Revised: January 12, 2011
Accepted: March 19, 2011
Published online: April 27, 2011
AIM: To estimate the prevalence of cardiovascular events in Primary biliary cirrhosis (PBC) and to determine whether this risk is higher within specific subgroups of patients with PBC.
METHODS: We included 180 patients with PBC (cases) and 151 patients seen for HCV infection (controls). Medical records were reviewed and statistical analyses were performed as appropriate.
RESULTS: When compared to controls, PBC patients were older, leaner and had higher serum levels of total cholesterol, high density lipoprotein and low density cholesterol. There were more females in the PBC group (91.7% vs 43%, P < 0.001). More control subjects had smoked than the PBC patients (63.6% vs 35%, P < 0.001). The prevalence of hypertension, diabetes, coronary artery disease and stroke was similar between the two groups. Seven percent of controls and 10% of cases developed any type of cardiovascular disease (P = 0.3). Only 36.7% were asymptomatic at diagnosis. Three cardiovascular events were documented among asymptomatic patients (4.5%) and fifteen among symptomatic patients (13.2%; P = 0.06). Among PBC patients with fatigue, 10 (13.5%) had a cardiovascular event compared to 7 (6.7%) among patients without fatigue (P = 0.1).
CONCLUSION: Asymptomatic PBC patients do not have a greater frequency of cardiovascular disease; nor do patients suffering with fatigue.
- Citation: Doycheva I, Chen C, Pan JJ, Levy C. Asymptomatic primary biliary cirrhosis is not associated with increased frequency of cardiovascular disease. World J Hepatol 2011; 3(4): 93-98
- URL: https://www.wjgnet.com/1948-5182/full/v3/i4/93.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i4.93
PBC is a chronic cholestatic disease characterized by progressive immune-mediated destruction of interlobular bile ducts[1]. Serum cholesterol is frequently elevated in patients with primary biliary cirrhosis, albeit in a variable fashion[2]. Most of the cholesterol elevation is due to lipoprotein X, a lipoprotein fraction within the low density cholesterol (LDL) region[3]. Typically, we observe an increase in LDL cholesterol that parallels disease severity as a result of the progressive loss of LDL receptors in the liver, thus leading to failure of clearance by the hepatocytes[4]. The high density lipoprotein (HDL), on the other hand, tends to be higher in the earlier stages of disease and lower with more advanced PBC[5].
In the general population, hyperlipidemia is well known to predict increased morbid-mortality from cardiovascular disease[6], the leading cause of death in the United States. Thus, especially as PBC affects middle-aged patients, the presence of hyperlipidemia in this population was initially thought to be associated with an increased risk of cardiovascular disease. However, a causation link between hyperlipidemia and cardiovascular disease in PBC could not be established, even when ultrasound imaging of the carotids was used for measurement of the intima-media thickness as a marker for subclinical cardiovascular disease[2,7]. This controversy has been revisited due to[1] evidence that patients with asymptomatic PBC may have an increased non liver-related mortality in comparison to patients with symptomatic PBC and[2] evidence that fatigue may be associated with increased cardiovascular deaths.
Thus, in the present study we aimed to investigate the frequency of cardiovascular disease [coronary artery disease (CAD), transient ischemic attack (TIA) and stroke (CVA)] in patients with PBC in comparison to controls and to determine if asymptomatic patients with PBC had an increased frequency of cardiovascular events compared to those symptomatic at initial diagnosis.
Our cohort included consecutive patients with PBC seen for the first time at the University of Florida (Gainesville, Florida) between January 1, 1997 and December 31, 2005. Cases were identified by ICD code and subsequent chart review from a database of patients with cholestatic liver diseases seen at the Hepatology clinic at the University of Florida. A diagnosis of PBC required either histological confirmation and the presence of chronic cholestasis with or without positive antimitochondrial antibody (AMA) or a positive AMA titer above 1:40 associated with persistent elevation of serum alkaline phosphatase and without significant elevation of transaminases (less than 3 times upper limit of normal), even in the absence of a previous liver biopsy. Mild and advanced histological disease was defined as stage 0-2 and 3-4 respectively based on the Metavir scoring system. As a control group, we studied consecutive patients with chronic hepatitis C referred for HCV treatment at our institution during the same period of time.
The following information was collected for each patient: demographic data, laboratory and liver histology information, body mass index (BMI), smoking habits, history of hypertension, diabetes, coronary artery disease including acute myocardial infarction and angina, transient ischemic attack , ischemic and hemorrhagic stroke, date of liver transplant if performed, clinical presentation at initial diagnosis, medication history, date and cause of death if available. Patients were defined as initially asymptomatic if they did not have any symptoms attributable to PBC at the time of diagnosis, according to their recollection and information from the medical records. Symptoms attributable to PBC include fatigue without identifiable cause, pruritus, jaundice, gastrointestinal bleeding due to esophageal or gastric varices, hepatic encephalopathy, ascites or lower extremities edema.
The Mayo risk score was calculated, when possible, for each PBC patient with variables obtained at the time of initial presentation at the University of Florida. Mayo risk score = 0.871 × loge (bilirubin in mg/dL) + -2.53 × loge (albumin in g/dL) + 0.039 age in years + 2.83 loge (prothrombin time in seconds) + 0.859 edema[8]. Patients were followed as a historical cohort until the date of last visit, date of death or date of liver transplant.
Data are presented as means ± SD or median with range for continuous variables and as frequencies for categorical variables. Categorical variables were analyzed by the Chi-Square (c2) or Fisher’s exact test. Continuous variables were analyzed by the student-t test or Mann-Whitney nonparametric test. All reported P values were two-sided and a P value of less than 0.05 was considered to be statistical significant. All statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL).
A total of 180 patients with PBC (“cases”) and 151 patients with chronic hepatitis C (“controls”) were included in the study. Cases were followed for an average of 2.9 ± 2.8 years (range 0-11) and controls for an average of 3.2 ± 2.3 years (range 0-10). Table 1 shows baseline clinical characteristics of cases and controls. The majority of the cases were females and Caucasian was the predominant race in both groups. Compared with controls, cases were older (median age 56.3 years vs 49.8 years) and had higher levels of serum total cholesterol (median cholesterol 216 mg/dL vs 157.5 mg/dL), low density lipoprotein [(LDL), median LDL 127 mg/dL vs 80 mg/dL] and high density lipoprotein [(HDL), median HDL 54 mg/dL vs 48 mg/dL], whereas both groups had similar serum triglyceride levels. Despite being small in number, significantly more cases were on statin therapy than controls (Table 1).
| Variables | Controls | PBC | P value |
| n = 151 | n = 180 | ||
| Age (years) | 49.8 (20-72) | 56.3 (22-74) | < 0.001 |
| Female gender | 65 (43) | 165 (91.7) | < 0.001 |
| Caucasian | 123 (81.5) | 158 (87.8) | 0.1 |
| AMA | 0 (0) | 137 (77.8) | |
| BMI (kg/m2) | 28 (17-50) | 26.8 (17.6-51) | 0.09 |
| Hypertension | 49 (32.5) | 48 (26.8) | 0.3 |
| Diabetes | 21 (13.9) | 26 (14.5) | 0.9 |
| Smoking | 96 (63.6) | 62 (35) | < 0.001 |
| Stage of diseasea | 0.001 | ||
| 0-2 | 28 (21.1) | 64 (38.6) | |
| 3-4 | 105 (78.9) | 102 (61.4) | |
| Total cholesterol (mg/dL) | 157.5 (73-320) | 216 (81-2055) | < 0.001 |
| LDL (mg/dL) | 80 (20-189) | 127 (12–539) | < 0.001 |
| HDL (mg/dL) | 48 (20-101) | 54 (17-163) | 0.001 |
| Triglyceride (mg/dL) | 117 (19-2334) | 104 (32-736) | 0.2 |
| Statin use | 2 (1.3) | 15 (8.3) | 0.004 |
| CAD | 8 (5.3) | 14 (7.8) | 0.4 |
| TIA | 2 (1.3) | 1 (0.6) | 0.6 |
| Stroke | 0 (0) | 3 (1.7) | 0.3 |
| Any CVD | 10 (6.6) | 18 (10) | 0.3 |
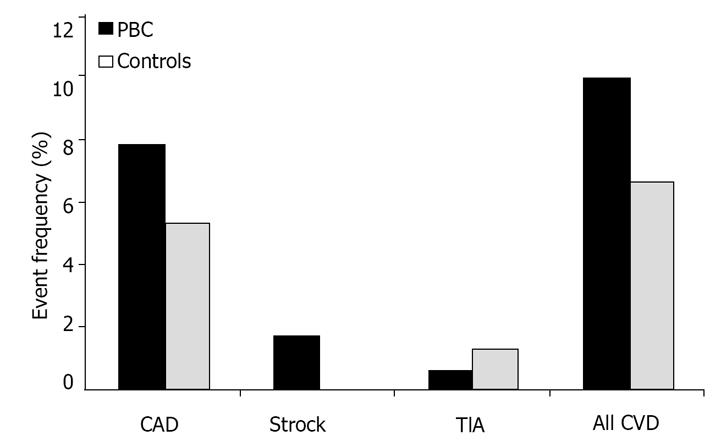
As far as risk factors for cardiovascular disease, almost twice as many patients in the control group had smoking habits compared to patients with PBC. However, no difference was observed with respect to rates of hypertension, diabetes, overweight status or obesity. Approximately 7% of controls and 10% of cases developed a cardiovascular disease such as CAD, TIA or stroke. Despite having more significant hyperlipidemia, a well established risk for cardiovascular diseases, cases did not have significantly more cardiovascular events than controls (Table 1 and Figure 1).
Advanced fibrosis defined as a Metavir score = 3 was more common among controls than cases (78.9% vs 61.4%, P = 0.001, Table 1). Cases with advanced fibrosis had lower serum triglyceride levels compared to cases with mild to moderate fibrosis (Table 2). Otherwise, there was no significant difference of serum total cholesterol, HDL or LDL levels between cases with mild to moderate and cases with advanced fibrosis (Table 2).
| Stage 0-2 | Stage 3-4 | P value | |
| Total cholesterol (mg/dL) | 229.5 (129-2055) | 210 (81-612) | 0.3 |
| HDL (mg/dL) | 56 (20-102) | 53 (20-163) | 0.4 |
| LDL (mg/dL) | 130.5 (52-239) | 127 (12-539) | 0.9 |
| Triglyceride (mg/dL) | 128 (47-736) | 98.5 (32-462) | 0.008 |
Cases were further divided into two subgroups based on the presence or absence of symptoms. Approximately two thirds of cases were symptomatic. Among the 114 symptomatic patients, 73 (64%) had fatigue and 57 (50%) had pruritus. There was a trend toward higher serum cholesterol level in asymptomatic patients (P = 0.06) (Table 3). Nevertheless, there was no significant difference in other characteristics, including stage of disease, between the two subgroups (Table 3). Despite a trend toward more of “any cardiovascular disease” in the symptomatic subgroup (P = 0.06), there was no significant difference in the frequency of individual cardiovascular events between the two subgroups (Table 3).
| Variables | Asymtomatic | Symptomatic | P value |
| n = 66 | n = 114 | ||
| Age (years) | 56.7 (31-74) | 56.0 (22-73) | 0.4 |
| Female gender | 58 (87.9) | 107 (93.9) | 0.2 |
| Caucasian | 60 (90.9) | 98 (86) | 0.3 |
| AMA | 50 (78.1) | 87 (77.7) | 1 |
| BMI | 26.2 (19-43) | 27 (17.6-51) | 0.4 |
| Hypertension | 19 (29.2) | 29 (25.4) | 0.6 |
| Diabetes | 12 (18.5) | 14 (12.3) | 0.3 |
| Smoking | 18 (28.6) | 44 (38.6) | 0.2 |
| Stage of fibrosisa | 0.6 | ||
| 0-2 | 21 (35.6) | 43 (40.2) | |
| 3-4 | 38 (64.4) | 64 (59.8) | |
| Total cholesterol (mg/dL) | 223.5 (84-2055) | 209.5 (81-612) | 0.06 |
| LDL (mg/dL) | 134 (34-505) | 123 (12-539) | 0.2 |
| HDL (mg/dL) | 57 (17-163) | 53 (20-118) | 0.5 |
| Triglyceride (mg/dL) | 105 (32-527) | 104 (32-736) | 0.6 |
| Statin use | 8 (12.1) | 7 (6.1) | 0.2 |
| CAD | 3 (4.5) | 11 (9.6) | 0.22 |
| TIA | 0 | 1 (0.9) | 1 |
| Stroke | 0 (0) | 3 (2.6) | 0.3 |
| Any CVD | 3 (4.5) | 15 (13.2) | 0.06 |
When cases were divided into those with and without self reported fatigue, a trend towards higher serum total cholesterol levels was observed in cases without fatigue. This trend was not accompanied by significant differences with respect to cardiovascular risk factors or number of events between the two groups (Table 4).
| With fatigue | Without fatigue | P value | |
| n = 74 | n = 104 | ||
| Age (years) | 54.3 (22-73) | 57.1 (31-74) | 0.4 |
| Female gender | 71 (95.9) | 92 (88.5) | 0.08 |
| Caucasian | 63 (85.1) | 93 (89.4) | 0.4 |
| AMA | 57 (77) | 79 (78.2) | 0.9 |
| BMI (kg/m2) | 27 (17.6-39.2) | 26.2 (17.6-51) | 0.4 |
| Hypertension | 18 (24.3) | 29 (28.2) | 0.6 |
| Diabetes | 10 (13.5) | 15 (14.6) | 0.8 |
| Smoking | 26 (35.1) | 36 (35.6) | 0.9 |
| Stage of fibrosisa | 0.7 | ||
| 0-2 | 26 (37.1) | 38 (40.4) | |
| 3-4 | 44 (62.9) | 56 (59.6) | |
| Total cholesterol (mg/dL) | 203 (83-612) | 217 (81-2055) | 0.06 |
| LDL (mg/dL) | 123.5 (29-539) | 131 (12-505) | 0.2 |
| HDL (mg/dL) | 55 (20-118) | 54 (17-163) | 0.5 |
| Triglyceride (mg/dL) | 110 (32-462) | 104 (32-736) | 0.6 |
| Statin use | 6 (8.1) | 9 (8.7) | 0.9 |
| CAD | 7 (9.5) | 7 (6.7) | 0.5 |
| TIA | 1 (1.4) | 0 (0) | 0.4 |
| Stroke | 2 (2.7) | 0 (0) | 0.2 |
| Any CVD | 10 (13.5) | 7 (6.7) | 0.1 |
Our study suggests that hyperlipidemia in patients with PBC is not associated with higher risk for atherosclerotic events when compared to a control group consisting of patients with non-cholestatic liver disease. PBC patients had higher total cholesterol, LDL and HDL with similar incidence of cardiovascular and cerebrovascular events. Furthermore, we found no difference in the rate of cardiovascular events between symptomatic and asymptomatic patients and no difference in cardiac events in patient with fatigue.
Reports on the effect of hyperlipidemia associated with PBC on the risk of cardiovascular mortality in this population are conflicting. In a study by Crippin et al, 312 patients with PBC were followed prospectively for a median of 7.4 years and the risk of death related to atherosclerotic disease was not increased in patients with hyperlipidemia[9]. Alloca et al assessed intima-media thickness (IMT) with carotid artery ultrasound as a surrogate marker for subclinical atherosclerosis[7]. In this study, the control population consisted of a combination of healthy individuals and patients with hepatitis C. The investigators demonstrated increased IMT values in controls with hyperlipidemia but not in patients with PBC and corresponding serum levels of total cholesterol. Our findings are in agreement with these studies.
We also evaluated confounding risk factors for cardiovascular disease in both PBC patients and controls: although there were more smokers in the controls and a female predominance in the PBC group, hypertension, diabetes and obesity occurred with similar frequency. Interestingly, the lower smoking rate in PBC is in contrast with case control studies which have found excess smoking in patients with PBC [10-12]. This finding is likely to be related to the fact that our controls consisted of patients with HCV instead of the general population.
All PBC patients in our study were on ursodeoxycholic acid (UDCA), the current mainstay of therapy. UDCA is known to have cholesterol-lowering effect due to improvement of cholestasis and modifications of cholesterol metabolism. Both effects are most likely due to changes in endogenous bile acid composition[13]. However, data is lacking regarding the effect of UDCA on the development and progression of cardiovascular disease (CVD). In addition, 8.3 % of patients in the PBC group had received or were receiving statins at the time of diagnosis compared to only 1.3 % of the control group. A recent study by Stojakovic and colleagues demonstrated a possible beneficial effect of low dose atorvastatin in patients with additional risk factors for CVD and early stage of disease[14]. They followed 19 patients treated with 10 mg of atorvastatin for one year and observed reduced cholesterol levels and endothelial inflammation, as well as improved vascular function as reflected by flow-mediated dilation of the brachial artery. Importantly, atorvastatin was safe in that population. Nevertheless, a reduction in risk of cardiovascular events and mortality in patients with PBC and hyperlipidemia treated with statins has not been demonstrated yet; therefore the small number of events in the present study cannot be attributed to the use of statins by a minority of subjects.
One third of PBC patients in our study were asymptomatic at the time of diagnosis and there was no increased mortality from CAD in this population. We found that asymptomatic patients have slightly higher cholesterol levels in comparison with symptomatic ones. Prince et al reached a similar conclusion with respect to cholesterol levels[15]. However, that study also found a significant increase in ischemic heart disease among asymptomatic patients (14.9%) in comparison to symptomatic patients (5.9%) with PBC. Although it is possible that these differences are due to the smaller population size in the present study, the finding of increased cardiovascular deaths in asymptomatic PBC patients has not been validated by other studies[16].
Among the symptomatic patients, 64% had fatigue as one of the presenting symptoms. The patients with fatigue had a similar lipid profile and rate of atherosclerotic events in comparison to those without fatigue. These results are in agreement with the findings from Bjornsson et al who followed 208 patients with PBC and found that fatigue was a predictor of liver-related mortality and need for transplantation but not of non-liver-related mortality[17]. Only the older patients had an increase in cardiovascular complications. In contrast, Jones et al found that patients with severe fatigue have a higher rate of cardiac deaths than liver-related mortality in a four year follow up[18]. In subsequent studies, the authors hypothesized that this increased mortality due to cardiovascular events was related to autonomic dysfunction in patients with PBC[19]. Further studies investigating the role of fatigue as a predictor for CVD are needed.
Our study is limited by its retrospective nature and small number of events. Also, our control group consisted of hepatitis C patients and a very recent study showed that HCV infection is associated with lower lipid levels[20], but this information was not available at the time of initiation of the study. In addition, there are more males in the control group compared to the cases. One could initially consider that with more males there is an increased risk of cardiovascular events and that is the reason why no difference was seen in comparison to cases. However, it is important to notice that cases were older and most were post-menopausal, thus with a risk of cardiovascular events similar to that encountered in males.
Despite these limitations, we found that hyperlipidemia associated with PBC does not seem to be associated with an increased risk for atherosclerotic events. Thus, the need for treatment should be assessed on an individual basis and take into account the presence of additional known risk factors. Furthermore, our study did not show an increased risk for CVD among asymptomatic patients or those with fatigue. Further investigation involving a larger patient population followed for a longer period of time is warranted to confirm these findings, perhaps including multiple centers.
Primary biliary cirrhosis (PBC) is a chronic cholestatic disease associated with hyperlipidemia. In comparison to the general population, hyperlipidemia in PBC is not clearly associated with increased risk for cardiovascular disease. Approximately 50% of patients are asymptomatic at diagnosis; fatigue is the most common symptom in early stage.
Previous studies have shown that patients with asymptomatic PBC and those presenting with fatigue only may have increased cardiovascular mortality.
This study did not find a difference in the frequency of cardiovascular events between symptomatic and asymptomatic patients with PBC. In addition, patients with fatigue were not at an increased risk of cardiovascular events compared to PBC patients without fatigue.
The need for treatment of hyperlipidemia in PBC patients should be assessed on individual basis and according to the presence of additional known risk factors.
Cardiovascular events included coronary artery disease presenting with myocardial infarction or angina, transient ischemic attack and stroke.
This is an important research question and is very relevant. The disease primary biliary cirrhosis is rare in many parts of the world and the research is novel. The paper reads well and adds to the current body of literature.
Peer reviewer: Krishnan Rajeshwari, Professor, Department of Pediatrics, Maulana Azad Medical College, Bahadur Shah Zafar Marg, New Delhi 110002, India
S- Editor Zhang HN L- Editor Roemmele A E- Editor Zhang L
| 2. | Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, Podda M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265-269. |
| 3. | Chang PY, Lu SC, Su TC, Chou SF, Huang WH, Morrisett JD, Chen CH, Liau CS, Lee YT. Lipoprotein-X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res. 2004;45:2116-2122. |
| 4. | Gylling H, Farkkila M, Vuoristo M, Miettinen TA. Metabolism of cholesterol and low- and high-density lipoproteins in primary biliary cirrhosis: cholesterol absorption and synthesis related to lipoprotein levels and their kinetics. Hepatology. 1995;21:89-95. |
| 5. | Jahn CE, Schaefer EJ, Taam LA, Hoofnagle JH, Lindgren FT, Albers JJ, Jones EA, Brewer HB Jr. Lipoprotein abnormalities in primary biliary cirrhosis. Association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology. 1985;89:1266-1278. |
| 6. | Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700-1707. |
| 7. | Allocca M, Crosignani A, Gritti A, Ghilardi G, Gobatti D, Caruso D, Zuin M, Podda M, Battezzati PM. Hypercholesterolaemia is not associated with early atherosclerotic lesions in primary biliary cirrhosis. Gut. 2006;55:1795-1800. |
| 8. | Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, Dickson ER. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 1999;19:115-121. |
| 9. | Crippin JS, Lindor KD, Jorgensen R, Kottke BA, Harrison JM, Murtaugh PA, Dickson ER. Hypercholesterolemia and atherosclerosis in primary biliary cirrhosis: what is the risk? Hepatology. 1992;15:858-862. |
| 10. | Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-1202. |
| 11. | Howel D, Fischbacher CM, Bhopal RS, Gray J, Metcalf JV, James OF. An exploratory population-based case-control study of primary biliary cirrhosis. Hepatology. 2000;31:1055-1060. |
| 12. | Prince MI, Ducker SJ, James OF. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut. 2010;59:508-512. |
| 13. | Balan V, Dickson ER, Jorgensen RA, Lindor KD. Effect of ursodeoxycholic acid on serum lipids of patients with primary biliary cirrhosis. Mayo Clin Proc. 1994;69:923-929. |
| 14. | Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Sourij H, Stauber RE, Winkler K, Marz W. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis. 2010;209:178-183. |
| 15. | Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865-870. |
| 16. | Van Dam GM, Gips CH. Primary biliary cirrhosis in The Netherlands. An analysis of associated diseases, cardiovascular risk, and malignancies on the basis of mortality figures. Scand J Gastroenterol. 1997;32:77-83. |
| 17. | Bjornsson E, Kalaitzakis E, Neuhauser M, Enders F, Maetzel H, Chapman RW, Talwalkar J, Lindor K, Jorgensen R. Fatigue measurements in patients with primary biliary cirrhosis and the risk of mortality during follow-up. Liver Int. 2010;30:251-258. |
| 18. | Jones DE, Bhala N, Burt J, Goldblatt J, Prince M, Newton JL. Four year follow up of fatigue in a geographically defined primary biliary cirrhosis patient cohort. Gut. 2006;55:536-541. |
| 19. | Newton JL, Hudson M, Tachtatzis P, Sutcliffe K, Pairman J, Burt JA, Jones DE. Population prevalence and symptom associations of autonomic dysfunction in primary biliary cirrhosis. Hepatology. 2007;45:1496-1505. |
| 20. | Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030-1037. |