INTRODUCTION
Coronary artery disease (CAD) is a leading cause of premature and permanent disability. CAD arises from gradual narrowing of coronary artery due to atherosclerotic deposits. The progressive narrowing of coronary artery eventually predisposes the patient to myocardial ischemia, a condition in which coronary blood flow decreases to a level below what is needed to meet the demand for oxygen and nutrients. When coronary arterial lumen diameter is reduced by 50%, perfusion abnormalities can be detected but patients are usually asymptomatic. When the diameter is reduced by 70%, clinical symptoms occur during myocardial stress because tissue oxygenation is temporarily below what is needed for adequate heart function. In the advanced ischemic CAD, blood flow and tissue oxygenation are too low to sustain cardiac function at rest. As a result, myocardial infarction occurs. Therefore, rapid and accurate early detection of myocardial ischemia and infarction is highly desirable so that appropriate therapeutic regimens can be given before irreversible myocardial damage occurs.
Diagnostic radiotracers are small molecules labeled with a γ-emitter for single photon emission computed tomography (SPECT) or positron-emitter for positron emission tomography (PET). Nuclear cardiology plays a key role in CAD patient management[1-12]. Precise measurement of regional blood flow has significant clinical importance in identifying myocardial ischemia and infarction, defining the extent and severity of disease, assessing myocardial viability, establishing the need for medical and surgical intervention, and monitoring the effects of treatment. Thus, the radiotracer must be taken up by the myocardium proportionally to the blood flow in order to evaluate areas with reduced blood flow. If the patient has CAD, there will be an area of reduced radiotracer uptake in the myocardium, corresponding to the area of reduced blood flow. If the reduced uptake is worse under stress conditions than that at rest, the perfusion defect is most likely due to ischemia. Information gained during perfusion imaging studies can be used not only to identify CAD but also to give insight into the patient’s prognosis, such as the probability of a hard cardiac event (myocardial infarction or cardiac-related death).
Despite recent development of new non-invasive imaging technologies, such as stress echocardiography and coronary CT (computed tomography) angiography, and wider availability of PET, myocardial perfusion imaging with SPECT radiotracers remains the mainstay of non-invasive evaluations in patients with known or suspected coronary artery disease (CAD)[1-9]. It is the only available imaging technology that assesses the physiological consequence of coronary stenosis and can be combined with exercise and pharmacological stress[10].
The introduction of 201Tl in 1970’s was the turning point of widespread clinical use of myocardial perfusion imaging, and had a profound impact on therapeutic decision-making in patients with CAD over the last three decades. However, the combination of long half-life (t1/2 = 73 h), attenuation artifacts due to low abundance of γ-photons and the low count rate from dose constraints may result in suboptimal images in a significant proportion of perfusion imaging studies using 201Tl. In addition, 201Tl images should be taken as soon as it is injected into the patient due to its distribution and redistribution dynamics, which may not be suitable for situations where immediate imaging is not possible (for example, patients with acute myocardial infarction).
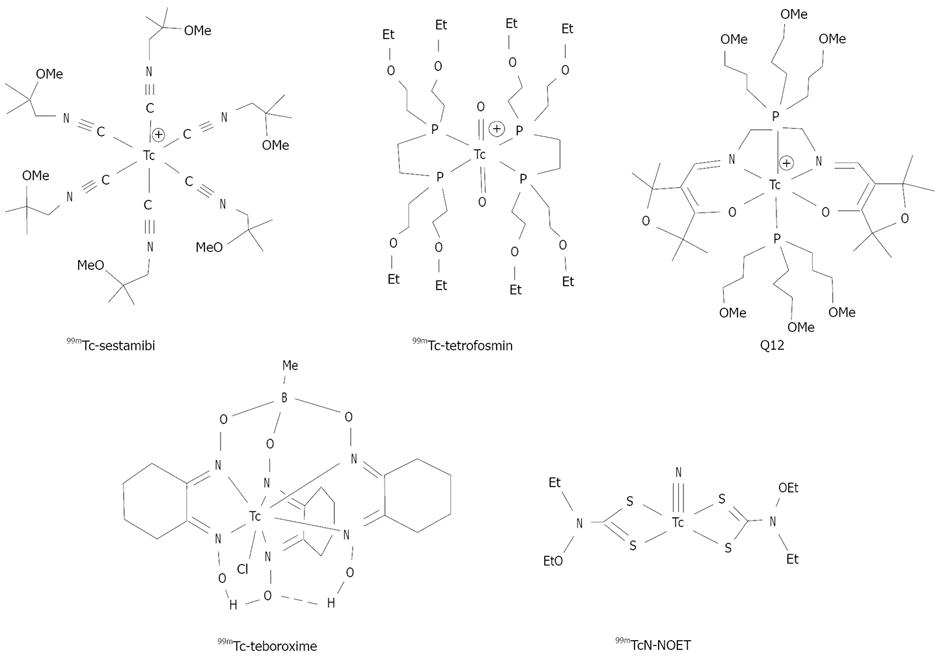
Compared to 201Tl, 99mTc yields relatively high-energy photons (~140 keV) and can be used at high doses due to its short-half life (t1/2 = 6.01 h). The use of 99mTc allows simultaneous assessment of myocardial perfusion and cardiac function in a single study[11]. The combination of half-life, optimal γ-energy and diverse coordination chemistry makes 99mTc the isotope of choice for development of myocardial perfusion radiotracers. In early 1980s, intensive efforts were focused on the development of 99mTc complex radiopharmaceuticals[1,2,5,13]. As a result, 99mTc-Sestamibi, 99mTc-Tetrofosmin, and 99mTc-Teboroxime (Figure 1) have been approved as commercial radiopharmaceuticals for myocardial perfusion imaging in nuclear cardiology. These cationic 99mTc radiotracers are highly lipophilic with cationic or neutral charge, contain at least two ether-like linkages (N-O-R or C-O-R), and are excreted though the hepatobiliary system due to their high lipophilicity.
Figure 1 99mTc radiotracers useful for heart imaging.
99mTc-Sestamibi, 99mTc-Tetrofosmin and 99mTc-Teboroxime have been approved as commercial radiopharmaceuticals for myocardial perfusion imaging.
An ideal perfusion radiotracer should have a high heart uptake with stable myocardial retention, which linearly tracks myocardial blood flow over a wide range. The uptake in the liver and lungs should be minimal so that diagnostically useful images can be obtained within 30 min post-injection. Despite their widespread applications, both 99mTc-Sestamibi and 99mTc-Tetrofosmin do not meet the requirements of an ideal perfusion imaging agent due to their high liver uptake and inability to track the increase in the myocardial blood flow well with roll-off at higher blood flow levels[14]. The intense liver uptake makes it difficult to interpret the heart activity in the inferior and left ventricular wall[1,2,5,12,13]. Because of their enterohepatic clearance, the gut uptake is often aggravated by pharmacological stress[14]. Despite intensive efforts to reduce this interference, photon scattering from the liver activity remains a significant challenge for diagnosis of heart diseases. Thus, it would be of great benefit to develop a new 99mTc perfusion radiotracer that has high heart uptake with the heart/liver ratio substantially better than that of 99mTc-Sestamibi and 99mTc-Tetrofosmin. This review article will summarize recent research efforts to minimize the liver uptake of cationic 99mTc radiotracers, and will focus on the use of ether and crown ether groups to improve their liver clearance kinetics. The main objective is to illustrate that minimizing liver radioactivity accumulation is critically important for new 99mTc radiotracers. The ultimate goal is to develop a new 99mTc perfusion radiotracer that will satisfy the unmet medical need and serve a large population of patients with known or suspected CAD.
IMPROVING LIVER CLEARANCE BY ETHER GROUPS
The usefulness of ether groups to reduce radiotracer liver uptake was first observed in development of 99mTc-Sestamibi[15-18]. Since then, many ether-containing ligands or chelators have been used to improve T/B ratios of cationic 99mTc complexes[19-22]. For example, studies on Q-series of 99mTc complexes showed that ethers on phosphine ligands could improve the heart uptake and imaging properties[19]. 99mTc-Sestamibi, 99mTc-Tetrofosmin and Q12 all contain six or more ether groups and show much less liver uptake as compared to 99mTcN-Noet and 99mTc-Teboroxime (Figure 1).
Ether-containing cationic 99mTc-nitrido complexes.
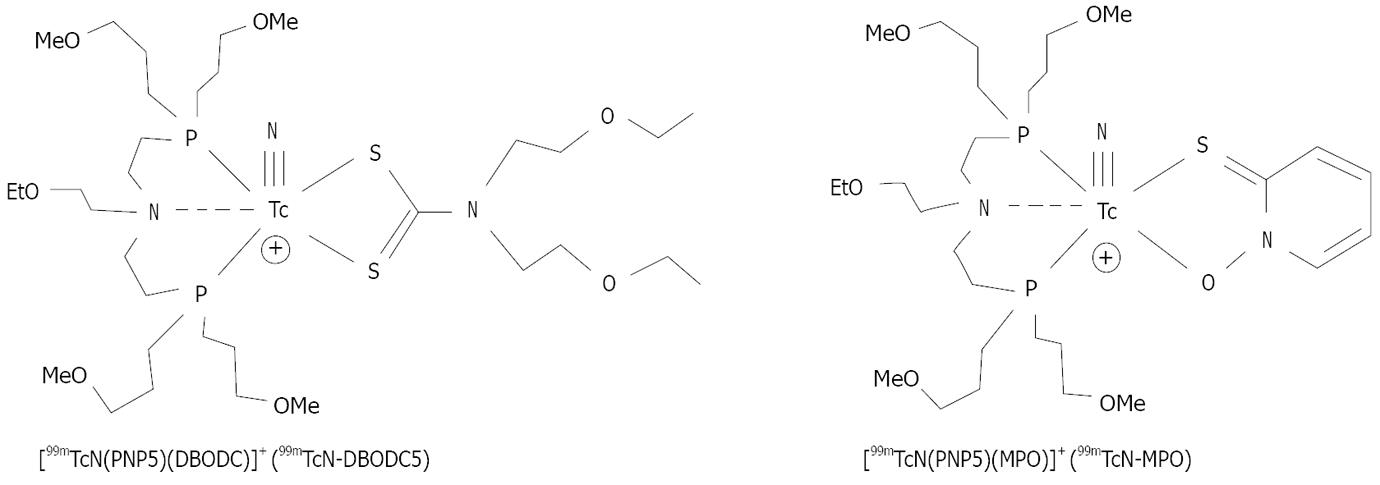
Duatti’s group reported a series of cationic 99mTc-nitrido complexes (Figure 2), which contain a [99mTc≡N]2+ core, a bidentate dithiocarbamate (DTC), a tridentate PNP-type bisphosphine[24,25]. It was found that the amine-N donor atom in the PNP bisphosphine chelator invariably is trans to the Tc≡N triple bond[24,25]. The nitrogen heteroatom is important to provide stabilization for the [99mTc≡N]2+ core. It is remarkable that the combination of DTCs and bisphosphines results in formation of cationic 99mTc-nitrido complexes of high yield and high radiochemical purity[24,25]. Results from animal studies show that DTCs and bisphosphines have significant impact on biodistribution properties of cationic 99mTc-nitrido complexes[26-29]. Among the cationic 99mTc-nitrido complexes evaluated in Sprague-Dawley (SD) rats, 99mTcN-DBODC5 (Figure 2) had a high heart uptake and was able to retain in the rat myocardium for more than 2 h[26,27]. The liver radioactivity accumulation was almost completely eliminated at 2 h p.i. with the heart/liver ratios being 10 × better than that of 99mTc-Sestamibi[26,27]. Imaging studies also showed that 99mTcN-DBODC5 had a fast liver clearance, and was able to give clear images of the heart as early as 15 min post-injection in SD rats[28]. The first-pass extraction fraction of 99mTcN-DBODC5 was between that of 99mTc-Sestamibi and 99mTc-Tetrofosmin[29]. 99mTcN-DBODC5 is currently under clinical investigation as a new myocardial perfusion radiotracer.
Figure 2 Cationic 99mTc-nitrido complexes with fast liver clearance and excellent heart/liver ratios.
Both 99mTcN-DBODC5 and 99mTcN-MPO are under clinical investigation as new myocardial perfusion imaging agents.
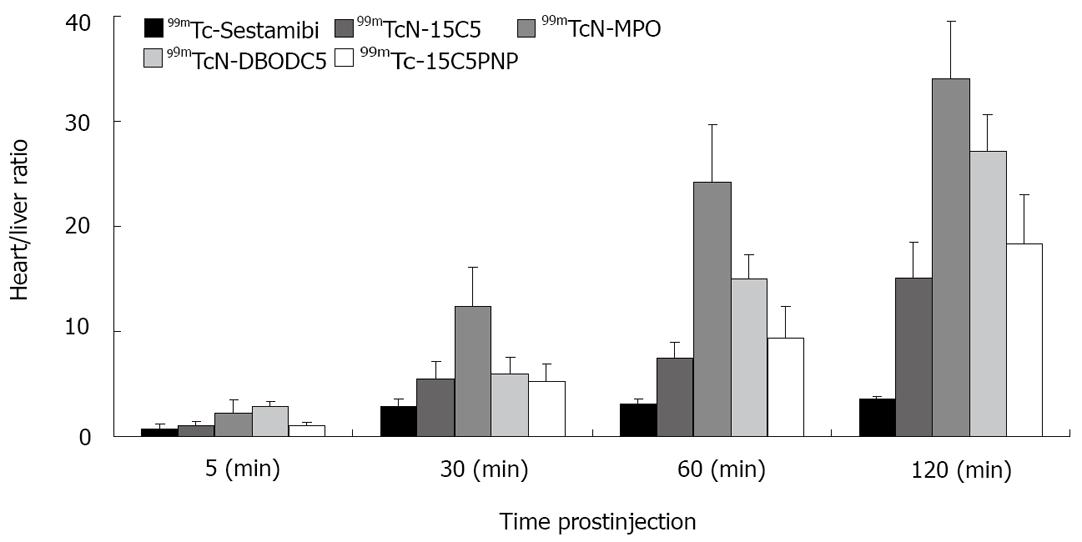
Other bidentate chelators have been used to replace DTC ligands in 99mTcN-DBODC[30-33]. The use of these bidentate chelators offers a great structural diversity, and allows easy modification of the lipophilicity and biological properties of cationic 99mTc radiotracers. For example, 2-mercaptopyridine oxide was used to prepare 99mTcN-MPO (Figure 2). 99mTcN-MPO and 99mTcN-DBODC5 shared the same basic structure, but differ in the bidentate π-donor chelating ligand. They also have very similar in vivo stabilities. Biodistribution studies in SD rats showed that 99mTcN-MPO had a high initial heart uptake (2.45 ± 0.58 %ID/g at 5 min post-injection), with a long myocardial retention (2.44 ± 0.46 %ID/g at 120 min post-injection)[46]. The heart uptake of 99mTcN-MPO was between that of 99mTc-sestamibi and that of 99mTcN-DBODC. The liver clearance was fast, resulting in excellent heart/liver ratios. The heart/liver ratio of 99mTcN-MPO at 30 min post-injection was 12.75 ± 3.34, which is ~4 × higher than that of 99mTc-sestamibi (2.90 ± 0.62) and 2 × higher than that of 99mTcN-DBODC (6.01 ± 1.45). By 120 min post-injection, the heart/liver ratio of 99mTcN-MPO increased to 27.60 ± 8.44, which is 8 × that of 99mTc-sestamibi (3.52 ± 0.34) and is slightly better than that of 99mTcN-DBODC (21.20 ± 3.39) at the same time point, although this difference is not significant within the experimental error.
Crown ether-containing cationic 99mTc-nitrido complexes
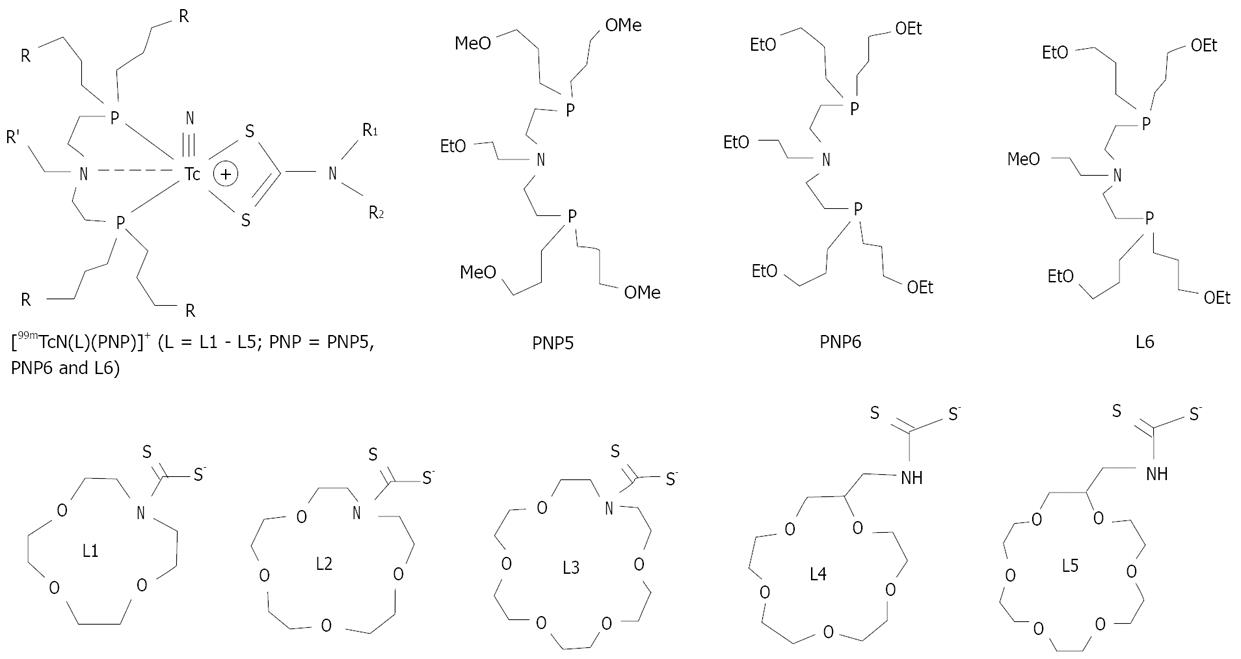
The crown ether-containing DTCs (Figure 3) are of particular interest because they are able to form stable cationic 99mTc-nitrido complexes in combination with bisphosphines, and the crown ether groups are able to balance the lipophilicity of cationic 99mTc-nitrido complexes without changing their overall molecular charge[31,32]. Both crown ether-containing DTCs and bisphosphines have a significant impact on the lipophilicity of their corresponding cationic 99mTc-nitrido complexes. For example, [99mTcN(L)(PNP6)]+ is much more lipophilic than [99mTcN(L)(PNP5)]+ because of the high lipophilicity of the four ethoxy groups in PNP6[31]. Results from biodistribution studies showed that most of the crown ether-containing cationic 99mTc-nitrido complexes had a relatively high initial heart uptake with a long myocardial retention[31]. It was also demonstrated that [99mTcN(L4)(L6)]+ (99mTcN-15C5) had the heart/liver ratios that were 4-5 times better than that of 99mTc-Sestamibi due to its faster liver clearance. The heart uptake and heart/liver ratios of 99mTcN-15C5 are comparable to that of 99mTcN-DBODC5[32]. The results from planar imaging and SPECT studies in dogs further confirm its faster liver clearance kinetics as compared to that of 99mTc-Sestamibi[33]. In dogs with acute myocardial infarction, 99mTcN-15C5 was able to detect the perfusion defect as early as 15-30 min.
Figure 3 Crown ether-containing DTCs, bisphosphines and their cationic 99mTc-nitrido complexes.
Crown ether-containing cationic 99mTc(I)-tricarbonyl complexes
The rich and diverse coordination chemistry of [99mTc(CO)3]+ offers a tremendous opportunity to develop new 99mTc(I)-tricarbonyl radiotracers[34-43]. The [99mTc(CO)3]+ core has also been widely used to prepare the target-specific 99mTc radiotracers[37-40]. In [99mTc(H2O)3(CO)3]+, all three water molecules are labile with respect to substitution[40,42]. Monodentate ligands, 2-methoxy-isobutylisonitriles (MIBI) and dimethyl-3-methoxypropylphosphine (DMMPP), have been used to prepare complexes [99mTc(L)3(CO)3]+ (L = DMMPP and MIBI)[22]. In Sprague-Dawley rats, [99mTc(DMMPP)3(CO)3]+ and [99mTc(MIBI)3(CO)3]+ showed high heart uptake. However, their heart/liver and heart/lung ratios are not as good as that of 99mTc-Sestamibi due to slow hepatobiliary excretion[22]. The major challenge is to maintain the “true” cationic nature of [99mTc(L)3(CO)3]+ (L = DMMPP and MIBI) in the blood circulation.
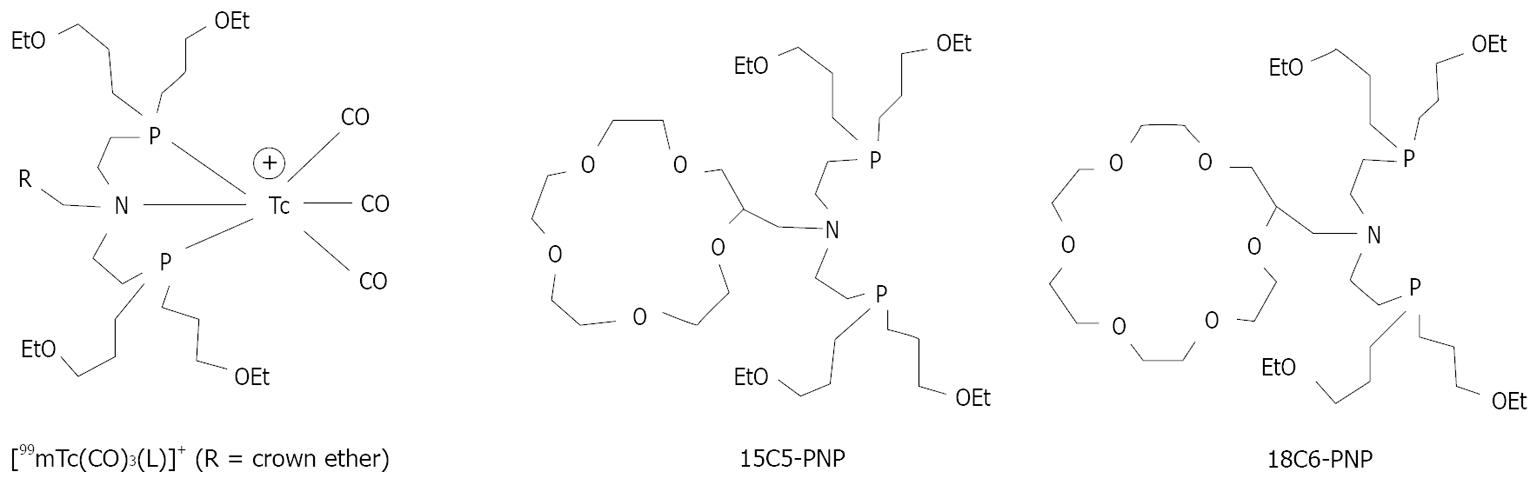
It has been reported that bidentate ligands form 99mTc(I)-tricarbonyl complexes with low solution stability, which may result in high protein binding and high background activity in blood[37,40]. The chloride in blood may react with [99mTc(L-L)(CO)3]+ to form neutral complexes [99mTc(L-L)(CO)3Cl] that has low heart uptake and slow hepatobiliary excretion. In contrast, the PNP bisphosphines are able to form highly stable cationic complexes [99mTc(CO)3(L)]+ (Figure 4: L = 15C5-PNP and 18C6-PNP)[44,45]. The tridentate bisphosphines are critical to maintain the cationic nature of 99mTc(I)-tricarbonyl complexes. Among the radiotracers evaluated in SD rats, [99mTc(CO)3(15C5-PNP)]+ (99mTc-15C5-PNP) had a high initial heart uptake with a long myocardial retention. It also showed a rapid clearance from liver and lungs. The heart/liver ratio of 99mTc-15C5-PNP is ~2.5× better than that of 99mTc-Sestamibi at 30 min post-injection. 99mTc-15C5-PNP is almost identical to 99mTcN-DBODC5 with respect to their heart uptake and heart/liver ratios[45]. Planar imaging studies also demonstrated that 99mTc-15C5-PNP had a much better liver clearance profile than 99mTc-sestamibi.
Figure 4 Cationic 99mTc(I)-tricarbonyl complexes with crown ether-containing PNP bisphosphines.
COMPARISON OF BIODISTRIBUTION CHARACTERISTICS
Figure 5 compares their heart/liver ratios in SD rats at 5, 30, 60 and 120 min post-injection. In general, the heart uptake of 99mTcN-15C5, 99mTcN-MPO, 99mTc-15C5PNP and 99mTcN-DBODC5 are comparable within the experimental error, and is slightly lower than that of 99mTc-Sestamibi, particularly at > 60 min post-injection. However, their heart/liver ratios are much better than that of 99mTc-Sestamibi at 30-120 min post-injection[46]. For example, the heart/liver ratio of 99mTcN-MPO (12.75 ± 3.34) at 30 min post-injection is almost twice of that of 99mTcN-DBODC5 (6.01 ± 1.45), and is ~4 times better than that of 99mTc-sestamibi (2.90 ± 0.22).
Figure 5 Comparison of heart/liver ratios between [99mTcN(L4)(L6)]+ (99mTcN-15C5), [99mTcN(mpo)(PNP5)]+ (99mTcN-MPO), [99mTc(CO)3(15CPNP)]+ (99mTc-15C5PNP), 99mTc-Sestamibi and 99mTcN-DBODC5 in SD rats.
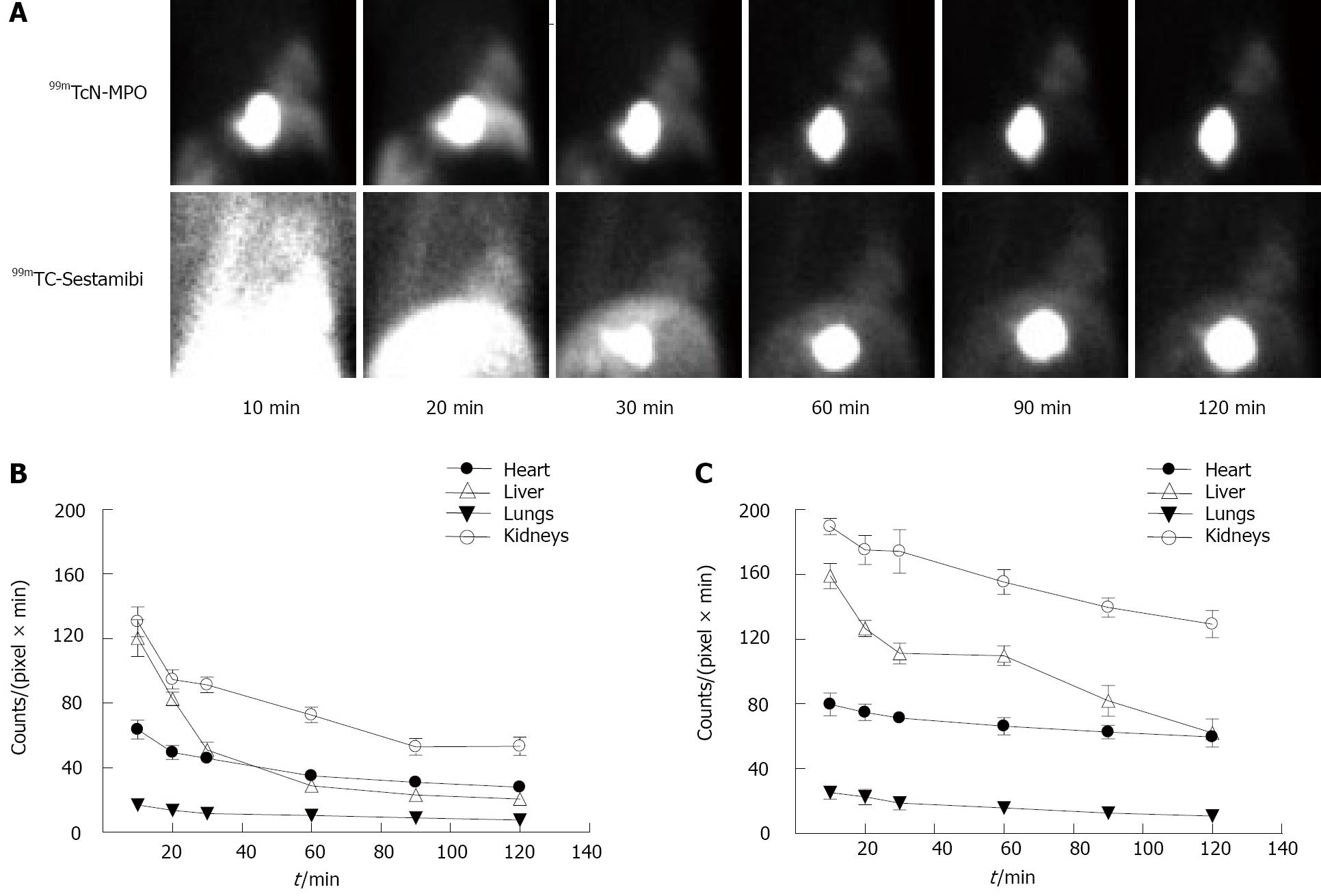
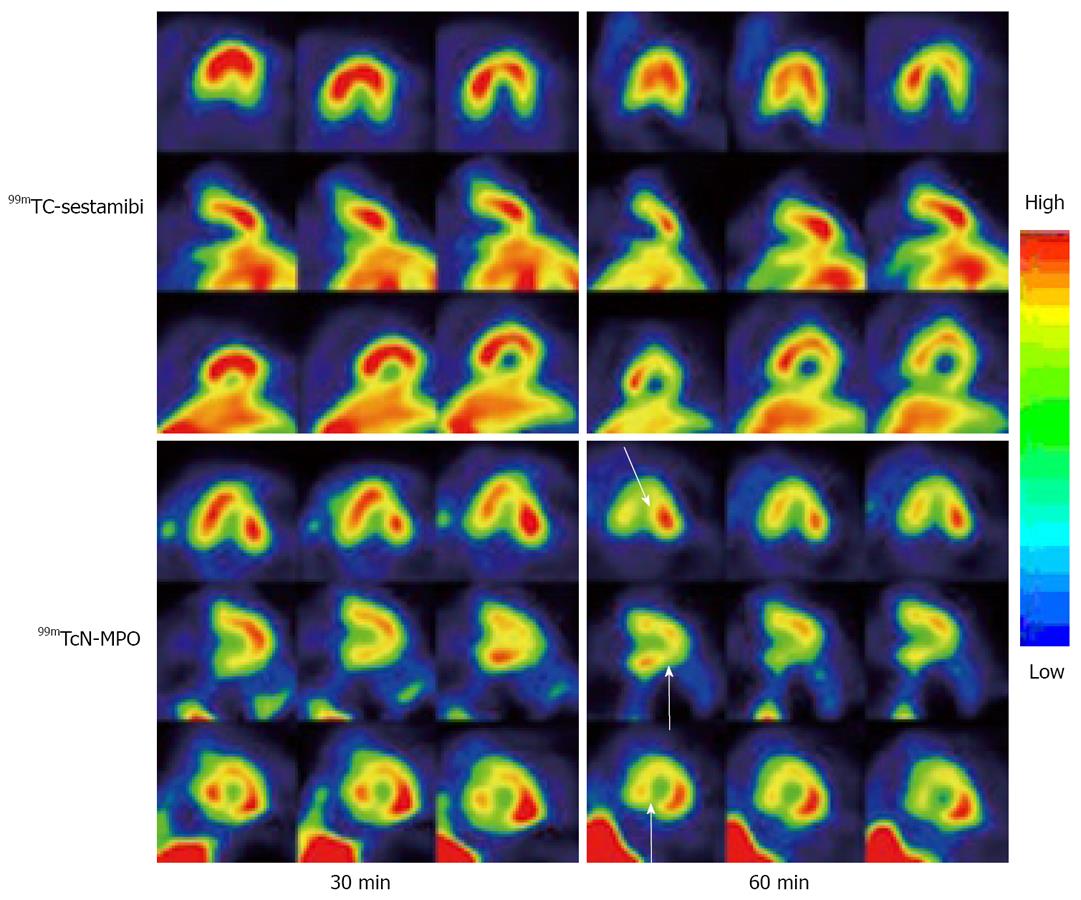
99mTcN-MPO was further evaluated in normal dogs in comparison with 99mTc-sestamibi. It was found that 99mTcN-MPO and 99mTc-sestamibi shared very similar blood clearance with < 50% of initial radioactivity remaining at 1 min, < 5% of initial radioactivity remaining at 30 min post-injection. The liver uptake in the dogs administered with 99mTcN-MPO decreased rapidly whereas a prolonged liver uptake was seen in all images of the dogs administered with 99mTc-Sestamibi (Figure 6). The heart/liver ratio of 99mTcN-MPO increased with time (0.53 ± 0.06 at 10 min and 1.22 ± 0.06 at 60 min post-injection), whereas the heart/liver ratio of 99mTc-Sestamibi remained relatively unchanged over the 2 h study period (0.50 ± 0.03 at 10 min and 0.60 ± 0.02 at 60 min post-injection). SPECT studies (Figure 7) in canines with acute myocardial infarction indicated that the perfusion defect could be visualized as early as 30 min after administration of 99mTcN-MPO but not 99mTc-Sestamibi. The combination of fast blood clearance, relatively high heart uptake with prolonged myocardial retention makes 99mTcN-MPO a better perfusion imaging radiotracer than 99mTc-Sestamibi. On the basis of preliminary results from the rats and dogs, 99mTcN-MPO was selected as a clinical candidate for human studies.
Figure 6 Planar images and organ clearance kinetics of normal dogs.
A: Planar images of normal dogs administered with 99mTcN-MPO and 99mTc-Sestamibi. Both radiotracers had similar initial myocardial uptake; B: Imaging quantification in normal dogs administered with 99mTcN-MPO; C: Imaging quantification in normal dogs administered with 99mTc-Sestamibi. The liver radioactivity of 99mTcN-MPO was markedly decreased within first 60 min whereas 99mTc-Sestamibi had a slower reduction in liver radioactivity over time. A mild myocardial washout was observed in the dogs administered with 99mTcN-MPO. No significant myocardial washout was seen in dogs administered with 99mTc-Sestamibi.
Figure 7 SPECT images of the same dog (with acute myocardial infarction) administered with ~10 mCi of 99mTc-Sestamibi and 99mTcN-MPO at 30 and 60 min p.
i. Arrows indicate the presence of perfusion defects due to myocardial infarction. For 99mTcN-MPO, the perfusion defects were clearly seen as early as 30 min post-injection due to its fast liver clearance. For 99mTc-Sestamibi, there was a significant between the perfusion defects and the liver radioactivity.
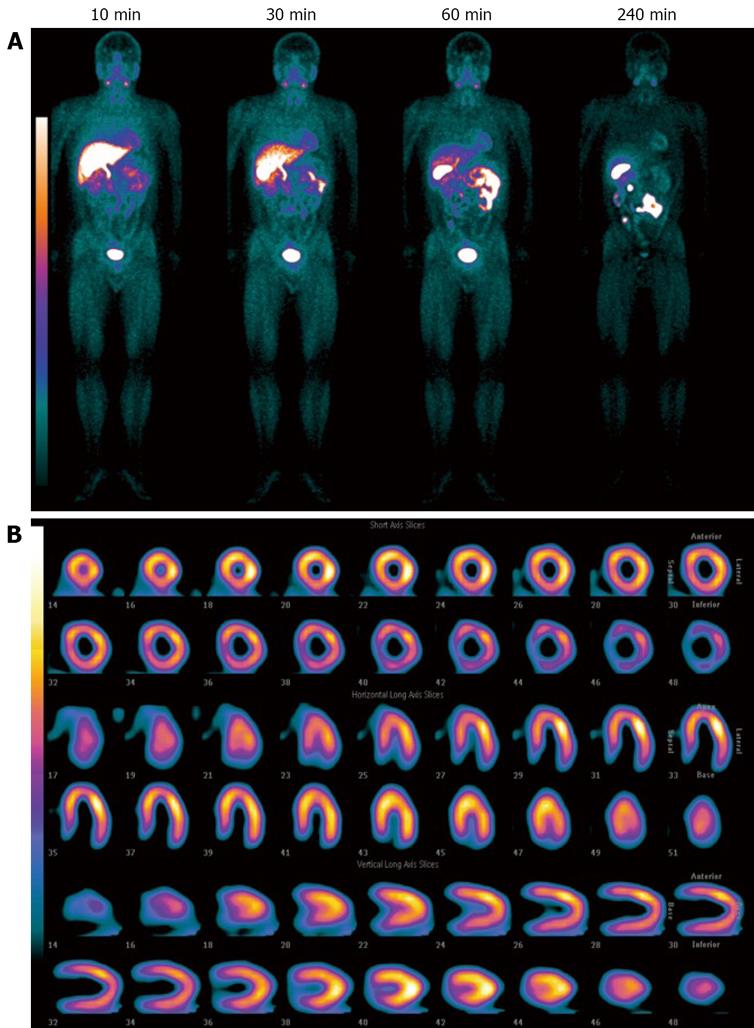
Safety parameters measured up to 24 h after injection of 99mTcN-MPO revealed no adverse reactions and clinically significant drug-related changes in healthy volunteers (unpublished data). The radiation dosimetry of 99mTcN-MPO is comparable to that of 99mTc-Sestamibi[16] and 99mTc-Tetrofosmin[17]. Figure 8 shows the whole-body images of a healthy volunteer administered with 99mTcN-MPO (~25 mCi) at 10, 30, 60 and 240 min post-injection. The radioactivity clearance was so fast that the heart was well separated from the left liver lobe at 10 min post-injection. Although its first-pass extraction fraction was between that of 99mTc-Sestamibi and 99mTc-Tetrofosmin[5,6], its fast liver clearance of 99mTcN-MPO will allow early visualization of the heart in patients with CAD. The rapid liver clearance may shorten imaging protocols and permit a more precise determination of perfusion defects in the inferoapical wall on myocardial images. 99mTcN-MPO is an excellent alternative to 99mTc-Sestamibi for myocardial perfusion imaging when the liver uptake makes it difficult to interpret the heart activity in the inferior and left ventricular wall in patients with known or suspected CAD.
Figure 8 The whole-body images of representative a healthy volunteer administered with 99mTcN-MPO.
A: Administered with 99mTcN-MPO (~25 mCi) at 10, 30, 60 and 240 min post-injection; B: Representative SPECT images of the heart after administration of 99mTcN-MPO (~25 mCi) at 60 min post-injection.
LOCALIZATION MECHANISM
Optimal log P values
Myocardium has the highest mitochondrial population that occupies up to 40% of the total volume of myocytes. Other mitochondria-rich organs include salivary glands, liver and kidneys. This may explain why most lipophilic 99mTc complex cations tend to have high uptake in mitochondria-rich organs, such as heart, liver and kidneys. For a 99mTc radiotracer to localize in mitochondria, it must be able to across plasma and mitochondrial membrane. Regardless of their charge, most small molecules are able to across the plasma membrane without significant difficulty. However, the mitochondrial membrane is only permeable to those molecules with appropriate molecular charge and molecular shape. While the contribution from mitochondrial potentials provides a driving force for mitochondrial localization of cationic 99mTc radiotracers, the lipophilicity might modulate their penetration capability across the lipophilic plasma and mitochondrial membranes. There is little information available with regard to the optimal lipophilicity for the heart-selectivity. Cationic 99mTc radiotracers with the log P values > 1.5 often shows a high protein binding and a slow clearance from non-cardiac organs while hydrophilic cationic radiotracers with log P < 0.5 usually show a low heart uptake with a fast washout from myocardium[30-32,45]. In both cases, the heart/liver ratio is low because of either high liver uptake or fast myocardial washout. On the basis of studies on cationic 99mTc-nitrido and 99mTc(I)-tricarbonyl complexes, it seems that cationic 99mTc radiotracers must have a log P value in the range of 0.9-1.2 in order to achieve a high heart uptake[30-32,45,46].
Subcellular distribution characteristics and mitochond-rial localization
To understand the myocardial localization mechanism, the subcellular distribution characteristics of 99mTcN-MPO[47] and 99mTcN-DBODC5[48] in SD rat hearts were analyzed in comparison with 99mTc-Sestamibi according to the literature[49,50]. It was clearly demonstrated that more than 85% of myocardial radioactivity localized in the mitochondria of myocytes for both 99mTcN-MPO and 99mTcN-DBODC5. There was no significant difference between 99mTcN-MPO, 99mTcN-DBODC5 and 99mTc-Sestamibi with respect to their mitochondrial radioactivity accumulation, and subcellular distribution characteristics.
MULTIDRUG RESISTANCE GENE EXPRESSION AND LIVER CLEARANCE KINETICS
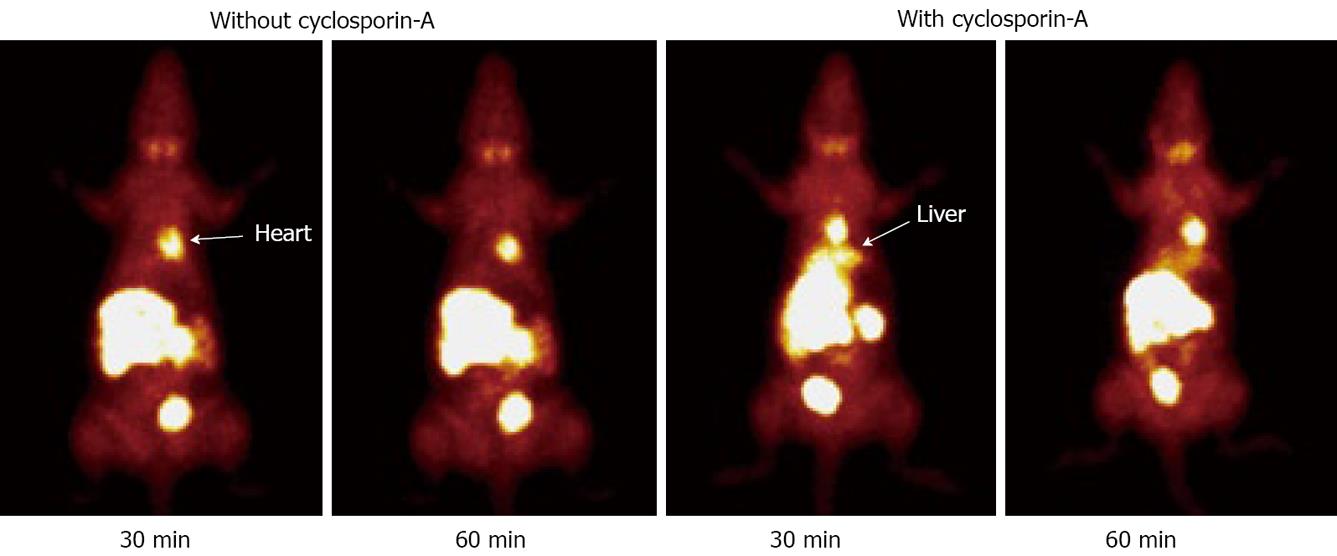
For the last two decades, many cationic 99mTc radiotracers have been evaluated for their potential as radiotracers for heart imaging. However, it is not clear why certain cationic 99mTc radiotracers show better liver clearance and heart/liver ratios than others, even though they may have a similar lipophilicity. It is well-documented that cationic 99mTc radiotracers, such as 99mTc-Sestamibi and 99mTc-Tetrofosmin, are also clinically useful for noninvasive imaging of multidrug resistance (MDR) in tumors of different origin[51-53,57,58]. It is well-documented that MDR1 P-glycoprotein (MDR1 Pgp) and multidrug resistance associated proteins (MRPs) are overexpressed in normal organs that are involved in excretory functions, including kidneys and liver[54-56]. For example, an increased 99mTc-Sestamibi uptake in normal liver of cancer patients has been reported after administration of P-glycoprotein inhibitors[59,60]. To demonstrate the correlation between the MDR and MRP transport function of hepatocytes and liver excretion kinetics of 99mTcN-MPO, both biodistribution and imaging studies were performed using the SD rats in the absence/presence of Cyclosporin A. It was found that the uptake of 99mTcN-MPO in the kidneys and liver was significantly increased, and the radioactivity excretion was delayed, in the presence of Cyclosporin A. Similar results were also obtained in planar images (Figure 9) of SD rats administered with 99mTcN-MPO with/without excess Cyclosporin A. Thus, the MDR/MRP transport function is most likely responsible of the fast efflux of 99mTcN-MPO from kidneys and liver.
Figure 9 Planar images of the SD rats administered with ~1.
0 mCi of 99mTcN-MPO in the absence and presence of cyclosporin-A at 30 and 60 min post-injection. Pre-treatment with cyclosporin-A (14 mg/kg) results in more liver radioactivity accumulation and slower liver clearance.