Published online Jun 27, 2011. doi: 10.4254/wjh.v3.i6.147
Revised: May 15, 2011
Accepted: May 22, 2011
Published online: June 27, 2011
AIM: To evaluate the impact of mass vaccination against the hepatitis B virus (HBV) in Egypt, and to search for vaccinee asymptomatic breakthrough HBV infection and its genotype.
METHODS: Seven hundred serum samples from vaccinated children and adults (aged 2-47 years) were used for quantitative and qualitative detection of HBsAb by ELISA. Three hundred and sixty serum samples representing undetectable or low or high HBsAb were screened for markers of active HBV infection (HBsAg, HBcAb (IgG) and HBeAb by ELISA, plus HBsAg by AxSYM) and HBV-DNA genotyping by nested multiplex PCR and by DNA sequencing.
RESULTS: It was found that 65% of children aged 2-4 years, and 20.5% aged 4-13 years, as well as 45% adults were good responders to HBV vaccination mounting protective level HBsAb. Poor responders were 28%, 59.5% and 34%, and non-responders were 7%, 20% and 21% respectively, in the three studied groups. Markers of asymptomatic HBV infections were HBsAg detected by ELISA in 2.5% vs 11.39% by AxSYM. Other markers were HBcAb (IgG) in 1.38%, HBeAb in 0.83%, and HBV-DNA in 7.8%. All had HBV genotype E infection.
CONCLUSION: It is concluded that HBV vaccine is efficient in controlling HBV infection among children and adults. The vaccine breakthrough infection was by HBV genotype E. A booster dose of vaccine is recommended, probably four years after initial vaccination.
- Citation: Abushady EA, Gameel MM, Klena JD, Ahmed SF, Abdel-Wahab KS, Fahmy SM. HBV vaccine efficacy and detection and genotyping of vaccineé asymptomatic breakthrough HBV infection in Egypt. World J Hepatol 2011; 3(6): 147-156
- URL: https://www.wjgnet.com/1948-5182/full/v3/i6/147.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i6.147
The Hepatitis B virus (HBV) is endemic in many developing countries, including Egypt[1-3]. As the majority of chronic carriers of HBV in the worldbecome soas a result of infections which occur prior to the age of 6, the Technical Advisory Group (TAG) of the World Health Organization has recommended that the HB vaccine must be added as a component of the Expanded Program of Immunization (EPI) in countries with a moderate to high prevalence[4]. The recombinant DNA-based vaccine, targeting the HBV major surface protein (r-HBsAg)has been incorporated in the national childhood vaccination program in Egypt since October 1992. Three doses of 10 μg r-HBsAg are given at 2, 4 and 6 mo of age[5,6]. The vaccination of infants, children and adolescents have produced high rates of seroconversion (95%) and induced adequate levels of HBsAb[7]. The accepted level of seroconversion is 10 or more mIU/L, which provides protection against HBV infection. While the success of the vaccine cannot be denied, it was noticed that vaccine efficiency was improved by the addition of preS1 and preS2 components. Universal vaccination of infants with the HBV vaccine has dramatically reduced infection, as well as the hepatitis-B surface antigen (HBsAg) carrier rate of chronic HBV, in addition to a significant decrease in the incidence of childhood hepatocellular carcinoma[1,8,9]. Protective immunity has been demonstrated in persons and populations up to 5 to 10 years post-vaccination, with associated decrease of asymptomatic breakthrough HBV infection[10-12]. In HBV endemic areas, postnatal or prenatal at the time of delivery mother-to-infant transmission of infection occurs frequently, especially if the maternal serum is hepatitis-Be antigen positive, which is the stage at which 90% of babies acquire HBV infection. Despite r-HBsAg vaccination, some of these infants become persistently infected and increase the worldwide HBV reservoir[13]. Furthermore, 5%-10% of the vaccinees display an inadequate antibody response following primary vaccination with triple doses of either plasma-derived or r-HBsAg vaccine, in addition to 3%-20% of non-responders, and may not be protected from subsequent exposure to HBV infection[6,13]. The introduction of a safe, effective, hepatitis B vaccine has led to universal infant vaccination, resulting in a reduced rate of perinatal HBV infection from infected mothers. Because of appropriate hepatitis B vaccination and passive immunoprophylaxis with hepatitis B immune globulin (HBIG) in infants of mothers with HBV infection, perinatal transmission has been reduced to less than 5% to 10%[14]. The configuration of the HBsAg used in current vaccine formulations contains a determinantwhich is located between amino acids (aa) 121-149 of the HBsAg immunogenic epitope, that trigger the production of polyclonal antibodies against the HBV major surface protein (HBsAb). The emergence of HBsAg variants, with mutations within aa 121-149 with altered antigencity, and binding to the HBsAb, has been reported in HBV from vaccinees in several areas of the world[15,16]. Using the currently available diagnostic assays, these variants may go undetected, and could potentially cause breakthrough infections in a vaccinated population, posing a potential threat to the long term success of HBV vaccination programmes[17-19]. Booster doses of Hepatitis B vaccine are recommended only in certain circumstances, for example, for hemodialysis patients, and for those with an ongoing risk of exposure. Annual anti-HBs testing and a booster dose should be administered when anti-HBs levels decline to < 10 mIU/mL. For other immuno-compromised persons, the need for booster doses has not been determined[20]. It has been appreciated that long-term immunity derives from immunological memory, which outlasts the loss of antibody levels.Hence subsequent testing and administration of booster doses is not required in successfully vaccinated immunocompetent individuals. With the passage of time and longer experience, protection has been shown to last for at least 25 years in those who showed an adequate initial response to the primary course of vaccinations[21].
In 1988, genotypes of HBV were proposed by a sequence divergence in the entire genome exceeding 8%, based on a comparison of 18 HBV isolates[22]. Four genotypes were recognized and they were given capital letters of the alphabet from A to D. In 1994, Norder et al[23] found an additional two HBV genotypes by means of the same criteria, and named them E and F; genotype G was reported in 2000[24], and genotype H was reported in 2002[25]. The vaccine “a” component of HBsAg is stable, due to conserved gene sequences encoding it in all HBV genotypes. On the other hand, the preS1, preS2 containing vaccines, which are more immunogenic, have high specific motifs. Thus there is a need to tailor the vaccine to the HBV genotype prevalent in the geographic areas of vaccination.
The objective of the current study was to evaluate the impact of mass childhood HBV vaccination in Egypt on asymptomatic HBV breakthrough infection in vaccinated individuals, and to determine the causative genotype.
This study was done in 2004, 12 years after the start of the vaccination programme. Laboratory testing was conducted from October 2004 to January 2007 at the Virology Laboratory, Microbiology Department, Faculty of Medicine, Al-Azhar University, the Virology Laboratory in the Military Central Laboratory and the Molecular Epidemiology Department NAMRU#3 Cairo, Egypt.
Six hundred serum samples from vaccinated children were collected (after obtaining the legal guardian consent). The six hundred children had received HBV vaccine [Engerix-B (Simksline “Sigma” licensed at 1989) from October 1992 until 1996. They received Euvax vaccine (Korea)] at 2, 4, and 6 mo of age, according to the vaccine schedule of the Egyptian Ministry of Health Population (MOHP). Serum samples from children aged 2- < 4 years of age were obtained from the Pediatric out-patient clinic at Al- Zahraa University Hospital, Cairo Governorate, Egypt. Serum samples of children aged from 4-13 years were obtained from the Maternal and Child Health Care Center in Qusena City, Menofya Governorate, or from Al- Zahraa University Hospital, Cairo Governorate, Egypt.
A hundred serum samples from vaccinated adults were collected (after obtaining individual consent) from Benha Teaching Hospital, the Motor Rehabilitation Institute and the Hearing and Speaking Institute, Qualyubia Governorate. All serum samples were collected between October 2004 and August 2005. Sera were divided according to the age of vaccinees into three groups:
Group I: two hundred children aged 2-<4 years. These children were considered healthy, with no history of medical or surgical problems, or risk factors for HBV infection, except for males who had beencircumcised.
Group II: four hundred children aged 4-13 years, some of whom had past surgical history (circumcision, tonsillectomy, para-umblical & inguinal herniorraphy).
Group III: one hundred sera from healthy adults, whose age ranged from 20-47 years. This group was without any history of risk for HBV infection, except that some females had a history of previous operations, such as caesarean section, tube dilatation and cervical curettage. All adults had received the 20 μgm r- HBsAg at a 0, 1, 6 mo interval schedule. The last dose of vaccine in the 90 adult volunteers was administered within theyear before inclusion in the study, six adults had had their last dose between one and four years previously, and four volunteers had had their last dose six years or more prior to the study. This study did not include another age and gender matched non-vaccinated subject, as almost all children had been vaccinated within the MOHP vaccine schedule.
BIO ELISA (BioKit, Barcelona, Spain) HBsAb kits were used to both quantitatively and qualitatively assess HBsAb in the 700 post-vaccination serum samples. A random selection of 360/585 serum samples that were either negative 82/360 or demonstrated low antibody titer 287/360 (< 10 mIU/L) together with 41/360 serum samples with high antibody titer (> 10 mIU/L), were subsequently screened for HBsAg, using two commercial kits; one to detect both wild and mutant strains (AxSYM from ABBOTT), and the other the ELISA Bioelisa for HBsAg (Bio-Kit, Barcelona, Spain). All serum samples positive for HBsAg were screened for the detection of HBcAb (IgG) (Bio-Kit, Barcelona, Spain) and HBeAb using (Diasorin, S.P.E Italy).
Serum DNA was extracted using Kaucner and Stinear 1998 heat shock method[26] . In some cases, DNA was extracted using the Qiagen DNA Blood mini kit, according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). Extracted DNA was subjected to HBV-DNA detection and genotyping using the nested multiplex PCR (nm PCR) method[27]. Briefly, for the first round PCR, the primer pair P1 sense (5’ TCACCATATTCTTGGGAACAAGA 3’) and S 1-2 antisense (5’ CGAACCACTGAACAAATGGC 3’) were used to amplify the conserved regions of the pre-S1 and S-gene (1063 bases). The reaction mixture contained 5 μL of extracted DNA in 25 μL 1 × PCR buffer containing 1.5MgCl2, 5 pmol of each primer completed 200 μmol/L of each of the four deoxynucleotides, 1U of AmpliTaq Gold DNA polymerase (Perkin-Elmer, Norwalk, Conn.) and completed to 50 μl with DEPEC treated sterile water. The samples were incubated at 95ºC for 10 min, followed by 40 ampilification cycles of 94ºC for 20 sec (denaturation), 55ºC for 20 sec (annealing), 72ºC for 1 min (extension), and then followed by further extension at 72ºC for 10 min. After that the product was kept at 4ºC. The second-round PCR was performed for each specimen using inner pair primers in two different combinations, Mix-A: B2:5’ GGCTCMAGTTCMGGAACAGT 3’ (nt 67-86, types A to E specific, sense), BA1R: 5’ CTCGCGGAGATTGACGAGATGT 3’ (nt 113-134, type A specific, anti sense), BB1R: 5’ CAGGTTGGTGAGTGACTGGAGA 3’ (nt 324-345, type B specific, antisense), BC1R: 5’ GGTCCTAGGAATCCTGATGTTG 3’ (nt 165-186, type C specific, antisense) for genotypes A, B and C using universal common primer B2 (sense) and specific primers A4, B5 and C6. Mix B: BD1: 5’ GCCAACAAGGTAGGAGCT 3’ (nt 2979-2996, type D specific, sense), BE1: 5’ CACCAGAAATCCAGATTGGGACCA 3’ (nt 2955-2978, type E specific, sense), BF1: 5’ GYTACGGTCCAGGGTTACCA 3’ (nt 3032-3051, type F specific, sense), B2R: 5’ GGAGGCGGATYTGCTGGCAA 3’ (nt 3078-3097, type D specific, antisense), for genotype D, E, and F using universal primer R10 (antisense) and specific primers D7, E8 and F9. In the second round PCR 5 μL of the first PCR product was added to each mix with the same components of the first round PCR. These were amplified for 40 cycles, with the following parameters: hot start at 95oC for 10 min, followed by 25 cycles of 94oC for 20 sec (denaturation), 58oC for 20 sec (annealing), 72oC for 30 sec (extension), and an additional 20 cycles of 94oC for 20 sec, 60oC for 20 se, 72oC for 30 sec, which was then followed by further extension at 72oC for 10 min, then the product was kept at 4oC. The products of this second round PCR were visualized by electrophoreses on 3% agarose gel, and are differentiated by the size of genotype-specific DNA bands, compared to a 50 base-pair DNA marker (Amersco).
To assess changes in the HBV S gene, the following primers were designed to ampilify the whole S gene, and used in a semi-nested PCR, HBsP1f (forward): 5’ GGAGYKGGAGCATTCGGS 3’, HBsP2f (forward) : 5’ GTTACAGGCGGGGTTTTTCTTG 3’ and HBsP4r (reverse): 5’ TCACACATCATCCATATARCTGAAAGC. The first round PCR was done using HBsP1f and HBsP4r, the reaction mixture consists of 5 μL of extracted DNA in 50 μL of a reaction buffer made of 30 pmol of each primer, 10 μmol/L of each of the four deoxynucleotides, 1 U of AmpliTaq Gold DNA polymerase, and 5 × PCR buffer containing 25 mol/L MgCl2. The samples were incubated at 95ºC for 10 min, followed by 35 cycles of 94ºC for 30 sec (denaturation), 60ºC for 1 min (annealing), 72ºC for 1 min (extension), followed by further extension at 72ºC for 10 min. The second round PCR was done in the same way as the first round, using HBsP2f and HBsP4r primers. To confirm the obtained genotype, and to determine the existence of any mutations, DNA sequencing of the S gene product was conducted using BigDye terminator technology and an ABI 377 fluorescent automated sequencer. DNA sequence was manually edited, using the software program Bioedit v7.0.5[28]. Sequence comparisons of the obtained Egyptian strain (a positive control chronic hepatitis patient EGYAZC P1P2P4 bankit 1229997 GQ25310e) were made using the program Clustal X[29] and HBV gene sequences retrieved from the Gene Bank: HBV genotypeE (AB183AB274977, LAGOS558AJ604967, CAR194AM494753, X75657, EU239226 PW6, 235-01DQ060830, MAL136AJ604992, CMR936 AB194948)[30] and HBV genotype A X75669, HBV genotype B X75660, HBV genotype C X75656, HBV genotype F X75658[23]. In addition, alignment of 87 nucleotide sequence obtained from preS1 genes from Egyptian HBV isolate (a sample from a vaccinated child EGYAZV B2 265R) and Gene Bank strains as indicated: HBV genotype E PW6 EU239226[31], HBV genotype A DQ788725[32], HBV genotype B FM211366[33], HBV genotype Cx75656 and HBV genotype D x75658[23] was done, and Phylogenetic analysis and distance calculations for molecular evolutionary analyses were conducted using MEGA version 3.1[34]. Phylogenies were constructed using the Neighbor-joining method and substitutions were modeled using the Kimura 2-parameter model, and phylogenetic analysis of selected sequences based onPreS1 and S fragments was done according to Felsetien(1988)[35].
The r-HBsAg vaccine used in Egypt appears to be efficient in inducing HBsAb immune response, which may, in turn, be efficient in controlling HBV infection in children and adults ( ≥ 10 mIU/mL), as 93% and 80% of children aged 2- < 4 years, 4-13 years respectively, and 79% adults acquired protective HBsAb. Having low HBsAb titre (less than 10 mIU/mL) were 28%, 59.5% and 34% in the three studied groups respectively (Table 1).
| Group IChildren (2- < 4 years)N=200 | Group IIChildren (4-13 years)N =400 | Group IIIAdults (20-47 years)N =100 | TotalNoN =700 | |
| Anti-HBs IgG | n (%) | n (%) | n (%) | n (%) |
| High +ve samples (10-99.9 mIU/mL) | 130 (65) | 82 (20.5) | 45 (45) | 257(36.7) |
| Low +ve samples (<10 mIU/mL) | 56 (28) | 238 (59.5) | 34 (34) | 328(46.9) |
| Negative samples (Non-responders) | 14 (7 ) | 80 (20 ) | 21 (21 ) | 115 (16.4) |
| Total positive samples | 186 (93) | 320 (80) | 79 (79) | 585 (83.6) |
The infection determined by detection of HBsAg by Bio ELISA test was 2.5% vs 11.39% by AxSYM. Active HBV replication by DNA amplification procedures was 6.11%, and other serological markers of infectivity were HBcAb (IgG) 1.38% and HBeAb 0.83% (Tables 2, 3) respectively.
| Anti-HBs IgG antibody level | Samples tested | HBs Ag | ||
| (ELISA) | (AxSYM) | |||
| n | Positive n (%) | Positive n ( %) | ||
| Group I Children (2-<4 years) | Low | 27 | 0 | 2 (7.4) |
| Negative | 8 | 0 | 3 (37.5) | |
| High | 20 | 0 | 0 | |
| Group II Children (4-13 years) | Low | 178 | 4 (2.25) | 17 (9.55) |
| Negative | 53 | 1 (1.88) | 11 (20.8) | |
| High | 14 | 0 | 1a (7.14) | |
| Group III Adults (20-47 years) | Low | 32 | 2 (6.25) | 2 (6.25) |
| Negative | 21 | 2 (9.52) | 5 (23.8) | |
| High | 7 | 0 | 0 | |
| Total | 360 | 9 (2.5) | 41b (11.39) | |
Among serum samples from children aged 2- < 4 years old, 65% had a high HBsAb titer, 28% had low titer, and 7 % did not have detectable HBsAb. The majority of these children had no markers of HBV infection by BioELISA test for HBsAg. Only 5/55 (9.09%) were infected as determined by detection of the HBsAg by AxSYM (Tables 1-3).
| Age group | anti-HBs IgG level | Number of serum samplesTested | HBV-DNA by nestedMultiplex PCR n (%) | HBc antibodyIgG n (%) | HBe IgG antibodyn (%) |
| Group I Children (2-<4 years) | Low | 27 | 0 | 0 | 0 |
| Negative | 8 | 0 | 0 | 0 | |
| High | 20 | 0 | 0 | 0 | |
| Group II Children (4-13 years) | Low | 178 | 4 (2.5) | 2 (1.2) | 0 |
| Negative | 53 | 14 (26.4) | 1 (1.9) | 1 (1.9) | |
| High | 14 | 0 | 0 | 0 | |
| Group III Adults (20-47 years) | Low | 32 | 2a (6.25) | 1 (3.1) | 1 (3.1) |
| Negative | 21 | 2a (9.52) | 1 (4.8) | 1 (4.8) | |
| High | 7 | 0 | 0 | 0 | |
| Total | 360 | 22 (6.11) | 5 (1.38) | 3 (0.83) |
In contrast, only 20.5% of children aged 4-13 years had a high HBsAb titer, 59.5% had a low HBsAb titer, and 20% did not have detectable HBsAb. Screening for HBV infection in this age group revealed that 2.04% and 12.24% were positive for HBsAg by BioELISA and AxSYM respectively. It was noticed that 7.14%, 9.55% and 20.75% of high, low and undetectable HBsAb were positive for HBsAg, 7.34% (2.25% in low HBsAb and 26.4% in undetectable HBsAb) for HBV-DNA, 0.81% for HBcAb and 0.4% for HBeAb (Tables 1-3).
Finally, within the adult age group 45% of the serum samples had a high antibody titer, 34% of samples had a low antibody titer and 21% were negative. In this age group, 6.66% and 11.66% were positive for HBsAg by ELISA and AxSYM, respectively, 5% were positive for HBcAb, and 3.33% were positive for HBeAb; HBV-DNA was detected in 6.66% of the serum samples (Tables 1-3).
On examining 41 high HBsAb positive samples, regardless of the assay, they were negative for all markers tested, except for one sample which was positive for HBsAg by AxSYM assay (Table 2 )
Out of 22 cases positive for HBV DNA; 4 cases of group III who were also HBsAg positive by both methods, and 18 cases of group II ( 5 of them were HBsAg positive by BioELISA 11 by AxYM and two were HBcAb positive), while 12 participants had HBV-DNA as the only marker for HBV infection. Considering HBV-DNA detection by nmPCR as a reference test, it was found that BioELISA specificity (100%), BioELISA sensitivity (96.29%), AxYM specificity (50%), AxYM sensitivity (96.65%).
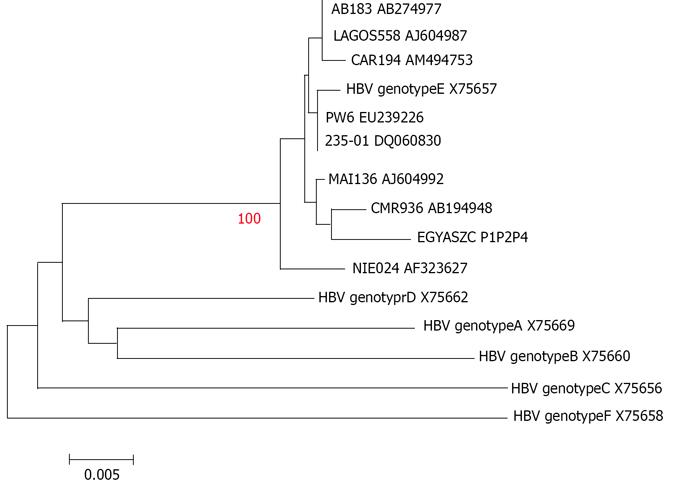
In all the HBV-DNA positive samples, genotype E positive control and samples were detected at 167bp specific for type E. The result was confirmed by DNA sequencing in the available PCR products (Figure 1 and 2). A correlation between HBsAg detection by BioELISA and HBV genotyping by nm PCR revealed that all samples positive by BioELISA (9 samples) and the 11 samples positive by AxYM were also positive by nm PCR. HBV DNA was detected in 12 samples that were HBsAg negative by both techniques.
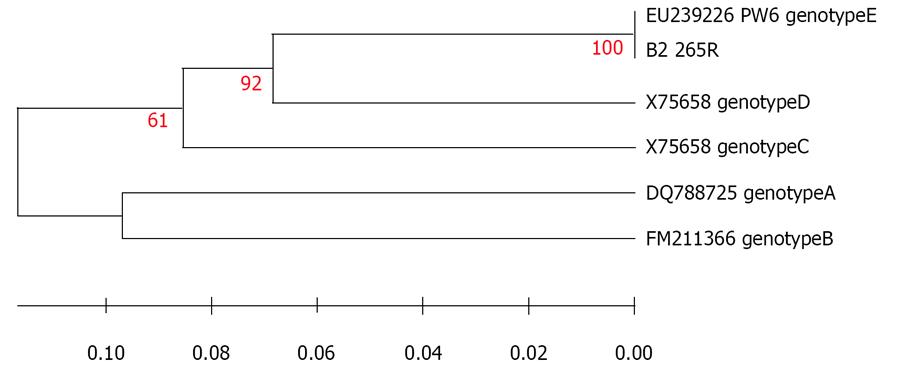
It was also found that tested subjects, either HBsAb positive or negative, were infected with the same genotype. Sequence comparisons of 565 nucleotide (genome position: ~155-720) obtained from HBV S- gene from Egyptian strain (a positive control chronic hepatitis patient) EGYAZC P1P2P4 and HBV/E gene sequences retrieved from the Gene Bank revealed only five nucleotide sequence differences (No 186, 201, 473, 515, 521) between them. The closest sequence was from CMR936 AB194948 (Figure 1). Furthermore, alignments of 87 nucleotide sequence (genome position: ~ 3048- 3135) obtained from preS1 genes from Egyptian HBV isolate (a sample from a vaccinated child) EGYAZV B2 265R and the Gene Bank strains indicated that there is 100 % similarity with HBV genotype E PW6 EU239226 (Figure 2).
Studies on vaccinated children during infancy and early childhood in countries with a high endemicity of chronic HBV infectionhave shown that more than 50% of participants had measurable anti-HBs levels of at least 10 mIU/L from 4 to 10 years after vaccination[36,37].
The main finding in this study was that 93% of children aged 2- < 4 years had responded to the vaccine, compared with 80% of children aged 4-13 years, and 79% of adults. In the present study, adults had a higher rate of high-titre antibodies (45%) than children (age 4-13 years) (20.5%), because the last vaccine dose in 90 adult volunteers was administered within a year before their inclusion in the study, six adults had their last dose between one and four years previously, and four volunteers had had their last dose six years or greater prior to the study. However, the children had received their doses since their birth, consequently they had had the vaccine administered between the ages of 4 to 13 years. It is well known that HBsAb level declines by time. The percentage of undetectable HBsAb level was lowest (7%) in 2- < 4 years old vaccinees, and rose to 20% and 21% as the age of the vacinee increased by the time of vaccination. This is comparable to McMahon et al, 2005[38] who found HBsAb response in 89% of the study population with 19% between 2 and 9.9 mIU/L and 70% had levels greater than 10 mIU/L. In the current study, 83.6% had detectable HBsAb, but only 36.7% of them had levels of ≥ 10 mIU/L. In the study of Zanetti et al, 2005[39], more than 60% of children and nearly 90% of recruits maintained protective HBsAb, and recorded undetectable concentrations in about 9% of children and 4% of recruits and detectable amounts lower than 10 mIU/L in 27% and 7% respectively, more than 10 years after vaccination. In Egypt, El-Sawy and Mohamed (1999)[40] tested the post-vaccination seroprotection rate in sera collected during one month (93.3%) and 5 years (53.3%). In Taiwan, the HBsAb was detected post- vaccination in 100% of 2 year- olds, and in 75% of 6 year old children[41]. In a study from Taiwan, they noted that a single dose of vaccine boosted the immune response in almost all individuals. The results led to the suggestion that booster doses may be necessary in seronegative subjects for at least 15 years after neonatal immunization. They believed that this applies to both hyper-endemic and low endemic areas of the world[42].
In the present study, detection of markers of HBV infec-tion in the form of HBsAg was 0% among children aged 2- < 4 years, 2% among children aged 4 -13 years and 6.66% among adults, as established by the ELISA method. Similarly Alam et al (2007)[43] found in their study that the frequency of HBV infection in the Pakistani population was higher in individuals aged from 20-40 years. In the present study, none of the high HBsAb- positive individuals were positive for HBsAg, supporting a previous observation that an HBsAb titer greater than 10 mIU/mL can be considered protective[44,45].
Population-based studies of HB immunization after 10-15 years follow-up showed a reduction in chronic HBsAg carrier prevalence from high (8% or greater) to low (< 2%) endemicity in immunized cohorts of infants[46]. The current results are comparable to the Shatat et al (2000)[47] study in Alexandria, Egypt, in which only one child out of 184 vaccinated 5 year old children who had received the full course of EPI r- vaccine was HBsAg positive, while El-Sawy and Mohamed (1999)[40] did not find HBsAg positive sera among 180 children with a one month to 5 year time lapse since their last dose of vaccination.Moreover, in a serosurvey in Alexandria, Reda et al (2003)[48] revealed that the number of HBsAg carriers is significantly lower among the vaccinated (0.8%), compared to the unvaccinated in 6 year old children (2.2%). All these findings, as well as the present study reflect the impact of HB vaccination in lowering the HBsAg carriage rate in Egypt, but it also raises several questions. Is the time schedule for the 2nd and 3rd dose of r- HB vaccine appropriate? How frequent is post- vaccination breakthrough HBV infection, and which genotype is associated with it?
The same observations have been recorded all over the world, in Gambia[49], in Taiwan[50], in Indonesia[51], in Senegal[52], in a hyper-endemic area in Southern Italy[53], in Chinese children[54] and in Saudi Arabia[55]. However, the current results have revealed a decrease in the titer of HBsAb as subjects grow older, associated with an increased probability of becoming infected over time. A higher rate of HBV-positive cases was observed among the non-responders, when compared to subjects who mounted an elevated level of anti-HBs IgG antibodies.
By comparison, the current results showed that the AxSYM system yielded significantly more positive results than the ELISA test with respect to the detection of HBsAg 11.39% by AxSYM in comparison to 2.5% by ELISA. This finding may be due to the emergence of mutant HBV that could not be detected by ELISA or false positive AxSYM results as noted previously[56].
It was found that 1.38% of the studied groups had HBcAb, and 0.83% had HBeAb. Since serological data were not obtained either before or after vaccination, it is impossible to conclude whether these individuals were already infected at the time of vaccination or whether they had been subsequently infected with the hepatitis B virus.
Considering viraemia, in the present study, 22 (6.11%) of all participants were positive for HBV DNA; 4 cases of group III were HBsAg positive by both methods, and 18 cases of group II ( 5 of them were HBsAg positive by BioELISA 11 by AxYM and two were HBcAb positive), so 12 participants had occult HBV infection. Similarly, McMahon et al (2005)[38] found that all detected cases of HBV DNA were HBsAb negative. In China, a higher result of HBV viraemia (36% of the vaccinated one year old children) was reported[57].
In the current study, HBV-DNA/HBsAg positive children may be either born to an HBV-positive mother or infected with an HBV mutant. It was noticed in study of Karthigesu et al (1999)[58] that vaccinated children may show serological evidence of breakthrough infections, particularly if they had a low HBsAb titer. They recorded that single-point mutation at nucleotide 421 of the S gene is associated with such breakthrough infections. It was recorded also by Coleman et al (2006)[59] that a child remained both DNA/HBsAg positive for > 12 years, despite having a protective HBsAb titer against the wild type virus that had a substitution mutation of glycine to arginine at HBsAg aa position 145.
The HBV genotype E recorded in this study has not previously been reported in Egypt; the most prevalent genotype in Mediterranean, Middle East and Egypt is the genotype D[60,61]. Sequence comparisons of the obtained Egyptian strain EGYAZC P1P2P4 and HBV/E gene sequences retrieved from the Gene Bank revealed that there were only five nucleotide sequence differences between them, the closest sequence was from CMR936 AB194948. Also alignments of 87 nucleotide sequence obtained from preS1 genes from the Egyptian HBV isolate EGYAZV B2 265R and the Gene Bankstrains indicated that there is a 100% similarity with HBV genotype E PW6 EU239226. The same conclusion was arrived at by Mulders et al (2003)[30], who reported that HBV genotype/E has low sequence diversity throughout the expanses of the HBV/E crescent, which covers almost 6000 km from Senegal to Angola. this suggest that it has a short evolutionary history in humans, and is incompatible with the evolution from the closest human virus genotype D. Transmission during childhood is supposed to be the most common mode of infection in Africa, and most children infected before the age of 6 mo become chronic carriers[62]. Early age of infection and high probability of chronic carrier status results in a high rate of transmission[63]. It was speculated from this study that the presence of this genotype in Egypt for the first time may be due to virus mutation in the “a” determinant that causes this vaccine escape mutant infection. Similarly, in Argentine they found that HBV genotype E was detected in two Argentinean sisters; one of them had been vaccinated against HBV[64].
HBV breakthrough infection was induced by a novel HBV genotype (E) with respect to that reported in Egypt (genotype D). The Hepatitis-B vaccine appears to be efficient in controlling HBV infection in children and adults. It was noticed that the HBsAb level decreases by age, with increased liability to get infected, and that those with undetectable HBsAb also had a higher rate of infection. Further studies are needed to evaluate the spread of this genotype in Egypt. Furthermore, the need for and timing of a boo-
ster dose should be studied (by whom and when?).
The apparent prevalence of hepatitis B virus (HBV) infection in Egypt has decreased after the Expanded Program of Immunization (EPI) vaccination program; however, the frequency of asymptomatic HBV carriers in response to the vaccination program needs to be determined.
Studies on vaccinated children during infancy and early childhood in countries having high endemicity of chronic HBV infection have shown that more than 50% of participants had measurable anti-HBs levels of at least 10 mIU/L 4 to 10 years after vaccination. Population-based studies in Alexandria, Egypt, of HB immunization after 10-15 years follow-up, showed a reduction in chronic HBsAg carrier prevalence from high (8% or greater) to low (< 2%) endemicity in immunized cohorts of infants. Thesame observations were recorded all over the world, in Gambia, in Taiwan, in Indonesia, in Senegal, in a hyper endemic area in Southern Italy, in Chinese children and in Saudi Arabia.
Considering viraemia and breakthrough infections, it was observed that there is a decrease in the titer of HBsAb as age progresses, with an increased probability to become infected over time. Similar results were recorded by McMahon et al, in China. Kato et al and Karthigesu et al all detected cases of positive HBV DNA in those who were HBsAb negative than in those who had HBsAb. The genotype of HBV in this study was genotype E, which has not previously been reported in Egypt; genotype D is the most prevalent in Mediterranean and Middle East and Egypt. Similarly in Argentina they found the HBV genotype E in two Argentinean sisters; one of them had been vaccinated against HBV.
Further studies are needed to evaluate the spread of the HBV genotype E in Egypt. The Hepatitis -B vaccine appears to be efficient in controlling HBV infection in both children and adults, so it is recommended that it should be given to the high risk groups all over the country. It was noticed that HBsAb level decreases with age leading to increased liability to get infected, and that those with undetectable HBsAb have is a higher rate of infection, so the need for a booster dose should be studied (to whom and when?). The number of HBsAg positive samples by AxSYM was higher than that of the BioELIS test. This raises the question of whether they were true or false positives? Further studies using different kinds of ELISA tests are needed to confirm or deny this observation.
This is an interesting study that investigated HBV vaccine efficacy in Egypt. It is readable and publishable.
Peer reviewers: Hatim Mohamed Yousif Mudawi, Associate Professor of Medicine, department of Internal medicine, Faculty of medicine, University of Khartoum, Sudan, PO BOX 2245, Khartoum, Sudan; Sang Hoon Ahn, MD, PhD, Professor, Department of Internal Medicine, Yonsei University College of Medicine, 250 Sungsanno, Seodaemun-gu, Seoul 120752, South Korea
S- Editor Zhang HN L- Editor Herholdt A E- Editor Zhang L
| 1. | Bassily S, Strickland GT, Abdel-Wahab MF, Esmat GE, Narooz S, el-Masry NA, Constantine NT, Struewing JP. Efficacy of hepatitis B vaccination in primary school children from a village endemic for Schistosoma mansoni. J Infect Dis. 1992;166:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | el-Sayed HF, Abaza SM, Mehanna S, Winch PJ. The prevalence of hepatitis B and C infections among immigrants to a newly reclaimed area endemic for Schistosoma mansoni in Sinai, Egypt. Acta Trop. 1997;68:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Heijtink RA, van Bergen P, van Roosmalen MH, Sünnen CM, Paulij WP, Schalm SW, Osterhaus AD. Anti-HBs after hepatitis B immunization with plasma-derived and recombinant DNA-derived vaccines: binding to mutant HBsAg. Vaccine. 2001;19:3671-3680. [PubMed] |
| 4. | West DJ, Calandra GB, Hesley TM, Ioli V, Miller WJ. Control of hepatitis B through routine immunization of infants: the need for flexible schedules and new combination vaccine formulations. Vaccine. 1993;11 Suppl 1:S21-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Mansour E, Baron ER. The expanded programme on immunization in Egypt 1984–1995. Cairo: Ministry of Health, Child Survival Project 1995; XXVIII. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | El Ghandoure S, El Sayed H, Abdel Hamid A, Gad S. Effectiveness of hepatitis- B vaccination in Egyptian Infant in Ismailia Governorate. Suez Canal Univ Med J. 1998;1:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Mansour E, Abdul-Rahim S, Batouty G, Zaghloul I, Abdel-Hadi S. Integration of hepatitis B immunization in the Expanded Program on Immunization of the Child Survival Project. J Egypt Public Health Assoc. 1993;68:487-494. [PubMed] |
| 8. | André F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine. 2000;18 Suppl 1:S20-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Banerjee A, Banerjee S, Chowdhury A, Santra A, Chowdhury S, Roychowdhury S, Panda CK, Bhattacharya SK, Chakravarty R. Nucleic acid sequence analysis of basal core promoter/precore/core region of hepatitis B virus isolated from chronic carriers of the virus from Kolkata, eastern India: low frequency of mutation in the precore region. Intervirology. 2005;48:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Ding L, Zhang M, Wang Y, Zhou S, Kong W, Smego RA. A 9-year follow-up study of the immunogenicity and long-term efficacy of plasma-derived hepatitis B vaccine in high-risk Chinese neonates. Clin Infect Dis. 1993;17:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Xu ZY, Duan SC, Margolis HS, Purcell RH, Ou-Yang PY, Coleman PJ, Zhuang YL, Xu HF, Qian SG, Zhu QR. Long-term efficacy of active postexposure immunization of infants for prevention of hepatitis B virus infection. United States-People’s Republic of China Study Group on Hepatitis B. J Infect Dis. 1995;171:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Shokrgozar MA, Shokri F. Enumeration of hepatitis B surface antigen-specific B lymphocytes in responder and non-responder normal individuals vaccinated with recombinant hepatitis B surface antigen. Immunology. 2001;104:75-79. [PubMed] |
| 13. | Jenson HB. Nonresponders to hepatitis B vaccine. Postgrad Med. 2000;107:97-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Tran T, Keeffe E. Management of the Pregnant Hepatitis B Patient. Current Hepatitis Reports. 2008;7:12–17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Fortuin M, Karthigesu V, Allison L, Howard C, Hoare S, Mendy M, Whittle HC. Breakthrough infections and identification of a viral variant in Gambian children immunized with hepatitis B vaccine. J Infect Dis. 1994;169:1374-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Hsu HY, Chang MH, Ni YH, Lin HH, Wang SM, Chen DS. Surface gene mutants of hepatitis B virus in infants who develop acute or chronic infections despite immunoprophylaxis. Hepatology. 1997;26:786-791. [PubMed] |
| 17. | Hsu HY, Chang MH, Ni YH, Chen HL. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut. 2004;53:1499-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 772] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 19. | Singh H, Aggarwal R, Singh RL, Naik SR, Naik S. Frequency of infection by hepatitis B virus and its surface mutants in a northern Indian population.. Indian J Gastroenterol. 2003;22:132-137. [PubMed] |
| 20. | Gabbuti A, Romanò L, Blanc P, Meacci F, Amendola A, Mele A, Mazzotta F, Zanetti AR. Long-term immunogenicity of hepatitis B vaccination in a cohort of Italian healthy adolescents. Vaccine. 2007;25:3129-3132. [PubMed] |
| 21. | Van Damme P, Van Herck K. A review of the long-term protection after hepatitis A and B vaccination. Travel Med Infect Dis. 2007;5:79-84. [PubMed] |
| 22. | Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Norder H, Couroucé AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 587] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 24. | Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol. 2000;81:67-74. [PubMed] |
| 25. | Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002;83:2059-2073. [PubMed] |
| 26. | Kaucner C, Stinear T. Sensitive and rapid detection of viable Giardia cysts and Cryptosporidium parvum oocysts in large-volume water samples with wound fiberglass cartridge filters and reverse transcription-PCR. Appl Environ Microbiol. 1998;64:1743-1749. [PubMed] |
| 27. | Naito H, Hayashi S, Abe K. Rapid and specific genotyping system for hepatitis B virus corresponding to six major genotypes by PCR using type-specific primers. J Clin Microbiol. 2001;39:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876-4882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29832] [Cited by in RCA: 27632] [Article Influence: 986.9] [Reference Citation Analysis (0)] |
| 30. | Mulders MN, Venard V, Njayou M, Edorh AP, Bola Oyefolu AO, Kehinde MO, Muyembe Tamfum JJ, Nebie YK, Maiga I, Ammerlaan W. Low genetic diversity despite hyperendemicity of hepatitis B virus genotype E throughout West Africa. J Infect Dis. 2004;190:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Zahn A, Li C, Danso K, Candotti D, Owusu-Ofori S, Temple J, Allain JP. Molecular characterization of occult hepatitis B virus in genotype E-infected subjects. J Gen Virol. 2008;89:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Märschenz S, Endres AS, Brinckmann A, Heise T, Kristiansen G, Nürnberg P, Krüger DH, Günther S, Meisel H. Functional analysis of complex hepatitis B virus variants associated with development of liver cirrhosis. Gastroenterology. 2006;131:765-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Fang ZL, Sabin CA, Dong BQ, Wei SC, Chen QY, Fang KX, Yang JY, Huang J, Wang XY, Harrison TJ. Hepatitis B virus pre-S deletion mutations are a risk factor for hepatocellular carcinoma: a matched nested case-control study. J Gen Virol. 2008;89:2882-2890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8936] [Cited by in RCA: 8082] [Article Influence: 404.1] [Reference Citation Analysis (0)] |
| 35. | Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1394] [Cited by in RCA: 1122] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 36. | Huang LM, Chiang BL, Lee CY, Lee PI, Chi WK, Chang MH. Long-term response to hepatitis B vaccination and response to booster in children born to mothers with hepatitis B e antigen. Hepatology. 1999;29:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Ni YH, Chang MH, Huang LM, Chen HL, Hsu HY, Chiu TY, Tsai KS, Chen DS. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796-800. [PubMed] |
| 38. | McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142:333-341. [PubMed] |
| 39. | Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 40. | el-Sawy IH, Mohamed ON. Long-term immunogenicity and efficacy of a recombinant hepatitis B vaccine in Egyptian children. East Mediterr Health J. 1999;5:922-932. [PubMed] |
| 41. | Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis. 2003;187:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Lu CY, Chiang BL, Chi WK, Chang MH, Ni YH, Hsu HM, Twu SJ, Su IJ, Huang LM, Lee CY. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology. 2004;40:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Alam MM, Zaidi SZ, Malik SA, Naeem A, Shaukat S, Sharif S, Angez M, Khan A, Butt JA. Serology based disease status of Pakistani population infected with hepatitis B virus. BMC Infect Dis. 2007;7:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med. 2004;351:2832-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1: Immunization of Infants, Children, and Adolescents. MMWR. 2005;54:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Kane MA. Status of hepatitis B immunization programmes in 1998. Vaccine. 1998;16 Suppl:S104-S108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Shatat HZ, Kotkat AM, Imam ZI. Follow-up study of the immunogenecity and efficiency of hepatitis B vaccine in Egyptian children. J Med Res Inst. 2000;21:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Reda AA, Arafa MA, Youssry AA, Wandan EH, Ab de Ati M, Daebees H. Epidemiologic evaluation of the immunity against hepatitis B in Alexandria, Egypt. Eur J Epidemiol. 2003;18:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Viviani S, Jack A, Hall AJ, Maine N, Mendy M, Montesano R, Whittle HC. Hepatitis B vaccination in infancy in The Gambia: protection against carriage at 9 years of age. Vaccine. 1999;17:2946-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Hsu HM, Lu CF, Lee SC, Lin SR, Chen DS. Seroepidemiologic survey for hepatitis B virus infection in Taiwan: the effect of hepatitis B mass immunization. J Infect Dis. 1999;179:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Ruff TA, Gertig DM, Otto BF, Gust ID, Sutanto A, Soewarso TI, Kandun N, Marschner IC, Maynard JE. Lombok Hepatitis B Model Immunization Project: toward universal infant hepatitis B immunization in Indonesia. J Infect Dis. 1995;171:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Maupas P, Chiron JP, Barin F, Coursaget P, Goudeau A, Perrin J, Denis F, Mar ID. Efficacy of hepatitis B vaccine in prevention of early HBsAg carrier state in children. Controlled trial in an endemic area (Senegal). Lancet. 1981;1:289-292. [PubMed] |
| 53. | Da Villa G, Piccinino F, Scolastico C, Fusco M, Piccinino R, Sepe A. Long-term epidemiological survey of hepatitis B virus infection in a hyperendemic area (Afragola, southern Italy): results of a pilot vaccination project. Res Virol. 1998;149:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, Wang SS, Li YP, Zhang KL. Long-term efficacy of plasma-derived hepatitis B vaccine: a 15-year follow-up study among Chinese children. Vaccine. 1999;17:2661-2666. [PubMed] |
| 55. | Al-Faleh FZ, Al-Jeffri M, Ramia S, Al-Rashed R, Arif M, Rezeig M, Al-Toraif I, Bakhsh M, Mishkkhas A, Makki O. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999;38:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Diepersloot RJ, van Zantvliet-van Oostrom Y, Gleaves CA. Comparison of a chemiluminescent immunoassay with two microparticle enzyme immunoassays for detection of hepatitis B virus surface antigen. Clin Diagn Lab Immunol. 2000;7:865-866. [PubMed] |
| 57. | Kato H, Nakata K, Hamasaki K, Hida D, Ishikawa H, Aritomi T, Nakao K, Kato Y, Yano M, Eguchi K. Long-term efficacy of immunization against hepatitis B virus in infants at high-risk analyzed by polymerase chain reaction. Vaccine. 1999;18:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Karthigesu VD, Allison LM, Ferguson M, Howard CR. A hepatitis B virus variant found in the sera of immunised children induces a conformational change in the HBsAg “a” determinant. J Med Virol. 1999;58:346-352. [PubMed] |
| 59. | Coleman PF. Surveillance for hepatitis B surface antigen mutants. J Med Virol. 2006;78 Suppl 1:S56-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, Robertson BH, Locarnini S, Magnius LO. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 649] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 61. | Zekri AR, Hafez MM, Mohamed NI, Hassan ZK, El-Sayed MH, Khaled MM, Mansour T. Hepatitis B virus (HBV) genotypes in Egyptian pediatric cancer patients with acute and chronic active HBV infection. Virol J. 2007;4:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 283] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 63. | Medley GF, Lindop NA, Edmunds WJ, Nokes DJ. Hepatitis-B virus endemicity: heterogeneity, catastrophic dynamics and control. Nat Med. 2001;7:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Mathet VL, Cuestas ML, Ruiz V, Minassian ML, Rivero C, Trinks J, Daleoso G, León LM, Sala A, Libellara B. Detection of hepatitis B virus (HBV) genotype E carried--even in the presence of high titers of anti-HBs antibodies--by an Argentinean patient of African descent who had received vaccination against HBV. J Clin Microbiol. 2006;44:3435-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |