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharm Res. 2010;27:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Andreassen PR, Ho GP, D'Andrea AD. DNA damage responses and their many interactions with the replication fork. Carcinogenesis. 2006;27:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Guilford JM, Pezzuto JM. Natural products as inhibitors of carcinogenesis. Expert Opin Investig Drugs. 2008;17:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1319] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 26. | Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1944] [Cited by in RCA: 2037] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 27. | Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 930] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 28. | Abdel-Hamid NM, Nazmy MH, Abdel-Bakey AI. Polyol profile as an early diagnostic and prognostic marker in natural product chemoprevention of hepatocellular carcinoma in diabetic rats. Diabetes Res Clin Pract. 2011;92:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Bowie AG, O'Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165:7180-7188. [PubMed] |
| 30. | Keiss HP, Dirsch VM, Hartung T, Haffner T, Trueman L, Auger J, Kahane R, Vollmar AM. Garlic (Allium sativum L.) modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J Nutr. 2003;133:2171-2175. [PubMed] |
| 31. | Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur J Pharmacol. 2009;606:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 33. | Schuchmann M, Galle PR. Apoptosis in liver disease. Eur J Gastroenterol Hepatol. 2001;13:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell. 2006;10:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2713] [Article Influence: 77.5] [Reference Citation Analysis (1)] |
| 36. | Luo D, Cheng SC, Xie H, Xie Y. Effects of Bcl-2 and Bcl-XL protein levels on chemoresistance of hepatoblastoma HepG2 cell line. Biochem Cell Biol. 2000;78:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem. 2001;276:27432-27440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Volkmann M, Schiff JH, Hajjar Y, Otto G, Stilgenbauer F, Fiehn W, Galle PR, Hofmann WJ. Loss of CD95 expression is linked to most but not all p53 mutants in European hepatocellular carcinoma. J Mol Med (Berl). 2001;79:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Takahashi M, Saito H, Okuyama T, Miyashita T, Kosuga M, Sumisa F, Yamada M, Ebinuma H, Ishii H. Overexpression of Bcl-2 protects human hepatoma cells from Fas-antibody-mediated apoptosis. J Hepatol. 1999;31:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Sun SY, Hail N, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 386] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 41. | Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene. 2006;25:3848-3856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Lee CY, Hsu YC, Wang JY, Chen CC, Chiu JH. Chemopreventive effect of selenium and Chinese medicinal herbs on N-nitrosobis(2-oxopropyl)amine-induced hepatocellular carcinoma in Syrian hamsters. Liver Int. 2008;28:841-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Wu LQ, Lu Y, Lu HJ, Zhao ZG, Yang M. Efficacy of intra-tumor injection of Kang-Lai-Te in treating transplanted hepatoma in rats. Hepatobiliary Pancreat Dis Int. 2004;3:580-584. [PubMed] |
| 45. | Xiao D, Pinto JT, Gundersen GG, Weinstein IB. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol Cancer Ther. 2005;4:1388-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Rotem R, Heyfets A, Fingrut O, Blickstein D, Shaklai M, Flescher E. Jasmonates: novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005;65:1984-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | You KR, Wen J, Lee ST, Kim DG. Cytochrome c oxidase subunit III: a molecular marker for N-(4-hydroxyphenyl)retinamise-induced oxidative stress in hepatoma cells. J Biol Chem. 2002;277:3870-3877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Iwasaka K, Koyama N, Nogaki A, Maruyama S, Tamura A, Takano H, Takahama M, Kochi M, Satoh K, Sakagami H. Role of hydrogen peroxide in cytotoxicity induction by ascorbates and other redox compounds. Anticancer Res. 1998;18:4333-4337. [PubMed] |
| 49. | Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H. Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake. J Cell Physiol. 2005;204:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Baader SL, Bruchelt G, Carmine TC, Lode HN, Rieth AG, Niethammer D. Ascorbic-acid-mediated iron release from cellular ferritin and its relation to the formation of DNA strand breaks in neuroblastoma cells. J Cancer Res Clin Oncol. 1994;120:415-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Menon M, Maramag C, Malhotra RK, Seethalakshmi L. Effect of vitamin C on androgen independent prostate cancer cells (PC3 and Mat-Ly-Lu) in vitro: involvement of reactive oxygen species-effect on cell number, viability and DNA synthesis. Cancer Biochem Biophys. 1998;16:17-30. [PubMed] |
| 52. | Head KA. Ascorbic acid in the prevention and treatment of cancer. Altern Med Rev. 1998;3:174-186. [PubMed] |
| 53. | Manivasagam T, Subramanian P, Suthakar G, Essa MM. The chemopreventive effect of diallyl disulphide on N-nitrosodiethylamine induced heptocarcinogenesis. J Appl Biomed. 2005;3:187–191. |
| 54. | Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4052] [Cited by in RCA: 4062] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 55. | Huang P, Oliff A. Signaling pathways in apoptosis as potential targets for cancer therapy. Trends Cell Biol. 2001;11:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Fidler IJ. Regulation of neoplastic angiogenesis. J Natl Cancer Inst Monogr. 2001;10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Lee SM, Li ML, Tse YC, Leung SC, Lee MM, Tsui SK, Fung KP, Lee CY, Waye MM. Paeoniae Radix, a Chinese herbal extract, inhibit hepatoma cells growth by inducing apoptosis in a p53 independent pathway. Life Sci. 2002;71:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Hart IR, Goode NT, Wilson RE. Molecular aspects of the metastatic cascade. Biochim Biophys Acta. 1989;989:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4077] [Cited by in RCA: 3940] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 61. | Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s-5059s. [PubMed] |
| 62. | Shin EC, Seong YR, Kim CH, Kim H, Ahn YS, Kim K, Kim SJ, Hong SS, Park JH. Human hepatocellular carcinoma cells resist to TRAIL-induced apoptosis, and the resistance is abolished by cisplatin. Exp Mol Med. 2002;34:114-122. [PubMed] |
| 63. | Khanna S, Roy S, Bagchi D, Bagchi M, Sen CK. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic Biol Med. 2001;31:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Kojima-Yuasa A, Hua JJ, Kennedy DO, Matsui-Yuasa I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003;73:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Tang FY, Nguyen N, Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int J Cancer. 2003;106:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Wang S, Zheng Z, Weng Y, Yu Y, Zhang D, Fan W, Dai R, Hu Z. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 2004;74:2467-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 2. Curr Oncol. 2006;13:99-107. [PubMed] |
| 69. | Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. Herb-drug interactions: a literature review. Drugs. 2005;65:1239-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 70. | Beijnen JH, Schellens JH. Drug interactions in oncology. Lancet Oncol. 2004;5:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 71. | Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991;4:391-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 659] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 72. | Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol Lett. 1995;82-83:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 299] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect. 1997;105 Suppl 4:965-970. [PubMed] [DOI] [Full Text] |
| 74. | Zou L, Harkey MR, Henderson GL. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002;71:1579-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 75. | Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of herbs with cytochrome P450. Drug Metab Rev. 2003;35:35-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 76. | Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22:2489-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 77. | McCune JS, Hatfield AJ, Blackburn AA, Leith PO, Livingston RB, Ellis GK. Potential of chemotherapy-herb interactions in adult cancer patients. Support Care Cancer. 2004;12:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Park WH, Lee SK, Oh HK, Bae JY, Kim CH. Tumor initiation inhibition through inhibition COX-1 activity of a traditional Korean herbal prescription, Geiji-Bokryung-Hwan, in human hepatocarcinoma cells. Immunopharmacol Immunotoxicol. 2005;27:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Park WH, Joo ST, Park KK, Chang YC, Kim CH. Effects of the Geiji-Bokryung-Hwan on carrageenan-induced inflammation in mice and cyclooxygenase-2 in hepatoma cells of HepG2 and Hep3B. Immunopharmacol Immunotoxicol. 2004;26:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Qian Y, Ling CQ. Preventive effect of Ganfujian granule on experimental hepatocarcinoma in rats. World J Gastroenterol. 2004;10:755-757. [PubMed] |
| 81. | Penza M, Montani C, Jeremic M, Mazzoleni G, Hsiao WL, Marra M, Sharma H, Di Lorenzo D. MAK-4 and -5 supplemented diet inhibits liver carcinogenesis in mice. BMC Complement Altern Med. 2007;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Li X, Shi ZM, Feng P, Wen ZY, Wang XJ. Effect of Qi-protecting powder (Huqi San) on expression of c-jun, c-fos and c-myc in diethylnitrosamine-mediated hepatocarcinogenesis. World J Gastroenterol. 2007;13:4192-4198. [PubMed] |
| 83. | Agarwal R, Katiyar SK, Lundgren DW, Mukhtar H. Inhibitory effect of silymarin, an anti-hepatotoxic flavonoid, on 12-O-tetradecanoylphorbol-13-acetate-induced epidermal ornithine decarboxylase activity and mRNA in SENCAR mice. Carcinogenesis. 1994;15:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Chatterjee ML, Agarwal R, Mukhtar H. Ultraviolet B radiation-induced DNA lesions in mouse epidermis: an assessment using a novel 32P-postlabelling technique. Biochem Biophys Res Commun. 1996;229:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Huang XY, Wang L, Huang ZL, Zheng Q, Li QS, Tang ZY. Herbal extract "Songyou Yin" inhibits tumor growth and prolongs survival in nude mice bearing human hepatocellular carcinoma xenograft with high metastatic potential. J Cancer Res Clin Oncol. 2009;135:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Fang SC, Hsu CL, Lin HT, Yen GC. Anticancer effects of flavonoid derivatives isolated from Millettia reticulata Benth in SK-Hep-1 human hepatocellular carcinoma cells. J Agric Food Chem. 2010;58:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Cao Y, Xia QH, Meng H, Zhong AP. Antitumor and synergistic effect of Chinese medicine "bushen huayu jiedu recipe" and chemotherapy on transplanted animal hepatocarcinoma. World J Gastroenterol. 2005;11:5218-5220. [PubMed] |
| 88. | Lin LW, Sun Y, He YM, Gao SD, Xue ES, Lin XD, Yu LY, Lin XF, Yang YH. Percutaneous intratumoral injection of traditional Chinese herbal compound medicine Star-99 in treatment of hepatocellular carcinoma of mice. Hepatobiliary Pancreat Dis Int. 2004;3:49-54. [PubMed] |
| 89. | Ha KT, Kim JK, Lee YC, Kim CH. Inhibitory effect of Daesungki-Tang on the invasiveness potential of hepatocellular carcinoma through inhibition of matrix metalloproteinase-2 and -9 activities. Toxicol Appl Pharmacol. 2004;200:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Chao JC, Chiang SW, Wang CC, Tsai YH, Wu MS. Hot water-extracted Lycium barbarum and Rehmannia glutinosa inhibit proliferation and induce apoptosis of hepatocellular carcinoma cells. World J Gastroenterol. 2006;12:4478-4484. [PubMed] |
| 91. | Lu Y, Wu LQ, Dong Q, Li CS. Experimental study on the effect of Kang-Lai-Te induced apoptosis of human hepatoma carcinoma cell HepG2. Hepatobiliary Pancreat Dis Int. 2009;8:267-272. [PubMed] |
| 92. | Chen T, Li D, Fu YL, Hu W. Screening of QHF formula for effective ingredients from Chinese herbs and its anti-hepatic cell cancer effect in combination with chemotherapy. Chin Med J (Engl). 2008;121:363-368. [PubMed] |
| 93. | Cui J, Nan KJ, Tian T, Guo YH, Zhao N, Wang L. Chinese medicinal compound delisheng has satisfactory anti-tumor activity, and is associated with up-regulation of endostatin in human hepatocellular carcinoma cell line HepG2 in three-dimensional culture. World J Gastroenterol. 2007;13:5432-5439. [PubMed] |
| 94. | Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. I. Immune restoration of local xenogeneic graft-versus-host reaction in cancer patients by fractionated Astragalus membranaceus in vitro. J Clin Lab Immunol. 1988;25:119-123. [PubMed] |
| 95. | Baig S, Alamgir M. Cell death induced by Morarah and Khaltita in hepatoma cancer cells (Huh-7). J Coll Physicians Surg Pak. 2009;19:644-648. [PubMed] |