INTRODUCTION
Acute liver failure (ALF), characterized by massive necrosis of the liver, is a syndrome defined by the onset of coagulopathy and hepatic encephalopathy and has a mortality rate ranging from 50% to 70%[1-6]. Most patients die of liver failure but some die because of bacterial infection or gastro-intestinal bleeding during the progressive decline in liver function. The prognosis of ALF has not dramatically improved, despite the introduction of supporting treatments such as plasma exchange, dialysis and antibiotics[7-10]. Although liver transplantation is the only effective treatment, it is seldom performed, mainly because of the rapid progression of liver failure and the shortage of donors.
Why has the prognosis of ALF not improved? It may be because the pathogenesis of ALF is poorly understood. With limited understanding of the underlying pathogenesis, we cannot evaluate ways to prevent the progression of liver failure. ALF is considered to be a syndrome, which means that there are various routes that lead to massive liver necrosis. Therefore, in the past, the possible roles of triggers such as hepatitis viruses and hepatotoxic materials have been extensively discussed. However, none of the underlying mechanisms have been clarified, except in the cases caused by acetaminophen[11-13].
Recently, several authors reported increased serum levels of macrophage-derived factors in patients with ALF, irrespective of the trigger, which suggests that activated macrophages play an important role in the progression of ALF[14-16]. These reports led us to categorize patients with ALF in terms of the relationship between the macrophage activation and disease progression and to develop a new treatment, transcatheter arterial injection therapy (TASIT), for patients in the early stage of ALF. Based on our analyses of the accumulated cases, this treatment has elicited good responses in many patients but specific conditions were required for the responders. In this article, we discuss the clinical usefulness of TASIT and our system to identify patients with ALF/acute liver injury who would likely benefit from TASIT.
ACTIVATED MACROPHAGES AND MICROCIRCULATORY DISTURBANCES
In the last decade, several authors have reported increases in macrophage-derived CD-163 and osteopontin in the serum of patients with ALF[14-16]. Using the hypothesis that these factors might be produced by activated macrophages in the liver, we pathologically examined a series of resected livers from patients with ALF who had undergone liver transplantation and liver biopsy samples from patients in the early stage of ALF with very high levels of serum alanine aminotransferase (ALT)[17]. Of note, we found diffuse proliferation of activated macrophages in the liver in the majority of these resected livers and biopsy samples.
To collect further evidence of macrophage activation, we measured the serum ferritin concentration in 100 patients with severe acute liver injury, including ALF. The etiology of acute liver injury included HAV (n = 9), HBV (n = 31), HCV (n = 3), drugs other than acetaminophen (n = 6), Wilson’s disease (n = 5), autoimmune hepatitis (n = 3) and 43 cases with indeterminate etiology. We found that the serum ferritin levels were elevated in most patients and, in about a half of them, the concentrations exceeded 10 000 ng/mL[17]. Although the serum ferritin levels are elevated in various diseases including infectious diseases and malignancies, such markedly high levels are comparable to those found only in macrophage activating syndrome[18-20]. Importantly, in some patients, the serum ferritin concentration was in the normal range or only slightly elevated, despite ALT levels exceeding 1 000 U/L. It is known that the serum ferritin concentration increases in acute hepatitis and that the elevated ferritin is derived from collapsed hepatocytes. However, the presence of patients with high ALT and normal ferritin levels strongly suggests that the remarkably elevated serum ferritin concentration in acute liver injury is mainly derived from activated macrophages and not from hepatocytes[21-26].
Because the activation of macrophages in the liver occurs during the early stage of ALF and worsens liver damage, it remains unclear how the activated macrophages are involved in the massive hepatocytic death. To answer this question, we focused on the production of lactate dehydrogenase (LDH) in the liver which increases in response to hypoxia[27-29]. Pathological examination of liver biopsy samples from patients in the early stage of ALF showed that hepatocytic LDH expression increased in correlation with macrophage proliferation. According to these findings, we believe that the terminal process underlying massive hepatocytic death in ALF might be intrahepatic hypoxia caused by microcirculatory disturbances in the liver. Although the steps involved in the progression from macrophage activation to hepatic microcirculatory disturbances are unclear, it is likely that the macrophages secrete cytokines that harm the endothelial cells, resulting in fibrin accumulation in the sinusoid as revealed in animal models[30].
TRANSCATHETER ARTERIAL STEROID INJECTION THERAPY
If the activation and proliferation of macrophages in the liver are the main causes of the massive hepatocytic death, a procedure that suppresses macrophage activity in patients in the early stage of ALF could prevent progression to severe liver failure. Based on this hypothesis, we developed a new treatment method called TASIT in 2005[31]. In this procedure, methylprednisolone is injected via the proper hepatic artery for 2 h (1 000 mg/d for 3 d). If severe coagulopathy is observed, once daily plasma exchange is added to the regimen.
In 2006, we reported our initial results for the first 17 patients who underwent TASIT and evaluated its efficacy and safety by comparison with the same number of patients who were admitted just before the introduction of TASIT or who rejected TASIT[31]. The patients enrolled in that study fulfilled at least one of the following criteria: (1) progressive and sustained prolonged prothrombin time (PT) [PT-international normalized ratio (INR) > 1.5 for 3 d]; (2) presence of ascites; or (3) presence of hepatic encephalopathy. Although the study was not a randomized trial, the prognosis of the patients treated with TASIT was dramatically improved compared with those without TASIT, with survival rates of 76% and 24% respectively. Furthermore, no complications, other than a transient elevation in the serum glucose concentration, were observed. For cases where TASIT was effective, the coagulopathy and encephalopathy improved rapidly (unpublished observations). The average duration of hospitalization for conservative survivors with TASIT was less than 2 wk. To date, a total of 71 patients with ALF or severe acute liver injury expecting to proceed to ALF have undergone TASIT and the conservative survival rate exceeds 70%. We have experienced no complications directly caused by the TASIT procedure except one case in which a limited puncture site hematoma was observed.
To clarify how the arterially injected steroids affect the activated macrophages in the liver, we investigated the pathological changes in some patients by comparing liver biopsy samples taken before and 1 wk after TASIT. This study revealed that the number of proliferated macrophages decreased after TASIT, as did LDH production, indicating that the efficacy of TASIT is dependent on correcting intrahepatic microcirculatory disturbances by suppressing macrophage activity.
CLASSIFICATION OF PATIENTS FOR APPROPRIATE TREATMENT
As described above, ALF is defined as a syndrome associated with coagulopathy and hepatic encephalopathy. Several liver-supporting treatments are recommended for patients who fulfilled the criteria of ALF but there are no guidelines for patients with coagulopathy but not encephalopathy. Because the transition from acute liver injury without encephalopathy to ALF is continuous, there are currently no methods to predict the prognosis of patients with severe acute liver injury and the likelihood of death as a result of ALF. Furthermore, there is no evidence to show that the mechanism of liver injury is qualitatively different between patients with versus without encephalopathy. Indeed, hepatic encephalopathy is an important symptom used to predict whether a patient should undergo liver transplantation. Nevertheless, we believe it is important to prevent the progression to ALF before the development of encephalopathy to improve the prognosis of these patients. In other words, the present definition of ALF encourages us to delay liver-supporting treatments until encephalopathy becomes overt. Thus, we believe that the definition of ALF should be reviewed and reconstructed on the basis of current evidence.
Importantly, the theory that over-activation of macrophages is involved in the progression of ALF might be common to most patients with ALF, regardless of the trigger. Therefore, it is reasonable to classify patients with acute liver injury, with or without encephalopathy, according to the grade of intrahepatic macrophage activation. If TASIT suppresses macrophage activation, such a classification would be clinically relevant as an indication for TASIT, replacing the traditional definition of ALF based on the existence of encephalopathy.
The next question to answer is how we can determine the grade/contribution of intrahepatic macrophage activation. Based on our clinical experience with TASIT, we have uncovered the following important findings: (1) Patients with high serum ferritin concentrations showed strong coagulopathy, regardless of the triggers; (2) The serum levels of LDH are correlated with those of ferritin; (3) In some patients, serum LDH and/or serum ferritin concentrations remained around the normal limit while serum ALT exceeded 1 000 U/L; (4) Among the patients with high levels of serum LDH and ALT activity, some showed an abrupt spontaneous decrease in LDH after observation for 6 to 12 h while ALT levels remained elevated; (5) TASIT was mainly effective in patients with a high serum ferritin concentration which was not influenced by the etiology of liver injury; (6) For patients with effective TASIT, the serum LDH concentration decreased abruptly compared with the changes in ALT levels; and (7) Some patients without excessively high ferritin or LDH progressed to liver failure when their ALT levels remained elevated for a long period.
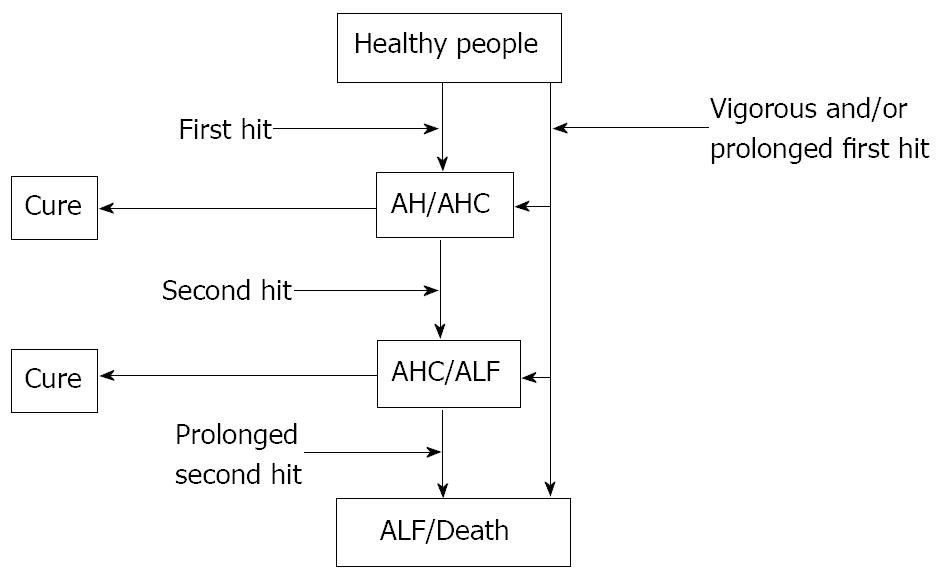
Based on these findings, we have proposed a mechanism underlying the progression of acute liver injury with coagulopathy/ALF (Figure 1). Acute hepatitis is caused by various triggers such as viruses, chemicals and autoimmune disorders. Most viruses and autoimmune disorders damage hepatocytes via cytotoxic T cells while hepatotoxic chemicals do so directly. We call these processes the “first hit”. Although the first hit is usually not sufficient to lead to ALF, a vigorous and/or prolonged first hit could result in ALF. In some patients experiencing the first hit, over-activation of macrophages occurs in the liver, leading to disturbed intrahepatic microcirculation and massive hepatocytic death. The grade of macrophage activation and the consequent microcirculatory disturbances can be estimated by the serum ferritin and LDH levels respectively[32]. We call the process involved in macrophage activation the “second hit”. The activation of macrophages spontaneously declines in some patients who may recover naturally. However, if macrophage activation is prolonged, the risk of death due to liver failure is greatly increased. The intensity and range of liver damage, represented by prolonged PT and elevated serum ALT concentrations, are determined by the sum of the first and second hits. TASIT is thought to be effective by preventing the second hit. Therefore, TASIT should be effective in patients whose liver failure is mainly caused by the second hit but not for those with first-hit-dominant liver failure.
Figure 1 Overview of acute liver failure.
Various triggers may directly harm the hepatocytes as a first hit, although this is usually not strong enough to lead to acute liver failure (ALF). In some patients who experience a first hit, over-activation of macrophages occurs in the liver as a second hit which leads to microcirculatory disturbances in the liver and massive hepatocyte death. The activated macrophages spontaneously decline in some patients but, if this activity is prolonged, the risk of death is substantially increased. Overall, the degree of liver damage is determined by the sum of the first and second hit. AH: acute hepatitis; AHC: acute hepatitis with coagulopathy.
CLASSIFICATION OF ACUTE LIVER INJURY WITH COAGULOPATHY
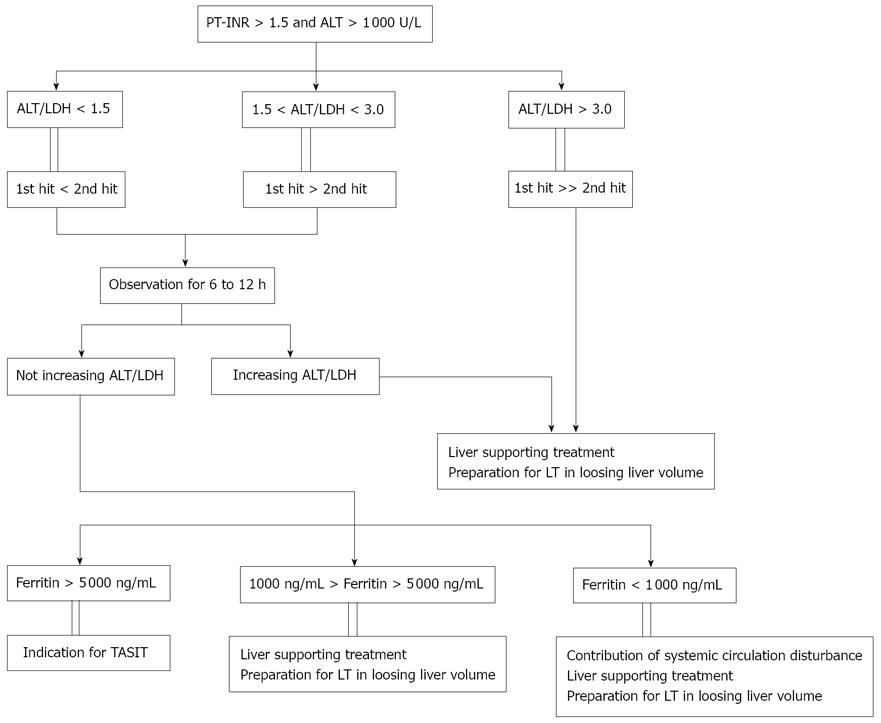
Patients admitted to our hospital with acute liver injury (ALT > 1 000 U/L) and coagulopathy (PT-INR > 1.5) show an elevated ALT/LDH ratio (Figure 2). Based on our experience, ratios < 1.5 represent liver injury mainly caused by the second hit while ratios of 1.5-3.0 indicate equal contributions of the first and second hit. Meanwhile, for patients with an ALT/LDH ratio > 3.0, the liver injury is due to a first-hit-dominant mechanism. In the third condition, we can provide liver-supporting treatment to prevent first hit and continue until liver transplantation can be performed.
Figure 2 A method to classify patients with acute liver failure (acute liver injury) based on the grade of intrahepatic macrophage activation.
First, patients are classified by their alanine aminotransferase (ALT)/lactate dehydrogenase (LDH) ratio which indicates the degree of hypoxia in liver. When a patient has a high serum concentration of LDH compared with that of ALT, the extent of the involvement of macrophage activation is estimated by the serum ferritin concentration. TASIT: transcatheter arterial steroid injection therapy. PT: prothrombin time; LT: liver transplantation.
For patients with an ALT/LDH ratio < 3.0, we monitor the ratio for 6-12 h. In some patients, the ratio increases abruptly during this period because of a decrease in the LDH concentration. This phenomenon likely reflects spontaneous improvement of the intrahepatic microcirculatory disturbances. In these patients, the serum ferritin levels are generally high although they decrease soon thereafter. Most patients with a rapid increase in the ALT/LDH ratio show improvements in coagulopathy but some still progress liver failure because the liver is thought to be severely damaged by the first hit. Most patients without an increase in the ALT/LDH ratio during the observation period present with remarkably elevated serum ferritin levels (> 5 000 ng/mL) and we perform TASIT in these patients. However some patients have relatively low serum ferritin levels (1 000-5 000 ng/mL) and the liver damage in these patients may be due to a first-hit-dominant mechanism. Therefore, we proceed with liver-supporting treatments and liver transplantation. In a few patients, the serum ferritin level is < 1 000 ng/mL but they have a low ALT/LDH ratio. We believe that systemic circulatory disorders may be present in these exceptional cases.
Our system is useful not only to determine the indication of TASIT or to prepare patients for liver transplantation in the early stage of the disease, but is also helpful to diagnose patients with rare etiologies. For example, we experienced a 40 year old man with an extremely high level of serum ferritin (55 775 ng/mL) despite a relatively high ALT/LDH ratio (2.6). The high ferritin level would normally suggest second-hit-dominant liver injury but this was not supported by the ALT/LDH ratio which should have been lower if ferritin was derived from the liver. We considered that most of serum ferritin was derived from extra-hepatic organs and we subsequently found that he was suffering from chronic active EB virus infection. Similarly, we diagnosed a 35 year old woman with acute-onset of Budd-Chiari syndrome after finding a low ALT/LDH ratio and a normal serum ferritin concentration in this patient. Both cases were diagnosed quickly after admission because of our system to manage acute liver injury and the use of a classification method based on the intrahepatic macrophage activation theory.
PROBLEMS TO BE CLARIFIED
We believe that our classification system is useful to better understand the processes underlying liver injury; however, there are several problems to be clarified. Firstly, we do not know what causes over-activation of intrahepatic macrophages. Most patients with acute hepatitis are cured without developing coagulopathy and over-activation of macrophages seldom occurs in these patients. Even in patients with macrophage over-activation, this activation spontaneously regresses in some patients. What determines the duration of macrophage over-activation? Both the host’s condition and the etiologies such as viruses and drugs may be responsible for the behavior of the macrophages. Secondly, it is unclear how activated macrophages cause microcirculatory disturbances. Although we believe that cytokines released from activated macrophages harm endothelial cells, no evidence for this has been reported to date. If we can clarify the underlying processes, we could develop more effective procedures than TASIT to suppress macrophage activation.
Although TASIT is certainly effective for patients with second-hit-dominant liver injury, the procedure has to be performed in the early stage of the disease, ideally during the period corresponding to the peak serum ALT level. Once the liver becomes obviously atrophic, it is difficult to rescue the patients, even with TASIT, most likely because a large amount of fibrin has accumulated in the sinusoids in the late stage of the disease. Clearly, a new approach other than TASIT is needed to overcome this problem. Indeed, for patients in whom liver failure proceeds in the absence of intrahepatic macrophage activation, there is still no effective method to prevent the disease progression. In our experience, TASIT was ineffective for patients with first-hit-dominant liver injury and liver transplantation was ultimately needed for these patients.
In our hospital, the overall prognosis of patients with acute liver injury and coagulopathy has improved because of our classification system and the introduction of TASIT. In Japan, ALF is caused by hepatitis viruses in more than half of the patients whereas acetaminophen is the major cause of ALF in European countries[33]. It is possible that TASIT is not effective for acetaminophen-induced liver injury because it may be a first-hit-dominant type. Therefore, further treatments are needed for such patients.
CONCLUSION
Recent discussions of ALF have mainly focused on the ability to predict prognosis because appropriate guidelines are needed to identify patients who will die without liver transplantation[34-37]. Accordingly, this trend is based on the notion that liver transplantation is the only effective method to rescue patients with ALF. Considering the limited supply of donors, liver transplantation cannot improve the prognosis of all patients with ALF. To reduce the number of patients who die from ALF, we must halt disease progression before ALF proceeds to the end-stage and we must develop new methods to treat patients and systems to classify patients to select the most appropriate treatment. Here, we have proposed such systems in response to these demands. We hope that further studies can elucidate the underlying mechanism involved in the development and progression of ALF and allow us to develop more effective systems and treatment for patients with acute liver injury.
Peer reviewer: Alex P Betrosian, MD, Third Department of Critical care, Athens University, Evgenidion Hopsital, 20 Papadiamantopoulou Str., Athens 11528, Greece
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N