Published online Jul 27, 2010. doi: 10.4254/wjh.v2.i7.251
Revised: July 7, 2010
Accepted: July 14, 2010
Published online: July 27, 2010
Advances in recent years in the understanding of, and the genetic diagnosis of hereditary hemochromatosis (HH) have changed the approach to iron overload hereditary diseases. The ability to use a radiologic tool (MRI) that accurately provides liver iron concentration determination, and the presence of non-invasive serologic markers for fibrosis prediction (serum ferritin, platelet count, transaminases, etc), have diminished the need for liver biopsy for diagnosis and prognosis of this disease. Consequently, the role of liver biopsy in iron metabolism disorders is changing. Furthermore, the irruption of transient elastography to assess liver stiffness, and, more recently, the ability to determine liver fibrosis by means of MRI elastography will change this role even more, with a potential drastic decline in hepatic biopsies in years to come. This review will provide a brief summary of the different non-invasive methods available nowadays for diagnosis and prognosis in HH, and point out potential new techniques that could come about in the next years for fibrosis prediction, thus avoiding the need for liver biopsy in a greater number of patients. It is possible that liver biopsy will remain useful for the diagnosis of associated diseases, where other non-invasive means are not possible, or for those rare cases displaying discrepancies between radiological and biochemical markers.
- Citation: Castiella A, Zapata E, Alústiza JM. Non-invasive methods for liver fibrosis prediction in hemochromatosis: One step beyond. World J Hepatol 2010; 2(7): 251-255
- URL: https://www.wjgnet.com/1948-5182/full/v2/i7/251.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i7.251
Liver biopsy with histological study and liver iron concentration quantification has long been the gold standard for the diagnosis and prognosis of hemochromatosis[1]. The great developments that have occurred in the field of iron overload diseases in the last 15 years - identification of the HFE gene and the mutations responsible of hemochromatosis, liver iron concentration (LIC) determination by Magnetic resonance imaging (MRI) and non-invasive liver fibrosis prediction with laboratory tests[1-3] - have all diminished the role of hepatic biopsy in hemochromatosis study. Consequently, it is usually only used for prognosis purposes[4]. The development of liver cirrhosis is crucial for hemochromatosis patients, as it changes both the prognosis and the management of the disease[5].
Nevertheless, liver biopsy has associated risks inherent to the technique, with a related mortality around 1/1 000-1/10 000[6,7], as well as wide variations in the results. Sampling error studies have shown that a single biopsy will miss cirrhosis in 10%-30% of patients and incorrectly classify fibrosis by at least one stage in 20%-30%[5,8]. In addition, both patients and hemochromatosis associations have their objections to the procedure[9]. Furthermore, liver fibrosis, and, in some cases, cirrhosis, may regress after treatment with phlebotomy[10]. Concerns about complications and sampling errors have resulted in a search for non-invasive tests for cirrhosis[5].
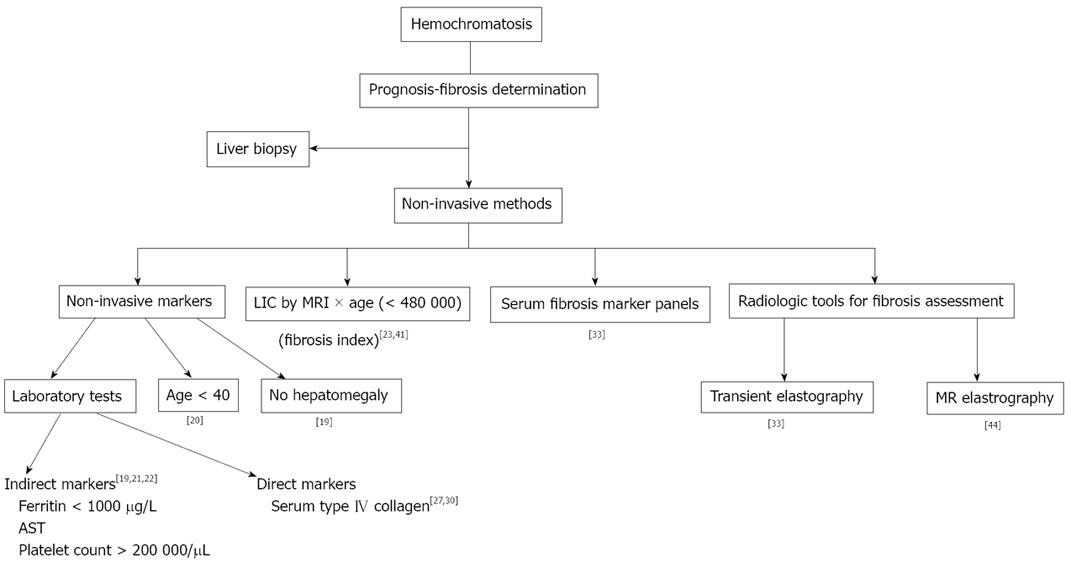
The liver is the most easily accessible tissue in which it is possible to determine the presence of iron overload. This is done by LIC measurement in hepatic biopsy[11], but there is a wide variation in measures of hepatic iron load, especially in patients with liver cirrhosis[12]. Liver biopsy no longer has a primary role in the diagnosis of hereditary hemochromatosis (HH) (Figure 1). In patients with HH, liver biopsy is nowadays performed for three main reasons[13]: (1) To determine the prognosis of the disease, calculating the fibrosis grade in the liver sample; a Serum Ferritin > 1 000 µg/L, raised aspartate aminotransferase (AST) and platelet count < 200 000/μL are considered markers of advanced liver disease, and liver biopsy has been recommended for these patients[1]; (2) To diagnose the presence of other diseases which produce iron overload, such as alcoholic liver disease and non-alcoholic speatohepatitis (NASH), and to determine their severity. When there is a coexistent pathology, liver biopsy may help to clarify the main cause of liver disease; and (3) To identify preneoplastic lesions, including iron-free foci[14] and dysplastic nodules. All of these three main reasons have been able to be assessed by non-invasive tools for quite some time[15-17].
Serological markers for non-invasive liver fibrosis determination are classified in two groups: (1) indirect markers, such as those which do not directly reflect the extracellular matrix metabolism, including transaminases, serum ferritin, and platelet count; and (2) direct markers, such as products of the extracellular matrix degradation or synthesis, e.g. procollagen-III N-terminal peptide (PIIINP), serum type IV collagen, laminin, hyaluronic acid, and tissue inhibitors of metalloproteinases (TIMP), etc[18].
Indirect markers: In the last decade, reports of non-invasive approaches for fibrosis prediction in HH have been published[19-23]. The most obvious of these are serum transaminases, but they are found to have normal levels in up to 50% of patients with cirrhosis[20].
Guyader et al[19] revised the clinical and laboratory variables of 197 HH (C282Y homozygote) patients in France. Their findings were validated in a group of 113 patients from Canada. No patient with high-degree fibrosis was found without hepatomegaly, raised AST, or serum ferritin > 1000 μg/L.
A study of 66 HH (C282Y homozygote) patients in the USA[20] revealed that age was an important feature, and that no high-degree fibrosis in patients younger than 40 was found. It has been previously suggested that patients over 45 run a greater risk of developing significant fibrosis or cirrhosis in HH[23,24].
More recently, Beaton et al[21] studied the non-invasive variables for cirrhosis prediction in 193 HH patients (C282Y homozygotes) in Canada, and the study was validated in a group of 162 patients from France. The combination of ferritin > 1 000 μg/L, platelets < 200 000/μL and raised AST correctly diagnosed the presence of cirrhosis in 77% of Canadian patients and in 90% of French patients. Morrison et al[22] proved in a USA multicenter study of phenotypic hemochromatosis patients that a ferritin value < 1 000 μg/L makes the presence of cirrhosis unlikely, regardless of patient age or transaminase values, thus avoiding the need for liver biopsy for a prognosis in these patients. Our group in the Basque Country[25,26], recently reported the utility of various non-invasive methods for fibrosis prediction in hemochromatosis, with 32 patients being included in the study of which nine presented with F3 or F4 fibrosis (four patients had cirrhosis). In our study, the combination of raised AST and a platelet count < 200 000/μL revealed a negative predictive value of 100% for high-degree fibrosis. Platelets alone had a 94% negative predictive value for high degree fibrosis; the four patients with cirrhosis had a platelet count < 200 000/μL, and three of them had a serum ferritin value < 1 000 μg/L. This is particularly unusual[27], revealing potential differences in different populations[26], but cirrhosis with a serum ferritin value < 1000 μg/L has also been reported by other groups in Canada and Australia[28,29]. Crawford et al[27] have evaluated the utility of current diagnostic algorithms for detecting cirrhosis (serum ferritin values, platelet count, and AST levels), in combination with serum markers of fibrosis (collagen type IV, hyaluronic acid), in predicting cirrhosis in HH patients. No patient with a serum ferritin < 1 000 μg/L were cirrhotic. A combination of a platelet count < 200 000/μL, ferritin > 1 000 μg/L, and raised AST failed to detect 30% (3/10) of the patients with cirrhosis (they did not have all the predicting factors), but none of the patients with cirrhosis had a platelet count > 200 000/μL and normal AST. We think that the combination of a platelet count < 200 000/μL and raised AST is very useful indication for cirrhosis prediction in HH[26].
Direct markers: Type IV collagen is an important component of the normal extracellular matrix, and serum components of type IV collagen are thought to primarily reflect matrix degradation. A serum type IV collagen level higher than 115 ng/mL has been found to be 100% sensitive in the prediction of underlying cirrhosis in HH[30]. Recently, the same group has reported similar 100% sensitivity with values > 113, but with only 56% specificity for cirrhosis[27]. Other fibrosis markers, like serum laminin and tissue inhibitor of metalloproteinase (TIMP-I) levels (or concentrations), seem to be of little value for fibrosis prediction in iron overloaded livers[30].
Jensen et al[31] showed that the serum procollagen IIIN-propeptide, previously studied by Colombo et al[32], and serum laminin, seem to be of little value in iron-loaded disorders.
Crawford et al[27] recently reported that serum hyaluronic acid with serum ferritin can accurately predict cirrhosis and thus reduce the need for liver biopsy in C282Y hemochromatosis. In their study, serum hyaluronic acid concentration > 46.5 ng/mL was 100% sensitive and 100% specific in identifying C282Y hemochromatosis patients with cirrhosis. In HH patients with serum ferritin values >1 000 μg/L, the measurement of hyaluronic acid is a noninvasive, accurate, and cost-effective method for the diagnosis of cirrhosis, and can assist in the clinical assessment of the eventual need, or not, for liver biopsy.
Serum fibrosis marker panels: In 2008, Adhoute et al[33] published a study about the diagnosis of liver fibrosis using FibroScan and other non-invasive methods in patients with hemochromatosis. They studied Fibro Test, Hepascore, APRI, FIB-4, Forns, Lok, and GUCI scores in 57 patients with HH, and 46 controls. No statistical difference was observed between the two groups in any of the non-invasive tests. In both groups, a significant correlation was found between FibroScan and Fibro Test values, Forns score, Hepascore and GUCI score. No correlation was found between Fibro Scan values and Lok score, FIB-4 score or APRI score. When comparing patients with a recent HH diagnosis (n = 10) and those with iron-depletion (n = 47), no significant differences were observed between the two groups for non-invasive methods for liver fibrosis evaluation, with the exception of the APRI and GUCI scores. A slight correlation was found between serum ferritin values and FibroScan. They concluded that biochemical markers and FibroScan may constitute reliable non-invasive means for liver fibrosis determination.
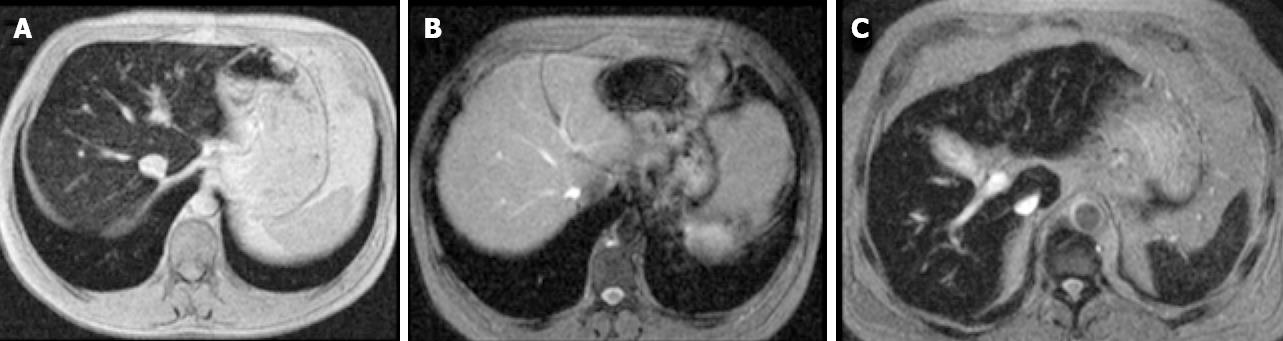
The risk of significant fibrosis or cirrhosis has been associated with the level of LIC[23]. Bassett et al[34] introduced the concept of a threshold for LIC above which cirrhosis was more likely, and Sallie et al[24] reported that, in addition to LIC, an age greater than 45 years may be a risk factor for significant fibrosis or cirrhosis. In 2005 Olynyk et al[23] showed that the duration of iron exposure by the liver increases the risk of significant fibrosis in HH, and considered patient’s age as a significant factor for fibrosis prediction. The product of age and LIC (fibrosis-index) obtained by liver biopsy or by MRI, with a 480 000 cut-off resulted in a 100% sensitivity and 86% specificity for the diagnosis of high degree- fibrosis (F3-F4)[23]. MRI can now be used for assessing iron load[35-38]; consequently, liver biopsy is no longer required for the evaluation of iron load[39,40], and the presence of iron in the reticuloendothelial system can be assessed by MRI of the spleen[40], thus discarding secondary hemochromatosis cases (Figure 2). This fibrosis index has been validated externally by our group[41]. The results we obtained were close to those in the original paper, but we think that this index must be taken into account in conjunction with other predictive parameters.
Transient elastography (FibroScan) is a new non-invasive, rapid, reproducible method, allowing assessment of liver fibrosis by measuring liver rigidity[42]. Adhoute et al[33] have studied the utility of FibroScan and other non-invasive methods in patients with hemochromatosis. They included 57 cases with 46 controls, obtaining a strong correlation between FibroScan and many biochemical markers, although ferritin levels did not correlate with FibroScan values. The prevalence of patients with FibroScan values higher than 7.1 kPa (cut-off level for significant fibrosis), was 22.8% in patients with hemochromatosis and 0% in the controls (P < 0.0001). However, the technique must be improved, because liver stiffness measurements are uninterpretable in nearly one in five cases of a large prospective series[43], mainly due to obesity, particularly increased waist circumference, and limited operator experience.
Recently, another non-invasive radiologic tool has been developed for liver fibrosis study: MR Elastography[44]. Large Az values for elasticity (> 0.990 for scores ≥ F2, ≥ F3, and F4) show that MR elastography was accurate in liver fibrosis staging and that it was superior to biochemical testing with APRIs. It seems that it will provide a higher technical success rate and a better diagnostic accuracy than ultrasound elastography and APRI for staging liver fibrosis[44]. To the best of our knowledge, this promising new non-invasive method has not yet been utilised for the study of hemochromatosis patients.
Based on the advances during the last few years, biochemical markers, LIC determination by MRI (Fibrosis index) and FibroScan and, probably, MR Elastography, all constitute reliable non-invasive means for detecting liver fibrosis. The role of liver biopsy in the study of hemochromatosis is decreasing. In future, it seems that lLiver biopsy will only be performed for diagnosis of associated diseases, or in patients where discrepancies between radiologic and biochemical markers exist. We think it is time to take a step forward and to reduce our “faith” in liver biopsy in favour of non-invasive methods for liver fibrosis prediction.
Peer reviewers: Waka Ohishi, MD, PhD, Senior Scientist, Chief, Division of Clinical Laboratories, Department of Clinical Studies, Radiation Effects Research Foundation, Hiroshima 732-0815, Japan; Regina Coeli dos Santos Goldenberg, Professor, Department of Carlos Chagas filho Biophysics Institute, Federal University of Rio de Janeiro, Rio de Janeiro 21941-902, Brazil
| 1. | Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383-3297. |
| 2. | Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R Jr, Ellis MC, Fullan A. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399-408. |
| 3. | Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, Deugnier Y. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357-362. |
| 4. | Castiella A, Alustiza JM, Artetxe J. Hereditary hemochromatosis. N Engl J Med. 2004;351:1263-1264; author reply 1263-1264. |
| 5. | Wood MJ, Skoien R, Powell LW. The global burden of iron overload. Hepatol Int. 2009;3:434-444. |
| 6. | Froehlich F, Lamy O, Fried M, Gonvers JJ. Practice and complications of liver biopsy. Results of a nationwide survey in Switzerland. Dig Dis Sci. 1993;38:1480-1484. |
| 7. | Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol. 1999;5:301-304. |
| 8. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. |
| 10. | Falize L, Guillygomarc’h A, Perrin M, Lainé F, Guyader D, Brissot P, Turlin B, Deugnier Y. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44:472-477. |
| 11. | Clark P, Britton LJ, Powell LW. The diagnosis and management of hereditary haemochromatosis. Clin Biochem Rev. 2010;31:3-8. |
| 12. | Villeneuve JP, Bilodeau M, Lepage R, Côté J, Lefebvre M. Variability in hepatic iron concentration measurement from needle-biopsy specimens. J Hepatol. 1996;25:172-177. |
| 13. | Hübschner SG. Role of liver biopsy in disorders of iron metabolism. Diagnostic Histopathology. 2008;14:577-585. |
| 14. | Deugnier YM, Charalambous P, Le Quilleuc D, Turlin B, Searle J, Brissot P, Powell LW, Halliday JW. Preneoplastic significance of hepatic iron-free foci in genetic hemochromatosis: a study of 185 patients. Hepatology. 1993;18:1363-1369. |
| 15. | Guyader D, Gandon Y, Sapey T, Turlin B, Mendler MH, Brissot P, Deugnier Y. Magnetic resonance iron-free nodules in genetic hemochromatosis. Am J Gastroenterol. 1999;94:1083-1086. |
| 16. | Alústiza JM, Castiella A. Liver fat and iron at in-phase and opposed-phase MR imaging. Radiology. 2008;641. |
| 17. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. |
| 18. | Marín Gabriel JC, Solís Herruzo JA. [Predicting liver fibrosis with non-invasive tests--a hope for the future]. Rev Esp Enferm Dig. 2008;100:605-610. |
| 19. | Guyader D, Jacquelinet C, Moirand R, Turlin B, Mendler MH, Chaperon J. Noninvasive prediction of fibrosis in C282Y homozygous hemochromatosis. Gastroenterology. 1998;115:929-936. |
| 20. | Bacon BR, Olynyk JK, Brunt EM, Britton RS, Wolff RK. HFE genotype in patients with hemochromatosis and other liver diseases. Ann Intern Med. 1999;130:953-962. |
| 21. | Beaton M, Guyader D, Deugnier Y, Moirand R, Chakrabarti S, Adams P. Noninvasive prediction of cirrhosis in C282Y-linked hemochromatosis. Hepatology. 2002;36:673-678. |
| 22. | Morrison ED, Brandhagen DJ, Phatak PD, Barton JC, Krawitt EL, El-Serag HB, Gordon SC, Galan MV, Tung BY, Ioannou GN. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med. 2003;138:627-633. |
| 23. | Olynyk JK, St Pierre TG, Britton RS, Brunt EM, Bacon BR. Duration of hepatic iron exposure increases the risk of significant fibrosis in hereditary hemochromatosis: a new role for magnetic resonance imaging. Am J Gastroenterol. 2005;100:837-841. |
| 24. | Sallie RW, Reed WD, Shilkin KB. Confirmation of the efficacy of hepatic tissue iron index in differentiating genetic haemochromatosis from alcoholic liver disease complicated by alcoholic haemosiderosis. Gut. 1991;32:207-210. |
| 25. | Castiella A, Zapata E, Otazua P, Fernández J, Alustiza JM, Ugarte M, Legasa L, Galardi A, Ugalde A, Barredo I. [Utility of various non-invasive methods for fibrosis prediction among Basque Country patients with phenotypic hemochromatosis]. Rev Esp Enferm Dig. 2008;100:611-614. |
| 26. | Castiella A, Zapata E, Otazua P. Hemochromatosis: platelets and aspartate aminotransferase are useful high-degree fibrosis marker. Hepatology. 2009;49:1781; author reply 1781-1782. |
| 27. | Crawford DH, Murphy TL, Ramm LE, Fletcher LM, Clouston AD, Anderson GJ, Subramaniam VN, Powell LW, Ramm GA. Serum hyaluronic acid with serum ferritin accurately predicts cirrhosis and reduces the need for liver biopsy in C282Y hemochromatosis. Hepatology. 2009;49:418-425. |
| 28. | Beaton M, Adams PC. Assessment of silent liver fibrosis in hemochromatosis C282Y homozygotes with normal transaminase levels. Clin Gastroenterol Hepatol. 2008;6:713-714. |
| 29. | Olynyk JK, Hagan SE, Cullen DJ, Beilby J, Whittall DE. Evolution of untreated hereditary hemochromatosis in the Busselton population: a 17-year study. Mayo Clin Proc. 2004;79:309-313. |
| 30. | George DK, Ramm GA, Walker NI, Powell LW, Crawford DH. Elevated serum type IV collagen: a sensitive indicator of the presence of cirrhosis in haemochromatosis. J Hepatol. 1999;31:47-52. |
| 31. | Jensen PD, Heickandorff L, Helweg-Larsen HM, Jensen FT, Christensen T, Ellegaard J. Serum procollagen III peptide concentration in iron overload. Eur J Haematol. 1996;57:157-164. |
| 32. | Colombo M, Annoni G, Donato MF, Conte D, Martines D, Zaramella MG, Bianchi PA, Piperno A, Tiribelli C. Serum type III procollagen peptide in alcoholic liver disease and idiopathic hemochromatosis: its relationship to hepatic fibrosis, activity of the disease and iron overload. Hepatology. 1985;5:475-479. |
| 33. | Adhoute X, Foucher J, Laharie D, Terrebonne E, Vergniol J, Castéra L, Lovato B, Chanteloup E, Merrouche W, Couzigou P. Diagnosis of liver fibrosis using FibroScan and other noninvasive methods in patients with hemochromatosis: a prospective study. Gastroenterol Clin Biol. 2008;32:180-187. |
| 34. | Bassett ML, Halliday JW, Powell LW. Value of hepatic iron measurements in early hemochromatosis and determination of the critical iron level associated with fibrosis. Hepatology. 1986;6:24-29. |
| 35. | Queiroz-Andrade M, Blasbalg R, Ortega CD, Rodstein MA, Baroni RH, Rocha MS, Cerri GG. MR imaging findings of iron overload. Radiographics. 2009;29:1575-1589. |
| 36. | Alústiza JM, Castiella A, De Juan MD, Emparanza JI, Artetxe J, Uranga M. Iron overload in the liver diagnostic and quantification. Eur J Radiol. 2007;61:499-506. |
| 37. | Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14:183-190. |
| 38. | Tziomalos K, Perifanis V. Liver iron content determination by magnetic resonance imaging. World J Gastroenterol. 2010;16:1587-1597. |
| 39. | Pietrangelo A. Non-invasive assessment of hepatic iron overload: are we finally there? J Hepatol. 2005;42:153-154. |
| 40. | Pietrangelo A, Corradini E, Ferrara F, Vegetti A, De Jong G, Luca Abbati G, Paolo Arcuri P, Martinelli S, Cerofolini E. Magnetic resonance imaging to identify classic and nonclassic forms of ferroportin disease. Blood Cells Mol Dis. 2006;37:192-196. |
| 41. | Castiella A, Emparanza JI. External validation for fibrosis predicting index in hereditary hemochromatosis. Am J Gastroenterol. 2005;100:2366-2367. |
| 42. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. |
| 43. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. |
| 44. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. |