INTRODUCTION
In the liver, the lipid content is regulated by dietary fatty acids or carbohydrates uptake, hepatic fatty acids biosynthesis, esterification, oxidation and export. Metabolic signals such as an excess of fatty acids, glucose or insulin can regulate the activity or abundance of key transcription factors to modulate hepatic lipid metabolism[1]. Many hepatic transcription factors have been identified as prospective targets for de novo lipogenesis and fatty acids oxidation including sterol regulatory element binding protein-1c (SREBP-1c), liver X receptor (LXRα), peroxisome proliferator-activated receptors (PPARα, δ, γ1, and γ2) and carbohydrate-responsive element-binding protein (chREBP)[2-5]. These factors integrate signals from various pathways and coordinate the activity of the metabolic machinery necessary for hepatic lipid metabolism with the supply of energy and fatty acids.
Recently, accumulating evidence suggests that endoplasmic reticulum (ER) stress response is critically involved in hepatic lipid metabolism. The presence of ER stress is evidenced in the liver of high fat diet-induced obese mice[6,7]. The specific type of fat deposited in the liver may directly induce ER stress response and precipitate the development of non-alcoholic fatty liver disease (NAFLD)[8]. The presence of increased circulating and/or hepatic saturated fatty acids but not polyunsaturated fatty acids may exacerbate hepatic steatosis, steatohepatitis and liver cell apoptosis through activating ER stress response[9-11]. These observations implicate a crucial role for the signaling pathways from the ER in the development and progression of hepatic lipid-associated diseases.
UNFOLDED PROTEIN RESPONSE
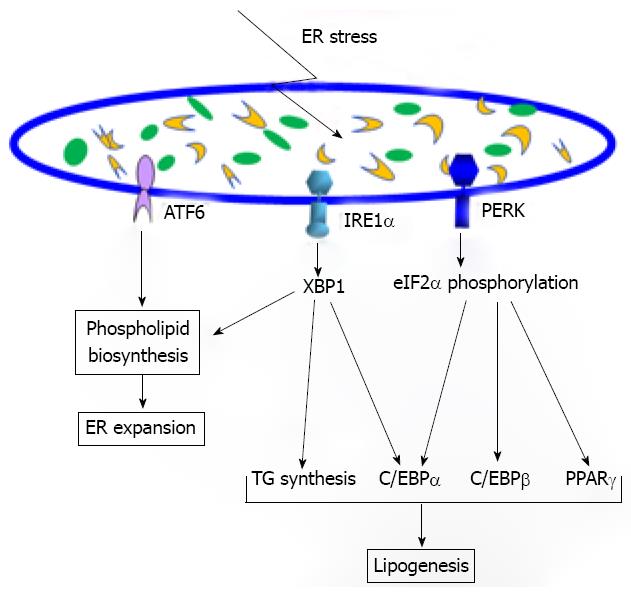
The ER is the network of interconnected membranous structures within the cytoplasm of eukaryotic cells contiguous with the outer nuclear envelope. The ER has been primarily recognized as a compartment for protein folding and assembly, a pool of free calcium and a site for lipid and sterol biosynthesis[12]. As a protein-folding compartment, the ER provides a high-fidelity quality control system to ensure that only correctly folded proteins can be transported out of the ER while unfolded or misfolded proteins are retained in the ER and eventually degraded. Under stress conditions, such as those that disrupt protein glycosylation, disulfide bond formation, ER and calcium channels, redox/oxidative stress, nutrient deprivation or viral infections, can cause accumulation of abnormally folded proteins or unassembled subunits[13-16]. The ER has evolved a highly specific signaling pathway termed Unfolded Protein Response (UPR) to help relieve the ER from the accumulation of unfolded or misfolded proteins (Figure 1). There are three ER transmembrane proteins that function as UPR transducers: inositol-requiring 1α (IRE1α), double-stranded RNA-dependent protein kinase (PKR)-like ER kinase (PERK) and activating transcription factor 6 (ATF6). It has been proposed that all three UPR transducers have the ER chaperone glucose regulate protein 78 (GRP78) bound to their ER luminal domains. This interaction allows GRP78 to act as a repressor of activation of the UPR transducers[17]. Under ER stress conditions, dissociation of GRP78 from the ER luminal domains of IRE1α, PERK and/or ATF6 allows them to be activated.
Figure 1 The involvement of the Unfolded Protein Response (UPR) signaling in lipid metabolism.
The UPR pathway through inositol-requiring 1α (IRE1α)/XBP1 or activating transcription factor 6 is involved in endoplasmic reticulum (ER) expansion by enhancing phospholipid biosynthesis under ER stress conditions. The IRE1α/XBP1 pathway also regulates lipogenesis by inducing expression of the key enzymes required for triglyceride synthesis under metabolic stress. Additionally, the IRE1α/XBP1 UPR branch can drive C/EBPα expression, facilitating adipogenesis and possibly lipogenesis. The UPR pathway through PERK/eukaryotic translation-initiation factor 2α can stimulate expression of the key lipogenic regulators C/EBPα, C/EBPβ, and PPARγ, promoting lipogenesis under metabolic stress.
Within minutes to hours after ER stress, the UPR transducer PERK-mediated translation attenuation occurs to prevent newly-synthesized proteins entering into the ER[18,19]. The activated PERK cytosolic domain leads to translational attenuation by phosphorylating α subunit of eukaryotic translation-initiation factor 2α (eIF2α). This procedure also causes cell cycle arrest in G1 phase by translational attenuation of the proteins involved in running the cell cycle. Under ER stress, IRE1α is also activated by homodimerization and autophosphorylation. Activated IRE1α functions as an endoribonuclease to splice the mRNA encoding a basic leucine zipper (bZIP) transcription factor XBP1[20-22]. In addition to its endoribonuclease activity, activated IRE1α can serve as a scaffold protein that recruits tumor-necrosis factor (TNF)-receptor-associated factor 2 (TRAF2) leading to activation of Jun amino-terminal kinases (JNK)-mediated inflammatory stress signaling pathway[23]. Upon UPR activation, ATF6 is released from the ER membrane and transits into the Golgi compartment where it is cleaved by site-1 protease (S1P) and site-2 protease (S2P) to release its functional fragment[24]. This fragment then moves into the nucleus and functions as a bZIP transcription factor to activate expression of UPR target genes. The primary role of the UPR is to protect the cell from ER stress by reducing the amount of proteins translocated into the ER lumen, increasing retrotranslocation and degradation of ER-localized proteins and augmenting the protein-folding capacity of the ER. However, under prolonged ER stress the role of the UPR will change from promoting cellular survival to the pathway of programmed cell death or apoptosis[25]. In recent years, the scope and consequences of ER stress and the UPR have significantly expanded. The UPR is essential for cells to augment ER protein folding capacity and remodel their secretory pathways in response to developmental demands and physiological changes[26]. In particular, the ER stress response can be triggered by metabolic factors and intrinsic feedback effectors. Prolonged or insufficient ER stress response may turn physiological mechanisms into pathological consequences[27,28]. Indeed, the UPR has been identified as fundamental cell signaling that is critical for health and disease.
UPR IN LIPOGENESIS
Recent evidence suggests that ER stress response is closely associated with lipid-associated metabolic disease. Three UPR pathways mediated through IRE1α, PERK and ATF6 were reported to be involved in the regulation of lipid metabolism. The UPR trans-activator XBP-1, the downstream target of IRE1α under ER stress, can regulate expression and activities of key enzymes in phospholipid biosynthesis[29]. Under ER stress, the activated form of XBP1 can increase the activity of the cytidine diphosphocholine (CDP-choline) pathway for biosynthesis of phosphatidylcholine and thus induce ER biogenesis[29,30]. Interestingly, a recent study revealed a distinguished role of XBP1 in de novo fatty acid synthesis in the liver[31]. The IRE1α/XBP1 UPR branch was activated in the liver of mice under the high-carbohydrate diet and directly controlled the expression of genes involved in fatty acid biosynthesis including the genes encoding acetyl CoA carboxylase 2 (Acc2), diacylglycerol acyltransferase 2 (Dgat2) and stearoyl CoA desaturase 1 (Scd1). Deletion of XBP1 in the mouse liver caused profound hypocholesterolemia and hypotriglyceridemia which were primarily due to diminished lipogenesis. Surprisingly, the regulation of lipogenesis by the UPR component IRE1α/XBP1 was unlikely to be related to the ER stress response. The activation of XBP1 by high dietary carbohydrates and its association with other UPR components in regulating lipid metabolism remains to be further elucidated. Additionally, a recent study demonstrated that the IRE1α-XBP1 UPR pathway is indispensable for adipogenesis[32]. XBP1-deficient mouse embryonic fibroblasts and 3T3-L1 cells with XBP1 or IRE1α knockdown exhibit profound defects in adipogenesis. Intriguingly, C/EBPβ, an early adipogenic regulator, induces Xbp1 expression by directly binding to its proximal promoter region. Subsequently, spliced XBP1 binds to the promoter of C/EBPα and activates its gene expression[32]. Since C/EBPα and C/EBPβ are also key regulators of lipogenesis, the interactions between C/EBP family transcription factors and the IRE1α-XBP1 UPR pathway may play a key role in adipocyte differentiation by regulating lipid metabolism and morphological as well as functional transformations during adipogenesis.
In addition to IRE1α/XBP1, the UPR transducer ATF6 was also involved in phospholipid biosynthesis and ER expansion as well as hepatic lipid homeostasis associated with acute ER stress[33,34]. ATF6α knockout mice displayed no obvious phenotype under normal condition but showed profound hepatic steatosis under acute ER stress induced by tunicamycin challenge[34]. Acute ER Stress altered expression of genes involved in maintaining energy and lipid homeostasis. Particularly, the expression of genes encoding microsomal triglyceride transfer protein (MTTP), sterol regulatory element binding protein (SREBP)-1, peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α) and peroxisome proliferator-activated receptor α (PPARα) was suppressed by ER stress in both wild type and ATF6α-null animals treated with tunicamycin, but this suppression was much greater in ATF6α-null animals. Furthermore, the cytosolic lipid droplet protein marker adipose differentiation-related protein (ADRP) was more significantly up-regulated in ATF6α knockout animals compared to the control animals. The results suggested that the increased lipid accumulation in the livers of ATF6α-deficient animals was partially due to a defect in fatty acid oxidation and possibly augmented by impaired lipoprotein secretion.
The UPR branch mediated through PERK/eIF2α was also implicated in regulating lipogenesis. In the high-fat-fed mice, PERK-mediated eIF2α phosphorylation was crucial for the expression of lipogenic genes and the development of hepatic steatosis by controlling expression of C/EBP family and PPARγ transcription factors[35]. Enforced expression of the eIF2α-specific phosphatase GADD34 can lead to lower liver glycogen levels and susceptibility to fasting hypoglycemia in lean mice and glucose tolerance and diminished hepatosteatosis in the high-fat-fed mice. Attenuated eIF2α phosphorylation resulted in lower expression of PPARγ and its upstream regulators C/EBPα and C/EBPβ suggesting that the translational control through phosphorylation of eIF2α is an important regulatory mechanism for lipogenesis under physiological ER stress. Additionally, the mammary gland lipogenesis was down-regulated in PERK-deficient mammary epithelial cells. SREBP1 expression was significantly down-regulated in the PERK-deficient mammary-gland cells[36]. Therefore, PERK-mediated UPR pathway likely regulates SREBP1-related de novo lipid synthesis in mammary gland. Supporting the role of ER stress response in lipogenesis, ER stress has been shown to activate the lipogenic transcription factor SREBP-1 and -2 leading to modulation of the lipogenic pathways[37,38]. Consistent with this observation, over-expression of GRP78/BiP, the master negative regulator of the UPR, in the liver of obese (ob/ob) mice can inhibit SREBP-1c cleavage and the expression of SREBP-1c and SREBP-2 target genes[39]. Hepatic triglyceride and cholesterol contents were reduced and insulin sensitivity was improved in GRP78-over-expressed mice. Together, these studies confirmed crucial roles of the UPR pathways in lipogenesis and the pathogenesis of lipid-associated metabolic disease.
CONCLUSION
A growing body of evidence suggested that UPR is critically involved in lipogenesis. The UPR pathways may represent attractive targets for future therapeutic intervention in modulating lipid metabolism associated with metabolic diseases. Indeed, recent studies demonstrated that small chemical chaperones such as 4-phenylbutyric acid (PBA) and taurine-conjugated ursodeoxycholic acid (TUDCA) can reduce ER stress in liver and adipose tissues and thus enhance insulin sensitivity and glucose tolerance in mouse models of severe obesity and type 2 diabetes (ob/ob)[7,40]. For future studies it is important to elucidate ER stress-associated mechanisms in regulating lipid metabolism under metabolic conditions. The resultant information will be important for designing novel strategies for the prevention and treatment of metabolic disease.
Supported partially by the American Heart Association (AHA) Scientist Development Award (0635423Z), the AHA Grant-in-Aid (09GRNT2280479), the Department of Defense Breast Cancer Research Program (BC095179P1) and the Karmanos Cancer Institute Pilot Grant
Peer reviewer: Nattiya Hirankarn, MD, Associated Professor, Immunology Unit, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Rama 4 road, Bangkok 10330, Thailand
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N