Revised: September 10, 2009
Accepted: September 17, 2009
Published online: February 27, 2010
AIM: To clarify the significance of combined resection of the spleen to dissect the No. 10 lymph node (LN).
METHODS: We studied 191 patients who had undergone total gastrectomy with splenectomy, excluding non-curative cases, resection of multiple gastric cancer, and those with remnant stomach cancer. Various clinicopathological factors were evaluated for any independent contributions to No. 10 LN metastasis, using χ2 test. Significant factors were extracted for further analysis, carried out using a logistic regression method. Furthermore, lymph node metastasis was evaluated for any independent contribution to No. 10 LN metastasis, using the same methods. The cumulative survival rate was calculated using the Kaplan-Meier method. The significance of any difference between the survival curves was determined using the Cox-Mantel test, and any difference was considered significant at the 5% level.
RESULTS: From the variables considered to be potentially associated with No. 10 LN metastasis, age, depth, invasion of lymph vessel, N factor, the number of lymph node metastasis, Stage, the number of sites, and location were found to differ significantly between those with metastasis (the Positive Group) and those without (the Negative Group). A logistic regression analysis showed that the localization and Stage were significant parameters for No. 10 LN metastasis. There was no case located on the lesser curvature in the Positive Group. The numbers of No. 2, No. 3, No. 4sa, No. 4sb, No. 4d, No. 7, and No. 11 LN metastasis were each found to differ significantly between the Positive Group and the Negative Group. A logistic regression analysis showed that No. 4sa, No. 4sb, and No. 11 LN metastasis were each a significant parameter for No. 10 LN metastasis. There was no significant difference in survival curves between the Positive Group and the Negative Group.
CONCLUSION: Splenectomy should be performed to dissect No. 10 LN for cases which have No. 4sa, No. 4sb or No. 11 LN metastasis. However, in cases where the tumor is located on the lesser curvature, splenectomy can be omitted.
- Citation: Aoyagi K, Kouhuji K, Miyagi M, Imaizumi T, Kizaki J, Shirouzu K. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol 2010; 2(2): 81-86
- URL: https://www.wjgnet.com/1948-5182/full/v2/i2/81.htm
- DOI: https://dx.doi.org/10.4254/wjh.v2.i2.81
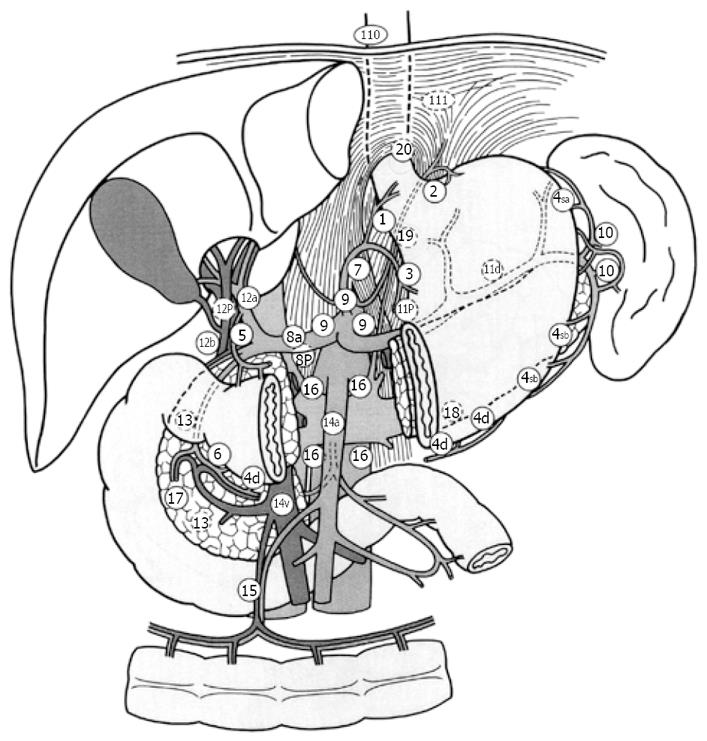
The lymph nodes at the splenic hilum (No. 10 LN) belong to the Group 2 lymph nodes of gastric cancer that involve the upper third of the stomach, according to the Japanese Classification of Gastric Carcinoma[1], and D2 lymph node dissection is the standard operation for gastric cancer in Japan. Therefore, splenectomy is widely performed to achieve complete D2 lymphadenectomy in Japan for macroscopically advanced gastric cancer located at the proximal part of the stomach. Extended lymphadenectomy, splenectomy, or a combination of both might theoretically improve prognosis by achieving better lymph node clearance[2,3], but none of these was associated with improved outcome in randomized trials specifically addressing this issue[4-7]. Moreover some complications in splenectomy, for example pancreatic fistula and left subdiaphragmatic abscess, sometimes occur[2,8-10], and splenectomy has been associated with increased morbidity after gastrectomy for gastric cancer[8,9,11-13]. Although most authors have recommended splenic preservation in the surgical treatment of gastric cancer[4,11,12], splenectomy is still considered for proximal gastric and gastroesophageal junction cancer, because the incidence of lymph node metastasis at the splenic hilum is thought to be higher in these tumors[2,3,14,15]. The aim of the present study was to clarify the significance of combined resection of the spleen with total gastrectomy to dissect No. 10 LN. Splenectomy has been more commonly performed in the resection of a proximal tumor, and therefore previous analyses may be biased. Here, we compared gastric cancer cases which underwent curative surgery with positive and negative No. 10 LN metastasis to total gastrectomy cases with splenectomy, clinicopathologically. The indication for splenectomy in patients with proximal and middle gastric cancer remain controversial. Here, we have investigated the characteristic findings in patients with lymph node metastasis to the splenic hilus, and report the indication for splenectomy.
Between 1990 and 2004, 2064 patients with gastric cancer underwent surgical resection at Kurume University Hospital. Here, we retrospectively studied the 191 cases of total gastrectomy with splenectomy and D2 or D3 dissection, excluding the cases of non-curative resection, those cases involving resection of multiple gastric cancer, and those of remnant stomach cancer. One hundred and twenty-four patients were men, and 67 were women. The mean age was 60.6 years, with an age range from 40 to 84 years. There were 7 patients at Stage IA, 18 patients at Stage IB, 40 patients at Stage II, 46 patients at Stage IIIA, 38 patients at Stage IIIB, and 42 patients at Stage IV. There were 20 cases with positive No. 10 LN metastasis (the Positive Group), and 171 cases with none (the Negative Group), as defined by the Japanese Classification of Gastric Carcinoma[1].
Various factors, including age, sex, site, location, tumor size, macroscopic type, histological type, depth of invasion, stromal volume, pattern of tumor infiltration, invasion of lymph vessel, venous invasion, N factor, Stage, and the number of lymph nodes with positive metastasis were evaluated for any independent contributions to No. 10 LN metastasis, using the χ2 test. The number of lymph node metastases was classified dependent on TNM staging, as follows, N0: no regional lymph nodes metastasis, N1: metastasis in 1 to 6 regional lymph nodes, N2: metastasis in 7 to 15 regional lymph nodes, and N3: metastasis in more than 15 regional lymph nodes. Significant factors were extracted for further analysis, carried out using a logistic regression method. Furthermore, lymph node metastasis was evaluated for any independent contribution to No. 10 LN metastasis, using similar methods as above. The statistical analyses were performed using a statistical analysis computer program (SPSSII for Windows, SPSS Japan Inc., Tokyo, Japan). The P value level of significance was set at 0.05.
The cumulative survival rate was calculated using the Kaplan-Meier method. The significance of any difference between the survival curves was determined using the Cox-Mantel test, and any difference was considered significant at the 5% level.
From the variables considered to be potentially associated with No. 10 LN metastasis, age, the number of sites, location, depth, invasion of lymph vessel, N factor, the number of lymph node metastases, and Stage were found to differ significantly between the Positive Group and the Negative Group (P = 0.017, P = 0.008, P < 0.001, P = 0.017, P = 0.008, P < 0.001, P = 0.003, and P < 0.001, respectively) (Table 1).
| Metastasis positive | Metastasis negative | Total | P value | ||
| 20 (10.5) | 171 (89.5) | 191 (100) | |||
| Gender | Male | 14 (70.0) | 111 (64.9) | 125 (65.4) | 0.651 |
| Female | 6 (30.0) | 60 (35.1) | 66 (34.5) | ||
| Age (years) | ≤ 60 | 7 ( 35.0) | 107 (62.6) | 114 (59.7) | 0.017 |
| > 60 | 13 (65.0) | 64 (37.4) | 77 (40.3) | ||
| Site | L | 1 (5.0) | 11 ( 6.4) | 12 (6.3) | 0.616 |
| M | 9 (45.0) | 58 (33.9) | 67 (35.1) | ||
| U | 10 (50.0) | 102 (59.6) | 112 (58.6) | ||
| Number of site | 1 | 5 (25.0) | 41 (24.0) | 46 (24.1) | 0.008 |
| 2 | 7 (35.0) | 78 (45.6) | 85 (44.5) | ||
| ≤ 3 | 8 (40.0) | 52 (30.4) | 60 (31.4) | ||
| Location | Less | 0 ( 0.0) | 73 (42.7) | 73 (38.2) | < 0.001 |
| Ant | 1 (5.0) | 15 (8.8) | 16 (8.4) | ||
| Post | 3 (15.0) | 32 (18.7) | 35 (18.3) | ||
| Great | 8 (40.0) | 13 (7.6) | 21 (11.0) | ||
| Cir | 8 (40.0) | 38 (22.2) | 46 (24.1) | ||
| Number of location | 1 | 7 (35.0) | 90 (52.6) | 97 (50.8) | 0.200 |
| 2 | 2 (10.0) | 28 (16.4) | 30 (15.7) | ||
| 3 | 3 (15.0) | 15 (8.8) | 18 (9.4) | ||
| 4 | 8 (40.0) | 38 (22.2) | 46 (24.1) | ||
| Tumor size (mm) | ≤ 100 | 11 (55.0) | 65 (38.0) | 76 (39.8) | 0.142 |
| > 100 | 9 (45.0) | 106 (62.0) | 115 (60.2) | ||
| Macroscopic type | 0 | 2 (10.0) | 13 (7.6) | 15 (7.9) | 0.503 |
| 1 | 1 (5.0) | 5 (2.9) | 6 (3.1) | ||
| 2 | 2 (10.0) | 44 (25.7) | 46 (24.1) | ||
| 3 | 10 (50.0) | 69 (40.4) | 79 (41.4) | ||
| 4 | 5 (25.0) | 30 (17.5) | 35 (18.3) | ||
| 5 | 0 (0.0) | 10 (5.8) | 10 (5.2) | ||
| Histological type | Differentiat | 3 (15.0) | 53 (31.0) | 56 (29.3) | 0.137 |
| Undifferentiated | 17 (85.0) | 118 (69.0) | 135 (70.7) | ||
| Depth | M, SM | 1 (5.0) | 10 (5.8) | 11 (5.6) | 0.020 |
| MP | 0 (0.0) | 12 (7.0) | 12 (6.3) | ||
| SS | 2 (10.0) | 17 (9.9) | 19 (9.9) | ||
| SE | 9 (45.0) | 111 (64.9) | 120 (62.8) | ||
| SI | 8 (40.0) | 21 (12.3) | 29 (15.2) | ||
| Ly | 0, 1 | 1 (5.0) | 48 (28.1) | 49 (25.7) | 0.025 |
| 2, 3 | 19 (95.0) | 123 (71.9) | 142 (74.3) | ||
| V | - | 2 (10.0) | 50 (29.2) | 52 (27.2) | 0.067 |
| + | 18 (90.0) | 121 (70.8) | 139 (72.8) | ||
| Stromal volume | Med | 3 (15.0) | 60 (35.1) | 63 (33.0) | 0.147 |
| Int | 8 (40.0) | 62 (36.3) | 70 (36.6) | ||
| Sci | 9 (45.0) | 49 (28.7) | 58 (30.4) | ||
| INF | α | 1 (5.0) | 42 (24.6) | 43 (22.5) | 0.127 |
| β | 6 (30.0) | 47 (27.5) | 53 (27.7) | ||
| γ | 13 (65.0) | 82 (48.0) | 95 (49.7) | ||
| N | 0 | 0 (0.0) | 60 (35.1) | 60 (31.4) | < 0.001 |
| 1 | 2 (10.0) | 50 (29.2) | 52 (27.2) | ||
| 2 | 12 (60.0) | 40 (23.4) | 52 (27.2) | ||
| 3 | 6 (30.0) | 18 (10.5) | 24 (12.6) | ||
| M | 0 (0.0) | 3 (1.8) | 3 (1.6) | ||
| Number of N | 0 | 0 (0.0) | 60 (35.1) | 60 (31.4) | 0.003 |
| 1-6 | 7 (35.0) | 55 (32.2) | 62 (32.5) | ||
| 7-15 | 6 (30.0) | 34 (19.9) | 40 (20.9) | ||
| 16- | 7 (35.0) | 22 (12.9) | 29 (15.2) | ||
| Stage | IA,IB | 0 (0.0) | 25 (14.6) | 25 (13.1) | < 0.001 |
| II | 1 (5.0) | 39 (22.8) | 40 (20.9) | ||
| IIIA | 1 (5.0) | 45 (26.3) | 46 (24.1) | ||
| IIIB | 4 (20.0) | 35 (20.5) | 39 (20.4) | ||
| IV | 14 (70.0) | 27 (15.8) | 41 (21.5) |
A logistic regression analysis was conducted for the eight parameters (age, the number of site, location, depth, invasion of lymph vessel, N factor, the number of lymph node metastases, and Stage) that had been found to be significant using the χ2 test. A logistic regression analysis showed that the location and Stage were each significant parameters of No. 10 LN metastasis (P = 0.003 and P = 0.006, respectively) (Table 2). There was no case locating on the lesser curvature in the Positive Group (Table 1).
| Parameters | Disease progression | ||
| OR | 95% CI | P value | |
| Age (yr) | 2.697 | 0.852-8.538 | 0.092 |
| Depth | 0.636 | 0.264-1.532 | 0.313 |
| Ly | 2.269 | 0.220-23.407 | 0.491 |
| N | 0.801 | 0.277-2.319 | 0.683 |
| Number of N | 1.062 | 0.446-2.531 | 0.892 |
| Stage | 4.840 | 1.576-14.864 | 0.006 |
| Number of sites | 0.541 | 0.246-1.191 | 0.127 |
| Location | 2.027 | 1.269-3.238 | 0.003 |
The numbers of No. 2, No. 3, No. 4sa, No. 4sb, No. 4d, No. 7, and No. 11 LN metastasis were found to differ significantly between the Positive Group and the Negative Group (P < 0.001, P = 0.001, P < 0.001, P < 0.001, P < 0.001, P = 0.019, and P < 0.001, respectively) (Table 3). A logistic regression analysis showed that No. 4sa, No. 4sb, and No. 11 LN metastasis were each significant parameters for No. 10 LN metastasis (P < 0.001, P = 0.006, and P = 0.002, respectively) (Table 4) (Figure 1).
| Metastasis positive | Metastasis negative | Total | P value | |
| No. 1 | 8 (40.0) | 41 (24.0) | 49 (25.7) | 0.121 |
| No. 2 | 11 (55.0) | 28 (16.4) | 39 (20.4) | < 0.001 |
| No. 3 | 18 (90.0) | 87 (50.9) | 105 (55.0) | 0.001 |
| No. 4sa | 12 (60.0) | 10 (5.8) | 22 (11.5) | < 0.001 |
| No. 4sb | 10 (50.0) | 16 (9.4) | 26 (13.6) | < 0.001 |
| No. 4d | 12 (60.0) | 34 (19.9) | 46 (24.1) | < 0.001 |
| No. 5 | 1 (5.0) | 10 (5.8) | 11 (5.8) | 0.878 |
| No. 6 | 4 (20.0) | 19 (11.1) | 23 (12.0) | 0.248 |
| No. 7 | 10 (50.0) | 43 (25.1) | 53 (27.7) | 0.019 |
| No. 8a | 5 (25.0) | 19 (11.1) | 24 (12.6) | 0.076 |
| No. 8p | 1 (5.0) | 8 (4.7) | 9 (4.7) | 0.949 |
| No. 9 | 2 (10.0) | 13 (7.6) | 15 (7.9) | 0.706 |
| No. 11 | 11 (55.0) | 19 (11.1) | 30 (15.7) | < 0.001 |
| No. 12a | 0 (0.0) | 3 (1.8) | 3 (1.6) | 0.550 |
| No. 12b | 1 (5.0) | 2 (1.2) | 3 (1.6) | 0.192 |
| No. 12p | 0 (0.0) | 3 (1.8) | 3 (1.6) | 0.550 |
| No. 13 | 1 (5.0) | 1 (0.6) | 2 (1.0) | 0.066 |
| No. 14v | 1 (5.0) | 3 (1.8) | 4 (2.1) | 0.337 |
| No. 16 | 4 (20.0) | 16 (9.4) | 20 (10.5) | 0.141 |
| Etc. | 0 (0.0) | 4 (2.3) | 4 (2.1) | 0.48 |
| Parameters | Disease progression | ||
| OR | 95% CI | P value | |
| No. 2 | 0.791 | 0.181-3.452 | 0.755 |
| No. 3 | 1.414 | 0.219-9.117 | 0.716 |
| No. 4sa | 18.377 | 4.071-82.962 | < 0.001 |
| No. 4sb | 8.447 | 1.844-38.688 | 0.006 |
| No. 4d | 1.098 | 0.267-4.518 | 0.897 |
| No. 7 | 1.085 | 0.277-4.253 | 0.907 |
| No. 11 | 10.096 | 2.320-43.941 | 0.002 |
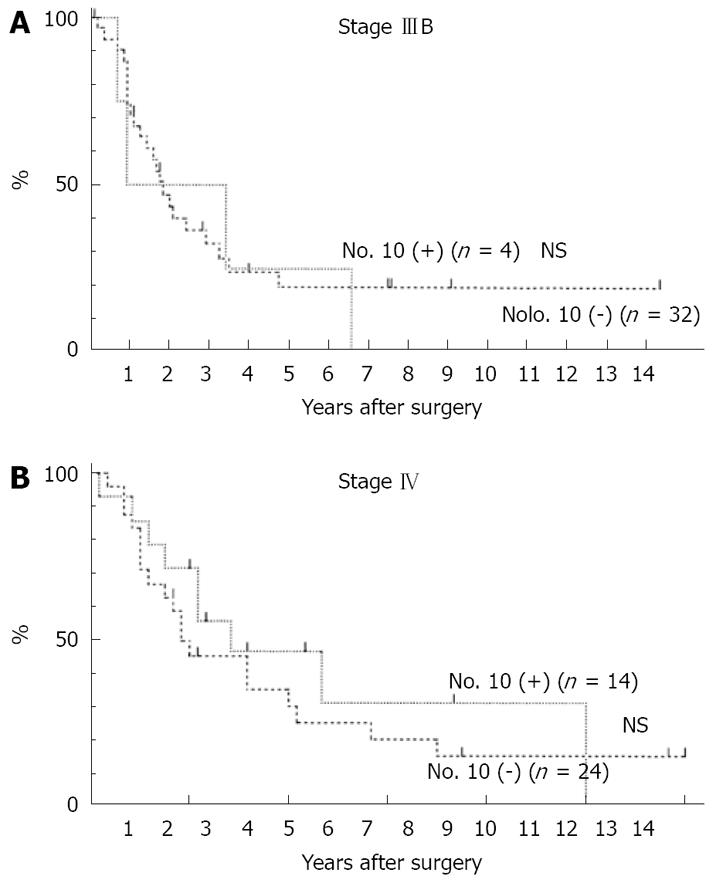
There was no significant difference in survival rate between the Positive Group and the Negative Group in Stage IIIB (Figure 2A), and in Stage IV cases (Figure 2B).
Resection of the spleen en bloc with the stomach for gastric cancer is still widely performed for a curative resection (R0). Fatouros et al[4] reported that an overestimation of the risk of residual disease in the splenic hilum nodes in the case of spleen preservation was seen in 94% of splenectomized patients, and preservation of the spleen may be associated with a reduced risk of early and overall recurrence, translating into a better survival in patients receiving curative surgery for gastric cancer. Pancreas-related abscess formation remains a strong factor in the mortality and morbidity rates[9,12,13]. The dissection of nodes along the distal splenic artery and nodes in the splenic hilum is an intraoperative risk factor for pancreas-related abscess formation. Distal pancreatectomy with splenectomy has a high risk of abscess formation. Pancreas-preserving splenectomy is a standard operation in Japan. However, splenectomy without dissection along the distal splenic artery also has a high risk to abscess formation. Some authors[9,13] have reported that splenectomy (with or without pancreatectomy) and nodal dissection are risk factors for operative morbidity but not mortality. Hartgrink et al[16] reported that the risk for morbidity and mortality was significant for pancreactomy and splenectomy, and that splenectomy and pancreatectomy should be performed only in case of direct invasion from the tumor into these organs. In considering the lymphatic pathway from the primary tumor to No 10 LN, metastasis in the lymph nodes along the lesser curvature (No. 3), the short gastric vessels, or gastroepiploic vessels (No. 4) may be good indicators of No. 10 LN metastasis[3]. In our study, there was no case with the cancer located on the lesser curvature in the Positive Group, and metastasis in the No. 4sa, No. 4sb, or No. 11 LN was a good indicator for No. 10 LN metastasis. The frequency of No. 10 LN metastasis was high in cases with cancer located on the greater curvature or posterior wall of the stomach. These results suggested that the lymphatic pathway along the posterior gastric artery, splenic artery, short gastric vessels, or gastroepiploic vessels were important for No. 10 LN metastasis. In our study, there was no significant difference in survival curves between the Positive Group and the Negative Group. These data show that dissection of the No. 10 LN has a survival benefit when curative surgery was performed. Ikeguchi et al[3] reported when curative surgery was performed, the survival of No. 10 positive patients was not different from that of No. 10 negative patients; therefore, for patients with an advanced gastric cancer located in the proximal part of the stomach, then D2 lymphadenectomy with splenectomy is recommended when patients show macroscopic evidence of serosal invasion with regional lymph node metastasis. Hartgrink et al[16] reported that the relevance of the dissection of the No. 10 and 11 LN has to be questioned since the survival benefit is small and the hospital mortality is significantly increased. Splenectomy and pancreatectomy are important risk factors for morbidity and mortality after D2 dissection, with adverse effects on survival as well[5]. A Japanese prospective randomized study on spleen preservation might be beneficial in patients with advanced gastric cancer who receive postoperative immunochemotherapy after total gastrectomy[7]. A randomized trial in Chile found no survival benefit from a splenectomy in patients with total gastrectomy, whereas morbidity was significantly increased[6]. Yamamoto et al[17] reported that total gastrectomy with splenectomy should be done for patients with T3 advanced gastric cancer and T2 advanced gastric cancer with multiple lymph node metastasis (more than 7 nodes), and recognized lymph node metastasis to the splenic hilus. Ikeguchi et al[3] reported that all cases with No. 10 LN positivity were macroscopically diagnosed as positive for serosal invasion or regional lymph node metastasis at the time of surgery. Sakaguchi et al[14] reported that splenectomy should be conducted in T2 cases with gross serosal change and T3, 4 cases. In our study, all cases with No. 10 LN metastasis showed regional lymph node metastasis. However, we detected No. 10 LN metastasis even in early gastric cancer, or cases with few lymph node metastases (less than 7 nodes), The effect of splenectomy on prognosis in patients with gastric cancer remains controversial. Splenectomy might facilitate a more complete lymph adenectomy by thorough clearance of the lymph nodes from the splenic hilum. Other surgeons have recommended splenectomy in patients with proximal or gastroesophageal junction cancer to address the increased likelihood of lymph node metastasis in the splenic hilum[2,3,14]. The method of No. 10 LN dissection without splenectomy is very difficult. Injury of the spleen and high bleeding volume occurred in many cases where No. 10 LN was dissected without splenectomy. Even if splenectomy was not performed, movement of the pancreas tail and spleen was needed to dissect No. 10 LN, so pancreatic fistula and pancreas related abscess formation were recognized in some cases. Moreover, the dissection of No. 10 LN may be incomplete in cases without splenectomy. Numerous retrospective as well as prospective randomized trials, however, have not demonstrated a prognostic benefit for splenectomy or extended lymph adenectomy. Some retrospective studies even demonstrated a worse survival after splenectomy[4,12]. Many cases with No. 10 LN were far advanced cancer which had peritoneal metastasis, liver metastasis, numerous lymph node metastasis, and distant metastasis. In these cases the benefit of dissection of No, 10 LN is small. However, dissection of No. 10 LN has survival benefit when curative surgery was performed. Therefore, we recommend splenectomy to dissect No. 10 LN in gastric cancer which involves the upper third of the stomach that is curatively resected, especially, when the tumor is located on the greater curvature or posterior wall of the stomach, or has No. 4sa, No. 4sb, or No. 11 LN metastasis. However, when the tumor is located on the lesser curvature, then splenectomy can be omitted.
The lymph nodes at the splenic hilum (No. 10 LN) belong to the Group 2 LN of gastric cancer that involves the upper third of the stomach, according to the Japanese Classification of Gastric Carcinoma, and D2 LN dissection is the standard operation for gastric cancer in Japan. Therefore, splenectomy is widely performed to achieve complete D2 lymphadenectomy in Japan for macroscopically advanced gastric cancer located at the proximal part of the stomach. Extended lymphadenectomy, splenectomy, or a combination of both might theoretically improve prognosis by achieving better lymph node clearance, but none of these was associated with improved outcome in randomized trials specifically addressing this issue. Moreover some complications in splenectomy, for example pancreatic fistula and left subdiaphragmatic abscess, sometimes occur, and splenectomy has been associated with increased morbidity after gastrectomy for gastric cancer. Although most authors have recommended splenic preservation in the surgical treatment of gastric cancer, splenectomy is still considered for proximal gastric and gastroesophageal junction cancer, because the incidence of lymph node metastasis at the splenic hilum is thought to be higher in these tumors.
The present study was to clarify the significance of combined resection of the spleen with total gastrectomy to dissect No. 10 LN.
This research clarified that splenectomy can be omitted in cases with the tumor located on the lesser curvature.
If we perform total gastrectomy curatively for proximal gastric cancer which is located on the lesser curvature, we can omit splenectomy to dissect No. 10 LN.
This is a retrospective study on the outcome of patients with gastric cancer after total gastrectomy and splenectomy. The authors have highlighted the survival benefit of dissection of No. 10 LN and the predictive factors for the metastatic No. 10 LN in patients with gastric cancer. The authors need to study a cohort of patients with histologically proven metastatic No. 10 LN with and without splenectomy.
Peer reviewer: Dr. Kelvin K Ng, PhD, Department of Surgery, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China
| 1. | Japanese Gastric Cancer Association: Japanese classification of gastric carcinoma. 13th ed. Tokyo: Kanehara 1999; 6-16. |
| 2. | Chikara K, Hiroshi S, Masato N, Hirotoshi A, Goro M, Hidetaka O. Indications for pancreaticosplenectomy in advanced gastric cancer. Hepatogastroenterology. 2001;48:908-912. |
| 3. | Ikeguchi M, Kaibara N. Lymph node metastasis at the splenic hilum in proximal gastric cancer. Am Surg. 2004;70:645-648. |
| 4. | Fatouros M, Roukos DH, Lorenz M, Arampatzis I, Hottentrott C, Encke A, Kappas AM. Impact of spleen preservation in patients with gastric cancer. Anticancer Res. 2005;25:3023-3030. |
| 5. | Degiuli M, Sasako M, Ponzetto A, Allone T, Soldati T, Calgaro M, Balcet F, Bussone R, Olivieri F, Scaglione D. Extended lymph node dissection for gastric cancer: results of a prospective, multi-centre analysis of morbidity and mortality in 118 consecutive cases. Eur J Surg Oncol. 1997;23:310-314. |
| 6. | Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401-407. |
| 7. | Okinaga K, Iinuma H, Kitamura Y, Yokohata T, Inaba T, Fukushima R. Effect of immunotherapy and spleen preservation on immunological function in patients with gastric cancer. J Exp Clin Cancer Res. 2006;25:339-349. |
| 8. | Otsuji E, Yamaguchi T, Sawai K, Okamoto K, Takahashi T. Total gastrectomy with simultaneous pancreaticosplenectomy or splenectomy in patients with advanced gastric carcinoma. Br J Cancer. 1999;79:1789-1793. |
| 9. | Weitz J, Jaques DP, Brennan M, Karpeh M. Association of splenectomy with postoperative complications in patients with proximal gastric and gastroesophageal junction cancer. Ann Surg Oncol. 2004;11:682-689. |
| 10. | Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Surgical treatment of advanced gastric cancer: Japanese perspective. Dig Surg. 2007;24:101-107. |
| 11. | Roukos D, Schmidt-Mathiesen A, Encke A. Adenocarcinoma of the gastric antrum: does D2 total gastrectomy with splenectomy improve prognosis compared to D1 subtotal gastrectomy? A long-term survival analysis with emphasis on Lauren classification. Surg Oncol. 1995;4:323-332. |
| 12. | Brady MS, Rogatko A, Dent LL, Shiu MH. Effect of splenectomy on morbidity and survival following curative gastrectomy for carcinoma. Arch Surg. 1991;126:359-364. |
| 13. | Wu CW, Chang IS, Lo SS, Hsieh MC, Chen JH, Lui WY, Whang-Peng J. Complications following D3 gastrectomy: post hoc analysis of a randomized trial. World J Surg. 2006;30:12-16. |
| 14. | Sakaguchi T, Sawada H, Yamada Y, Fujimoto H, Emoto K, Takayama T, Ueno M, Nakajima Y. Indication of splenectomy for gastric carcinoma involving the proximal part of the stomach. Hepatogastroenterology. 2001;48:603-605. |
| 15. | Kitamura K, Nishida S, Yamamoto K, Ichikawa D, Okamoto K, Taniguchi H, Yamaguchi T, Sawai K, Takahashi T. Lymph node metastasis in gastric cancer in the upper third of the stomach--surgical treatment on the basis of the anatomical distribution of positive node. Hepatogastroenterology. 1998;45:281-285. |
| 16. | Hartgrink HH, van de Velde CJ. Status of extended lymph node dissection: locoregional control is the only way to survive gastric cancer. J Surg Oncol. 2005;90:153-165. |
| 17. | Yamamoto M, Baba H, Kakeji Y, Endo K, Ikeda Y, Toh Y, Kohnoe S, Okamura T, Maehara Y. Postoperative morbidity/mortality and survival rates after total gastrectomy, with splenectomy/pancreaticosplenectomy for patients with advanced gastric cancer. Hepatogastroenterology. 2004;51:298-302. |