INTRODUCTION
Biliary tract cancers (BTC) are relatively rare tumors, and their prognosis is extremely poor. The median survival period of gallbladder (GB) cancer is 6 mo, and cholangiocarcinoma (CC) also has an unfavorable prognosis, with a median survival time of 3-9 mo. In particular, intrahepatic cholangiocarcinoma (ICC) generally presents at a more advanced stage than extrahepatic CC. Therefore, its prognosis is even worse. In the past, the efficacy of conventional systemic chemotherapy in advanced, unresectable BTC was negligible. However, after the use of gemcitabine (GEM), a novel nucleoside analog, encouraging results for tumor control rates and survival time have been recently reported for BTC[1-4]. Furthermore, a chemotherapeutic regimen using GEM combined with cisplatin has been demonstrated to be superior to GEM alone and/or a combination of fluoropyrimidines and cisplatin[5-9]. Chemotherapy using hepatic arterial infusion (HAI) is a promising option for advanced and multiple hepatocellular carcinoma (HCC) and unresectable metastatic liver tumors of various origins, and this therapeutic modality has resulted in superior tumor control rates compared to systemic chemotherapy[10-13]. However, there have been few reports on HAI therapy using GEM together with cisplatin for advanced BTC.
In this article, two cases of advanced ICC and unresectable GB cancer are reported, which showed favorable outcomes when treated with HAI therapy using a combination of GEM and cisplatin.
CASE REPORT
Case 1
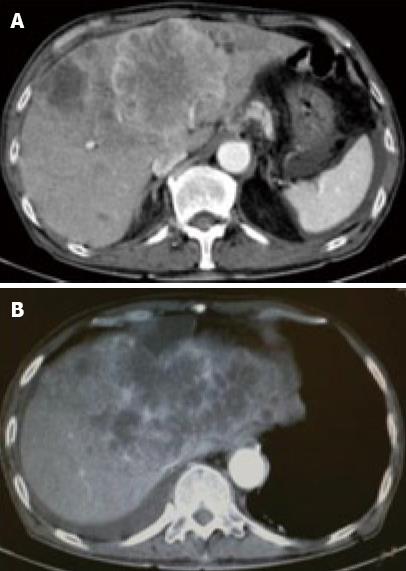
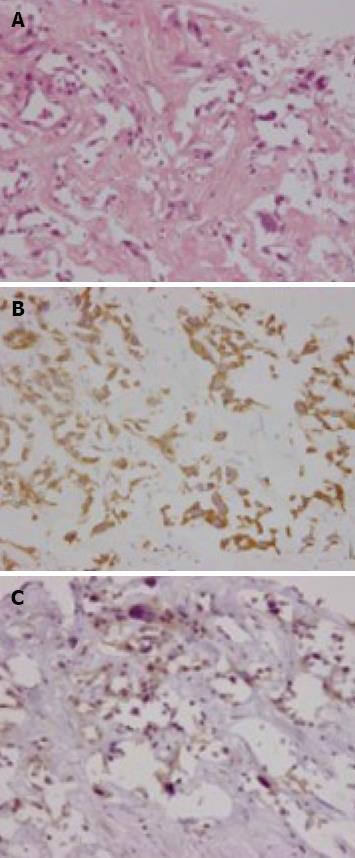
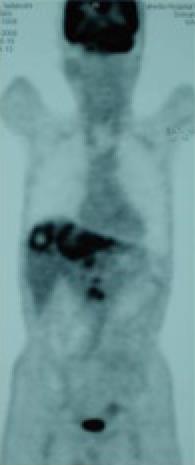
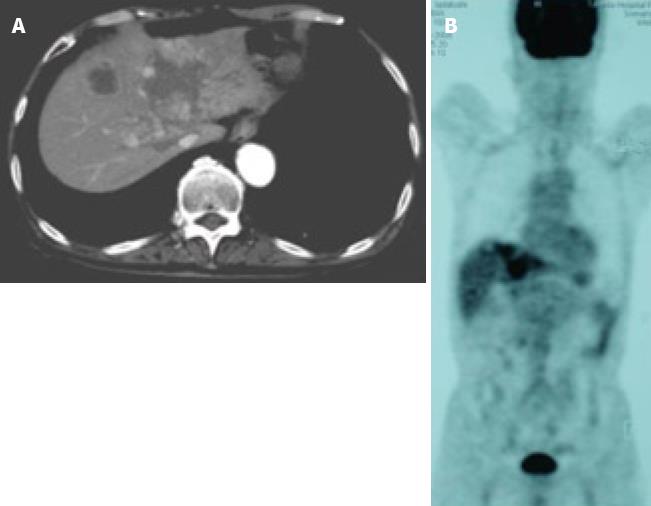
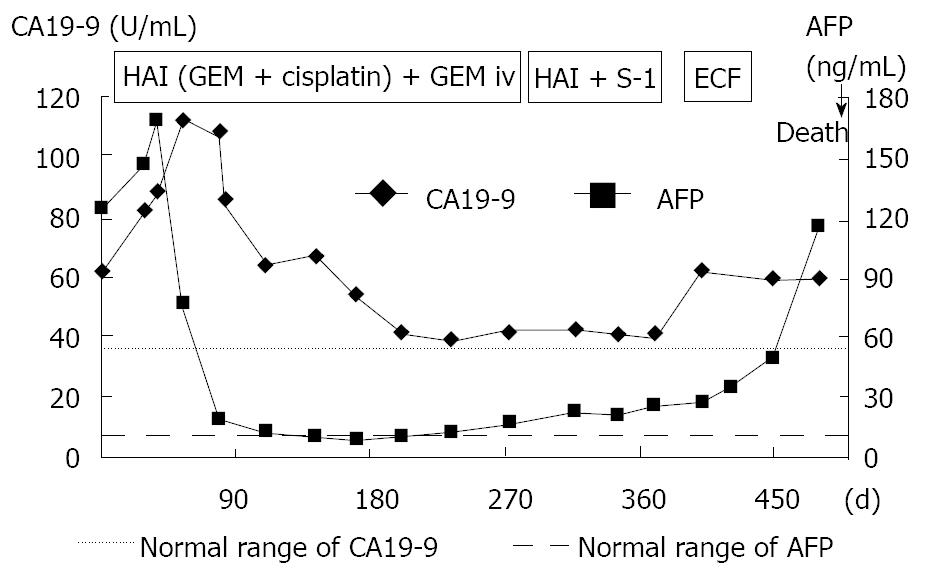
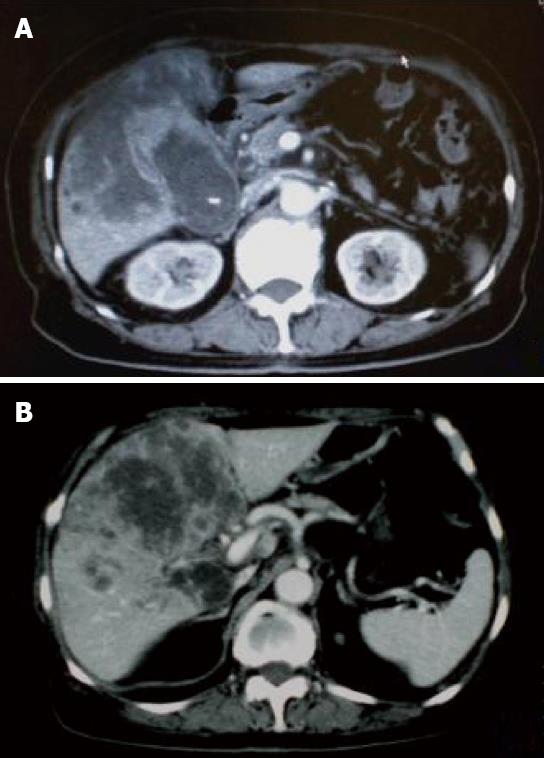
A 71-year-old man was admitted to our hospital with multiple hepatic tumors. A palpable liver was found in the right upper abdominal quadrant during physical examination, whereas no other relevant pathological findings were evident. The laboratory findings showed liver dysfunction. Among the serum tumor markers, α-fetoprotein (AFP) and carbohydrate antigen (CA) 19-9 were elevated (124.3 ng/mL, normal < 10; and 62 U/mL, normal < 37, respectively), but the level of carcinoembryonic antigen (CEA) was normal. Contrast- enhanced (CE) computed tomography (CT) of the abdomen revealed a huge liver tumor measuring 11 cm in diameter in the medial segment, and multiple small-sized tumors mainly located in the anterior and medial segment of the liver, which presented with ring enhancement in the arterial phase and central necrosis (Figure 1A and B). Multiple swollen lymph nodes (LNs) were noted in liver hilus and para- aorta, accompanied by ascites. No other tumors presented as primary foci in any other tissue outside the liver. Biopsied specimens from one of the hepatic tumors revealed a moderately-differentiated adenocarcinoma with widespread necrosis, which was not accompanied by hepatic cell components (Figure 2A). Additional histological examinations disclosed positive immunostaining for cytokeratin 7, and immuno-positive AFP in the adenocarcinoma cells (Figure 2B and C), suggesting that the hepatic tumors were AFP-producing cholangiocellular carcinomas. Under the diagnosis that the current case was an ICC of UICC TMN stage III C with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, intensive chemotherapy was implemented, using both HAI and intravenous (i.v.) administration. Following informed consent was given concerning the side effects of the therapy including interventional procedures and chemotherapeutic agents, 600 mg/m2 of GEM on d 1 and 8, and 10 mg/body of cisplatin on d 1 to 3 and 8 to 10 were infused via a subcutaneously implanted port which was connected to a catheter placed in the proper hepatic artery. Concurrently, 400 mg/m2 of GEM was administered intravenously on d 1 and 8, every 3 wk. The patient received 9 cycles of chemotherapy for 26 wk. Following completion of 2 cycles of chemotherapy, the huge tumor in the medial segment showed a reduction in diameter, and the ascitic fluid disappeared completely. However, an abnormal uptake on FDG-PET CT was observed in the hepatic tumors in both lobes, as well as the LNs in the liver hilus and para-aorta (Figure 3). After completion of 7 cycles of chemotherapy, almost all of the hepatic tumors had shrunk in size, and the swollen LNs were almost absent upon a CE-CT of the abdomen (Figure 4A). Meanwhile, a PET-CT revealed abnormal uptake only in the tumors in the medial segment, and no uptake in tumors in the right lobe and LNs (Figure 4B), suggesting that the tumor response was a partial response (PR), according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The elevated serum levels of CA19-9 and AFP were also decreased to almost the normal range after 8 cycles of chemotherapy (Figure 5). Following 9 cycles of chemotherapy, hematological toxicities such as severe thrombocytopenia (NCI-CTC, grade 3) and leukopenia (grade 2) were observed. Therefore, a modified chemotherapeutic regimen (80% of the initial dose of GEM on d 1, and 10 mg cisplatin on d 1 and 2) was carried out biweekly thereafter. Although a greater reduction of the abnormal uptake from the tumor in the medial segment was revealed on the PET-CT after the end of 11 cycles of chemotherapy, new abnormal uptake was found in the LN in the liver hilus. Therefore, the present case was diagnosed as a progressive disease (PD). A new chemotherapy regimen using GEM (480 mg/m2 for HAI and 320 mg/m2 for i.v. administration on d 2) and oral S-1 (80 mg/body for 7 to 14 d, consecutively) every 3 wk was started. Following the end of 4 cycles of the new regimen, the liver tumors and metastatic LNs became larger. An ECF regimen using epirubicin 40 mg/m2 and cisplatin 48 mg/m2 for HAI on d 1, and 5-FU 160 mg/m2 for i.v. administration for 4 consecutive days, was then carried out every 3 wk. However, systemic metastases (peritoneum, bone and lung) and massive ascites were soon observed. At 16 mo after the diagnosis, the patient died of multiple organ failure.
Figure 1 Contrast-enhanced (CE) computed tomography of the abdomen revealed a huge tumor, measuring 11 cm in diameter, which presented with ring enhancement in the arterial phase and central necrosis.
A: In the medial segment; B: Multiple small-sized tumors mainly located in the anterior and medial segments of the liver.
Figure 2 Biopsied specimens from one of the hepatic tumors.
A: A moderately-differentiated adenocarcinoma with wide-spread necrosis, which were not accompanied by hepatic cell components (HE × 200); B: Positive immunostaining for cytokeratin 7 (× 200) in the adenocarcinoma cells; C: Immuno-positive AFP (× 200) in the adenocarcinoma cells.
Figure 3 FDG-PET CT after the end of 2 cycles of chemotherapy revealed abnormal uptake in the hepatic tumors in both lobes and the LNs in the liver hilus and para-aorta.
Figure 4 Images after the end of 7 cycles of chemotherapy.
A: CE-CT showed a reduction in the size of a huge tumor seen in the medial segment; B: PET-CT revealed abnormal uptake only in the tumors in the medial segment, and no uptake was observed in tumors from the right lobe and the LNs in the liver hilus and para-aorta.
Figure 5 Clinical course and changes in the serum levels of CA19-9 and AFP.
Case 2
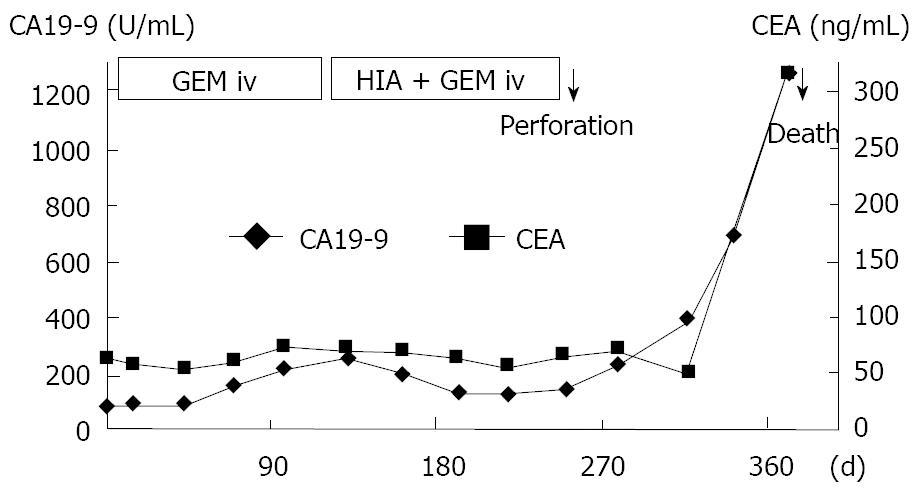
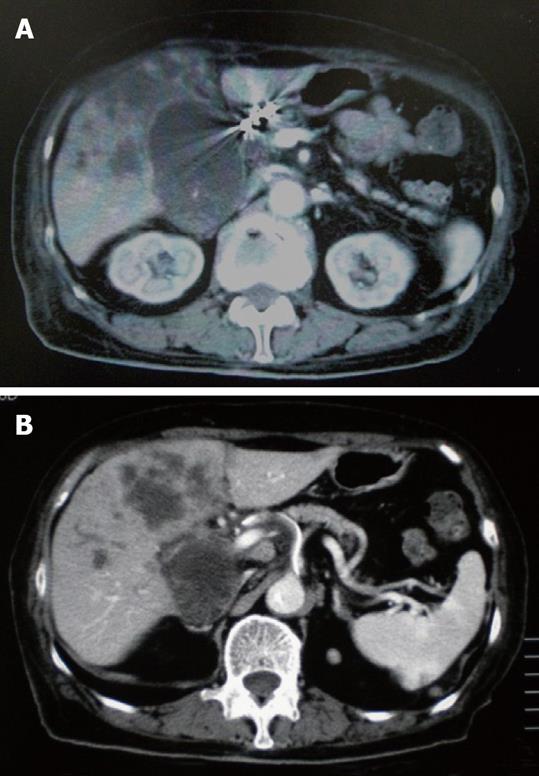
An 83-year-old woman presented to our hospital with abdominal discomfort and appetite loss. Physical examination revealed a swollen gallbladder but no other pathological findings. The laboratory data demonstrated liver dysfunction and an elevation in the serum levels of CA 19-9 and CEA (82.0 U/mL, normal < 37; 61.3 ng/mL, normal < 5, respectively). Ultrasonography and CE-CT of the abdomen revealed gall stones and a GB tumor, which invaded the adjacent liver directly. Multiple liver tumors and swollen LNs in the liver hilus were also observed. The patient’s PS was 1 and, based on the diagnosis that the current case was an advanced GB carcinoma (UICC TNM stage IV), chemotherapy using a 4 wk cycle of i.v. administration of 1000 mg/m2 of GEM on d 1, 8, 15 was started. After completion of 4 cycles of chemotherapy, the tumor increased in size in the GB, liver and LNs in the liver hilus (Figure 6A and B), and ascitic fluid appeared on CE-CT (RECIST, PD). The serum levels of CA 19-9 and CEA elevated gradually (Figure 7). Furthermore, metastasis in the lumbar vertebra was detected. Immediately thereafter, radiation therapy to the lumbar vertebrae was started. After informed consent was given, a heparin-coated catheter was placed in the common hepatic artery to supply all of the tumor vessels, including the GB and liver. Thereafter, the catheter was connected to the injection port. The gastroduodenal artery was occluded by steel coils to prevent gastroduodenal injury from anticancer drugs. Next, a new chemotherapeutic regimen comprising HAI (600 mg/m2 of GEM on d 1 and 8, and 10 mg/body of cisplatin on d 1 to 3 and 8 to 10) and the i.v. administration of GEM (400 mg/m2, d 1 and 8), was carried out every 3 wk for 3 cycles. After hematological toxicity (grade 2) occurred at the end of 3 cycles of chemotherapy, a modified regimen in which cisplatin administration was shortened to 2 d was instigated for 2 cycles. At the end of 3 cycles of chemotherapy with HAI, the tumors in the GB and liver decreased in size (Figure 8A and B), and the swollen LNs in the liver hilus regressed on the abdominal CT (RECIST, PR). However, following the 2 cycles of modified HAI chemotherapy, a perforation in the duodenum which was invaded directly by the GB cancer occurred. The chemotherapy was then discontinued, and 14 mo after the initial diagnosis, the patient was died of multiple organ failure.
Figure 6 CE-CT after the end of 4 cycles of i.
v. administration chemotherapy showed that the tumor increased in size. A: The GB tumor; B: Liver tumor.
Figure 7 Clinical course and changes in the serum levels of CA19-9 and CEA.
Figure 8 CE-CT after the end of 3 cycles of HAI chemotherapy showed a decrease in the size of tumor.
A: The GB tumor; B: Liver tumor.
DISCUSSION
In the past, there was no standard chemotherapy for advanced BTC i.e. GB cancer and CC. However, GEM has recently been used against BTC as the most active agent, and promising response rates and overall survival times with tolerable drug toxicities were demonstrated[1-4]. GEM has a synergistic and cytotoxic effect in vivo and in vitro, in combination with cisplatin[14,15] and capecitabine[16]. More recently, the superiority of combination chemotherapy using GEM plus cisplatin, as well as the combination of GEM with capecitabine or oxaliplatin, has been demonstrated among the GEM-only, GEM-based and 5-FU-based chemotherapeutic regimens for BTC[4,5,8,9,17-19]. The intra-arterial (i.a.) administration of GEM was used for the treatment of advanced pancreatic carcinoma by Shamseddine et. al. in 2005[20]. They reported that GEM in i.a. administration could be safely escalated to 1400 mg/m2 within a tolerable toxicity. On the other hand, in low dose FP (5-FU and cisplatin) therapy for advanced and multiple HCCs, cisplatin is administered intra-arterially at a dose of 10 mg/body for 5 consecutive days[13]. Based on the previous reports mentioned above and our judgment that the control of multiple liver tumors might decide the patient’s survival time, HAI therapy using GEM combined with cisplatin was performed. Following consideration of treatment efficacy against distant metastases (LNs and bone), the systemic administration of GEM was used together with HAI in both cases. In case 1, after the end of 7 cycles of chemotherapy, the liver tumors had regressed, and the swollen LNs and ascitic fluid disappeared completely. In case 2, because of the PD after 4 cycles of the i.v. administration of GEM, HAI therapy was started again, and consequently favorable tumor responses were achieved. The survival times of the current two cases were also similar.
A chemotherapeutic regimen using the i.a. administration of GEM together with hepatic embolization has been performed against advanced BTC, and respectable results were achieved[21,22]; the survival time for the group treated by GEM with microspheres was 20.2 mo, whereas that of the TACE group using a combination of GEM plus cisplatin was 13.8 mo. Arterial administration can deliver anti-cancer agents at high concentrations into liver tumors, and has a longer lasting cytotoxic effect. A previous report demonstrated that the peak plasma concentration of GEM after i.a. administration was reduced to ~1/7th of that observed using the systemic i.v. route, and that no grade 3 or 4 toxicity was documented after i.a. administration of up to 1400 mg/m2 of GEM[20]. Vogl et al[21] noted that the use of GEM doses (~1800 mg/m2) higher than the recommended 1000 mg/m2 was well tolerated if microspheres were used. Moreover, Gusani et al[22] showed that the median survival in unresectable CC treated with GEM-based TACE was not significantly different in patients with liver disease only, as compared to those with extra-hepatic disease. In 2008, HAI therapy using a combination of GEM and mitomycin against advanced CC revealed a poor tumor control rate, as compared to that against metastatic breast cancer and colorectal carcinoma[12]. Therefore, HAI therapy using GEM combined with cisplatin may be a useful and well- tolerated option for advanced BTC, especially in which multiple liver metastases are detected.
In recent years, targeted therapy has been carried out for advanced BTC as a second-line chemotherapy[23] and in phase II trials[24]. Chemotherapeutic regimens using cetuximab or bevacizumab combined with GEM and oxaliplatin have also been used against advanced BTC and, at present, passable tumor- responses have been achieved[23,24]. In conclusion, HAI therapy with GEM plus cisplatin might be an effective and well-tolerated option in advanced BTC, and in the future we recommend that clinical trials of HAI therapy using GEM-based platinum or capecitabine, with or without targeted agents, should be performed for advanced BTC.