Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.109093
Revised: June 10, 2025
Accepted: July 8, 2025
Published online: August 27, 2025
Processing time: 120 Days and 14.2 Hours
Hepatic osteodystrophy (HO) is a common and frequently untreated complica
Core Tip: Hepatic osteodystrophy (HO) is a common complication, manifested as osteoporosis or osteopenia, encountered in the evolution of chronic liver diseases (CLD). Despite being clinically significant, it often represents an underappreciated and underdiagnosed complication of CLD as systematic screening and management remain suboptimal. The general biology of HO, including its pathogenesis, diagnostic tools, and rationale for treatment, has been determined largely empirically from studies of postmenopausal women with osteoporosis. The treatment of HO is limited, reflecting an unmet need for the best possible management of this disorder. Bisphosphonates have been shown to be effective in selected group of patients with CLD.
- Citation: Pramanik S, Palui R, Ray S. Hepatic osteodystrophy: An underrecognized metabolic bone disease. World J Hepatol 2025; 17(8): 109093
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/109093.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.109093
Bone disease is a common complication of chronic liver disease (CLD). The term hepatic osteodystrophy (HO) refers to all metabolic bone complications encountered in CLD. However, osteoporosis is now widely accepted as the primary metabolic bone disease in primary biliary cholangitis (PBC) and likely CLD as a whole[1]. Despite being clinically signi
In patients with CLD, significant variability in skeletal alterations was observed, influenced by the etiology, duration, and stage of the liver condition[6]. Initially osteoporosis was described only with cholestatic live diseases (primary biliary cirrhosis and PBC), but recent data suggests approximately every second patient with viral hepatitis, hemochromatosis, and Wilson’s disease has osteoporosis or osteopenia[7]. A recent trial showed the prevalence of osteoporosis and oste
| Disease | Ref. | n | Age (Year) | Post-menopausal (% of women) | Cirrhosis (%) | Osteoporosis prevalence |
| Alcoholic liver disease | Kim et al[14], 2003 | 18 | 50 | 0 | 0 | 22 |
| Wilson disease | Weiss et al[7], 2015 | 14 | 36 | NR | 38 | 9 |
| HBV disease | Schiefke et al[15], 2005 | 13 | 49 | NR | 18 | 15 |
| HCV disease | Schiefke et al[15], 2005 | 30 | 49 | NR | 18 | 20 |
| MAFLD | Lee et al[9], 2016 | 6634 | Men > 40 and postmenopausal women | 50 | NR | 3.3% (male), 10.4 % (female) |
| Primary biliary cirrhosis | Seki et al[16], 2017 | 128 | 61 | 100 | 0 | 26 |
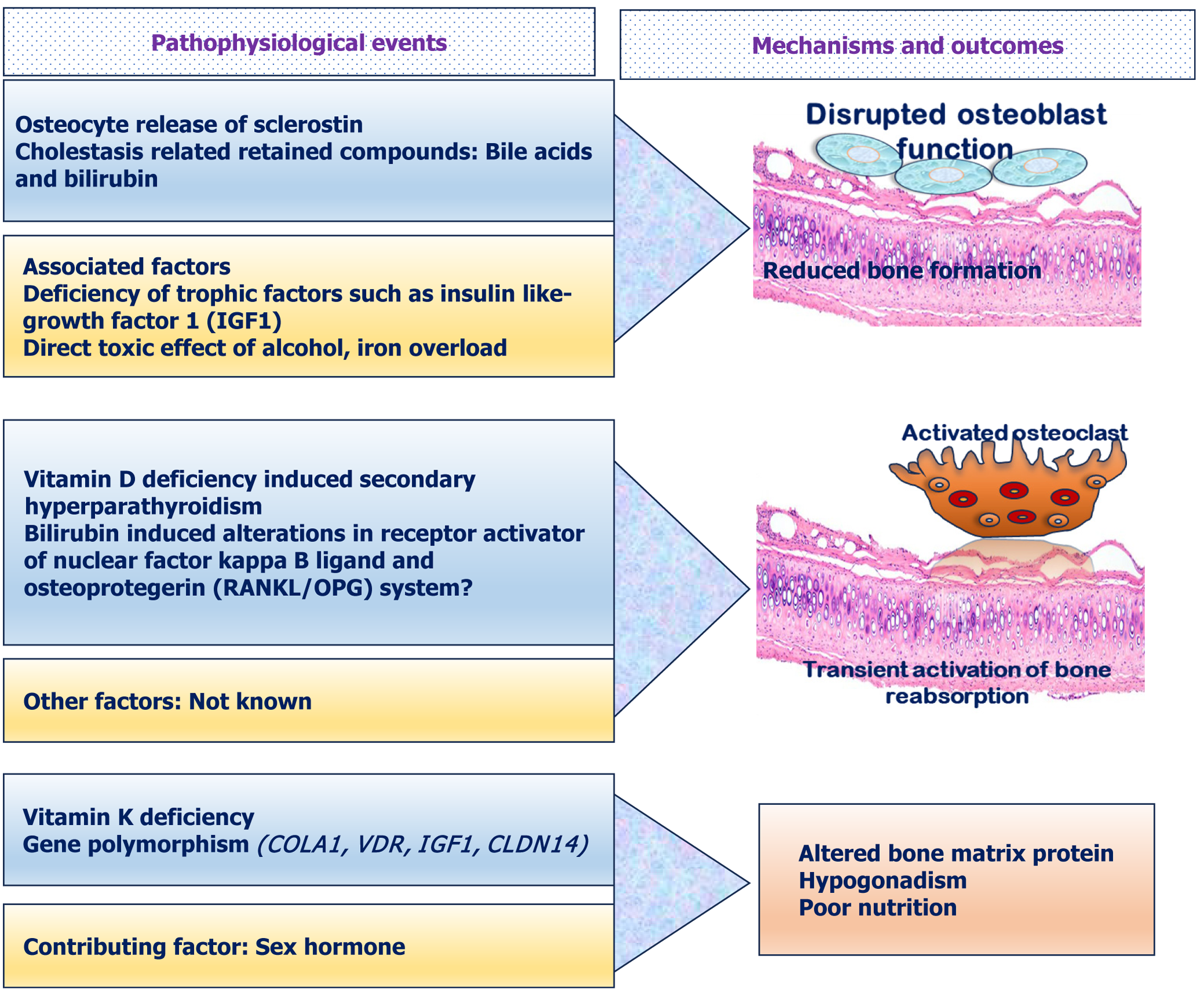
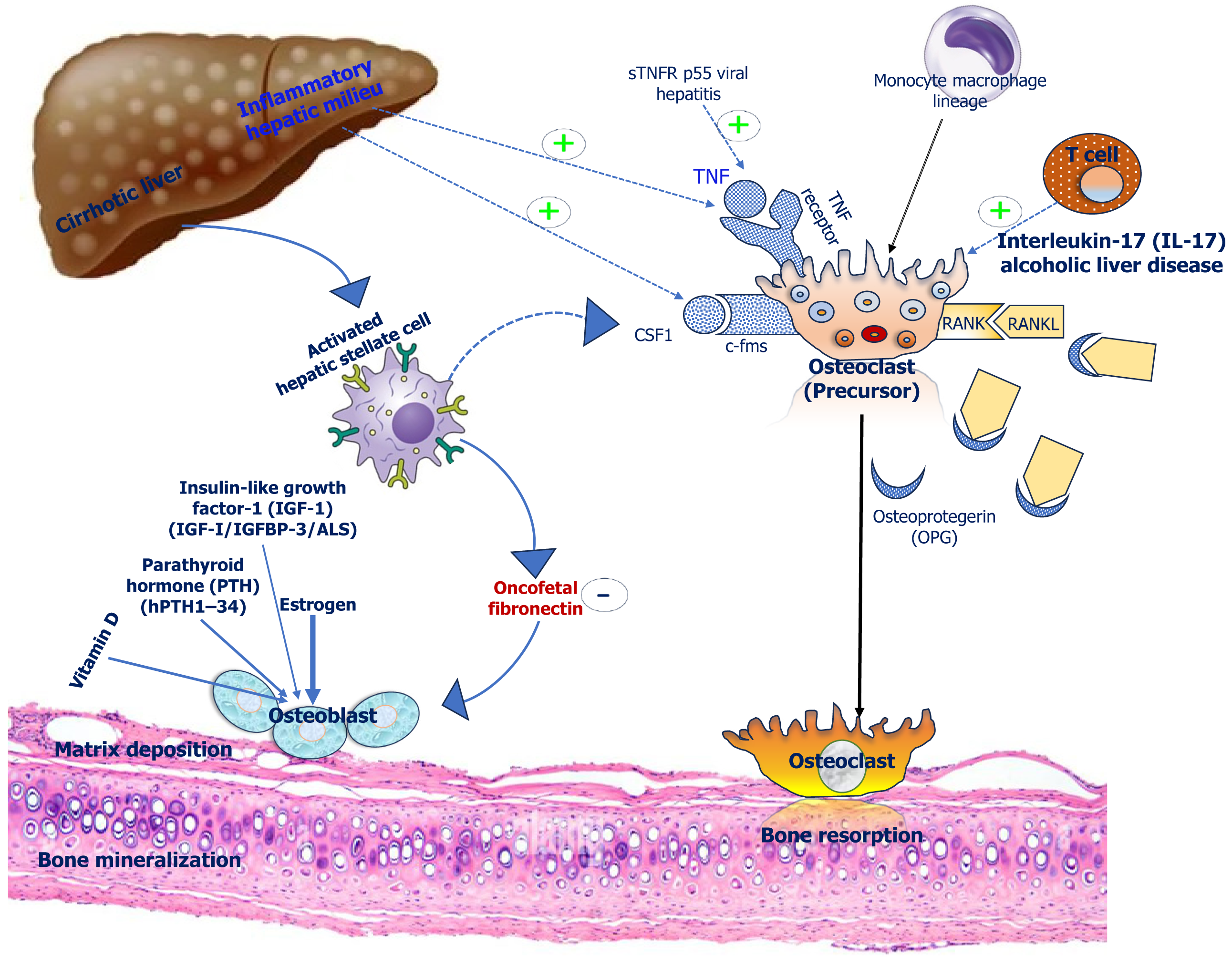
The pathogenesis of development of HO is complex and multifactorial[17]. Several interactive physiological effects contribute to the pathogenesis of HO. Both hepatokines (functional proteins secreted from hepatic tissue) like bone morphogenic protein 9, fibroblast growth factor 21, insulin like growth factor 1 (IGF1), hepcidin, feutin A etc., and osteokines (secreted from osseous tissue) like osteocalcin, osteopontin, sclerostin etc., interact together and can affect hepatic tissue as well as bone. This physiological crosstalk is collectively referred to as liver-bone axis[18]. This liver-bone interaction plays the pivotal role in the pathogenesis of HO. Initial studies for exploring the pathogenesis of HO were mainly done in cholestatic diseases. However, recent studies also evaluated extensively the possible factors behind the development of HO in other hepatic diseases including advanced CLD, metabolic dysfunction associated steatotic liver disease (MASLD) and ALD. Though there is evidence of significant heterogeneity, most of the studies found that the primary factor behind the low bone mass in HO is low bone formation rate in these patients. In addition, increased bone resorption rate and nutritional factors also play an important role in development of HO. The summary of the unifying mechanisms of pathogenesis is outlined in Figure 1.
Initial studies done in patients with primary biliary cirrhosis showed impaired osteoblastic function as suggested by decreased mean trabecular bone volume, mean wall thickness, mean osteoid seam width along with defective matrix synthesis[19]. Similar finding of low bone formation rate was also reported in other histomorphometric analysis studies done in cholestatic liver disease[20,21]. Low serum level of bone formation markers like osteocalcin were also reported in patients with primary biliary cirrhosis[22]. There is multiple hypothesis which can explain the low turn-over state of HO. Low IGF1 had been reported in patients with CLD[23]. Decreased synthesis of IGF-1, IGFBP-3 and growth hormone resistance play a role in the genesis of low bone mineral density (BMD) in these patients[24,25]. Sclerostin, a key negative regulator of the Wnt/β-catenin signalling pathway, inhibits bone formation by reducing osteoblastic activity[26]. Sclerostin binds to the LRP5/6 receptor and thus prevents the interaction of this LRP5/6 receptor with the WNT ligands and further activation of osteoblastic pathway[27]. Moreover, WNT signalling pathway also inhibits key mediators of osteoclastogenesis like receptor activator ratio of nuclear factor kappa ligand (RANKL), leading to inhibition of bone resorption[28]. Thus, sclerostin can also increase osteoclastogenesis by inhibiting this WNT signalling pathway. In histopathological examination of hepatic tissue of PBC, sclerostin was found to be expressed in the epithelium of the bile ducts and the expression level was higher in the presence of cholangitis or granuloma[29]. Moreover, serum level of sclerostin was reported to be higher in advanced liver disease[30]. Increased expression of sclerostin in osteocytes had also been reported in patient of ALD[31]. Thus, sclerostin is possibly one of the major mediators of low bone mass leading to the development of HO. In more advanced stage of cholestasis, the elevated levels of bilirubin and bile acids can also suppress the bone formation by inducing apoptosis of osteoblastic cells in patients with cholestatic liver disease[32,33].
In a few bone morphometric studies done in advanced liver disease, evidence of increased bone resorption had also been reported[34,35]. Decreased bone wall thickness and increased bone turnover were reported in patients with PBCs[35]. Secondary hyperparathyroidism due to vitamin D deficiency or alteration of vitamin D metabolism had been associated with PBC[36]. Osteoprotegerin (OPG)/RANKL can also play a pivotal role in activating osteoclast mediated bone resor
Among the other factors nutritional and genetic factors can also increase the risk of development of HD. Activation of osteocalcin is dependent on Vitamin K activity and increase in uncarboxylated osteocalcin had been associated with reduced BMD and increased in fracture risk[38]. Vitamin K deficiency is common in cholestatic disorders, and it can be another factor behind the low bone mass in liver diseases[39].
Alteration of vitamin D metabolism can also be one of the factors behind the deterioration bone metabolism in liver disease. In the skin 7-dehydrocholesterol is converted into vitamin D3. In cirrhosis, there is an increased expression of 7-dehydrocholesterol reductase which leads to increased degradation of 7-dehydrocholesterol[40]. Vitamin D binding protein (VDBP) is essential for proper circulation of vitamin D. Rodent studies have reported that VDBP levels are decreased with advancing CLD resulting in impairment of circulation of vitamin D and its metabolites in CLD[40]. Hyd
Hypogonadism can be seen in ALD, advanced cirrhosis, and hemochromatosis due to effect on both hypothalamic-pituitary and gonadal level and can cause bone loss by mainly reducing osteoblastic activity[46,47].
Varius genetic studies have explored the possible role of genetic mutations or polymorphism in development of HO. Genetic polymorphism of VDR gene had been reported to be associated with HO in PBC[48]. In a study from Spain, COLIA1 Sp1 and VDR polymorphism were reported to reduce bone mass in patients with PBC[49]. The polymorphism of IGF1 microsatellite repeat and CLDN14 genes were also reported to be associated with low bone mass in cholestatic liver disease[50,51].
Liver diseases are inflammatory conditions, and different pro-inflammatory cytokines can affect bone health in advanced liver diseases. CLD causes elevation of transforming growth factor-β (TGF-β) levels. Increased levels of TGF-β alters the composition of extracellular matrix thereby affecting bone flexibility. TGF-β shifts the extracellular matrix towards fibronectin which increases osteoclast activity. Oncofetal fibronectin, a o-glycosylated form of fibronectin is induced by TGF-β which directly interferes with formation of bone[17]. Similarly, vimentin expression is increased by TGF-β which suppresses the maturation of osteoprogenitor cells. Further, TGF-β interferes with bone morphogenetic protein signalling and blocks osteoblast maturation[17]. Proinflammatory cytokines like tumour necrosis factor α is increased in NAFLD and it can increase osteoclast activity as well as suppress osteoblast recruitment from progenitor cells[52]. Various other cytokines like interleukin (IL) 1, IL 8 were found to be associated in development of HO in cirrhotic patients[53]. The summary of effect of the various inflammatory cytokines are shown in the Figure 2.
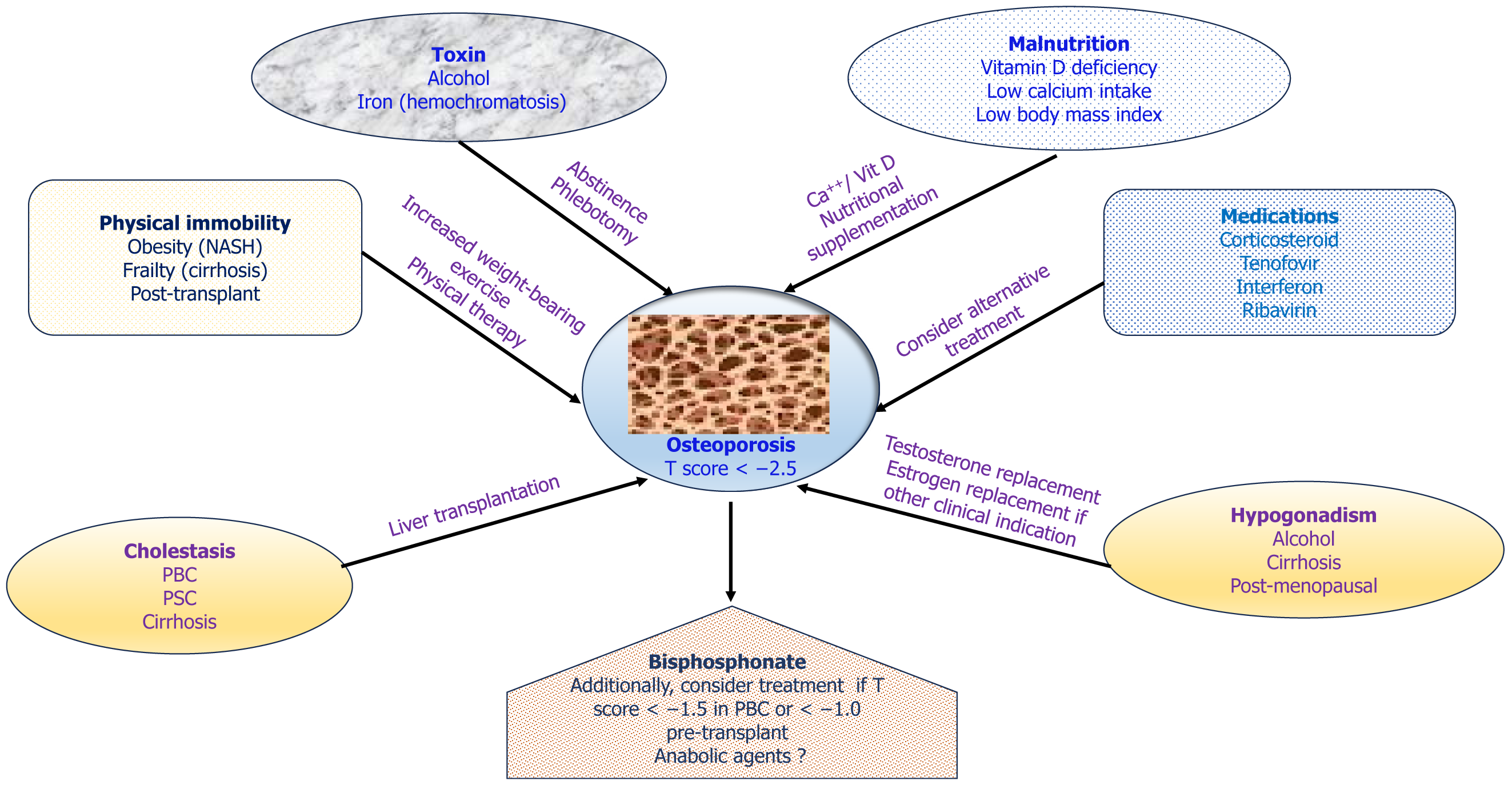
In addition to CLD per se, etiological factors behind the development of CLD can directly affect bone metabolism. Alcohol associated bone disease is a multifactorial disease[54]. Alcohol causes bone disease by direct effects on bone and mineral metabolism in addition to causing hepatic damage, nutrient deficiencies, hypogonadism and hypercortisolism[55,56]. In hemochromatosis, excess iron deposition can directly suppress bone formation by inhibiting osteoblasts and can also increase bone resorption rates[46,57,58]. The specific pathophysiological factors for different aetiologies like ALD, cholestatic liver disease, MASLD and viral hepatitis have been summarized in Table 2[52,59-72].
| Disease | Pathophysiological factors | Ref. |
| Alcoholic liver disease | Direct toxic effect of ethanol on osteoblast | [55-58] |
| Reduced IGF1/prostaglandins | ||
| Reduced VDBP and hydroxylation of vitamin D Increased proinflammatory cytokines (IL-1, IL-6, and TNF-α) | ||
| Alcoholic neuropathy and myopathy | ||
| Cholestatic liver disease | Enhanced IL-17 mediated bone loss | [59-61] |
| Reduced Vit D and VDBP | ||
| Increased oncofetal fibronectin inhibiting bone formation | ||
| MASLD | Increased proinflammatory cytokines (IL-1, IL-6, and TNF-α) | [48,62-64] |
| Increased RANKL and IL-17A mediated bone loss | ||
| Decreased Fetuin-A level leading to reduced bone mass | ||
| Reduced IGF-1, FGF21, and IGFBP1 | ||
| Increased osteopontin level mediated bone loss | ||
| Reduced LCAT and defect in reverse cholesterol transport | ||
| Viral hepatitis | Increased serum level of serum levels of soluble TNF receptor p55 | [65-68] |
| Increased RANKL mediated bone loss | ||
| Iron overload | ||
| Anti-viral drug mediated bone loss |
Other general risk factors like increasing age, low body mass index, malnutrition, lack of exercise, muscle wasting and smoking can also be responsible for significant bone loss in CLD[17]. Medications used in various liver diseases like corticosteroids, anti-viral agents (ribavirin), Cholestyramine, Calcineurin inhibitors and chemotherapeutic agents also have detrimental effects in the bone health[5,73-75].
High risk factors for osteopenia and osteoporosis can be classified into traditional risk factors and liver specific factors. Traditional risk factors such as older age, female gender, post-menopausal status, physical inactivity, alcohol con
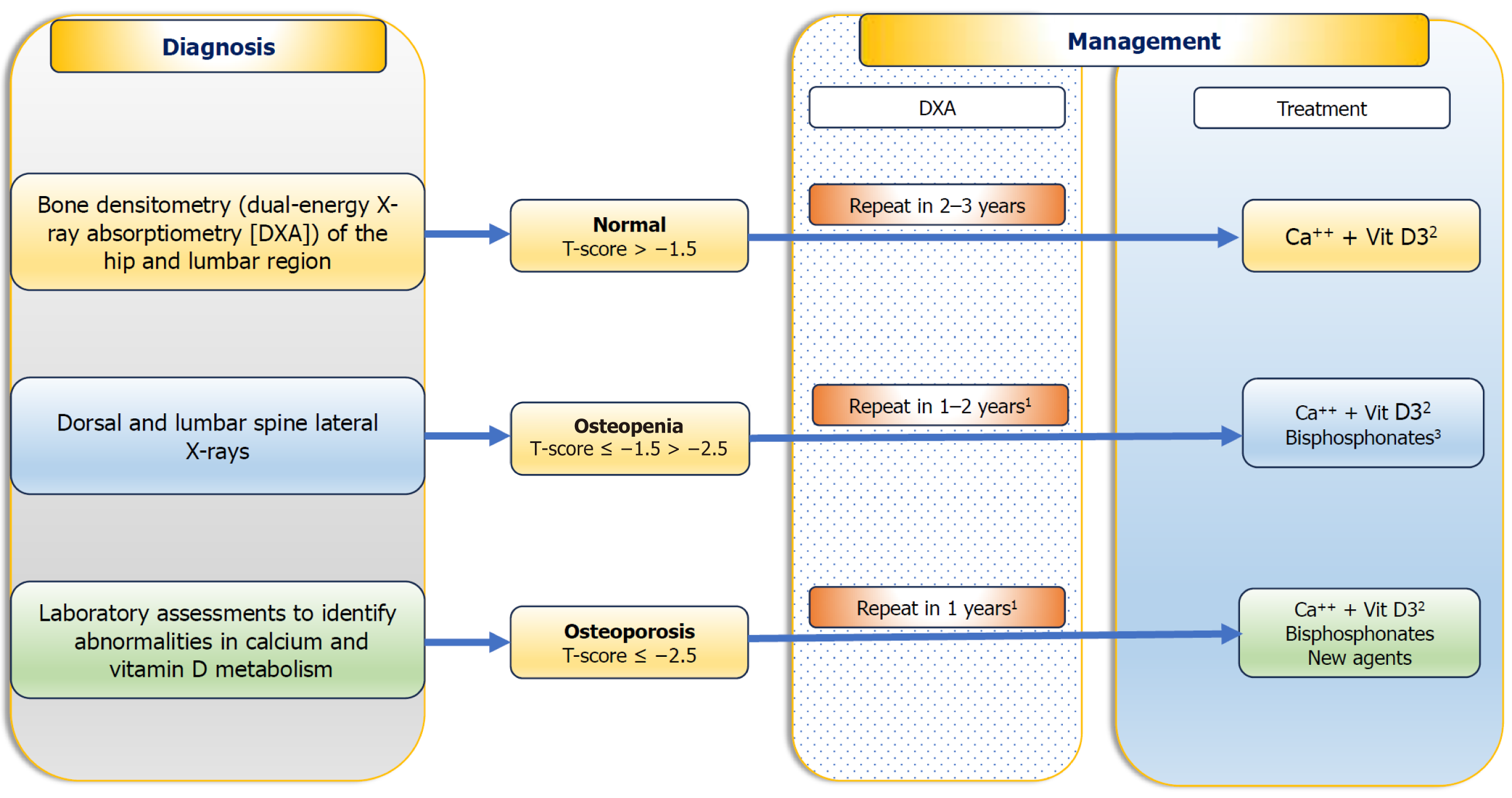
The gold standard for diagnosing HO is to assess BMD with dual X-ray absorptiometry (DXA) at the lumbar vertebrae and femoral neck. The World Health Organization (WHO) defines osteoporosis as a T-score below -2.5, indicating BMD more than 2.5 standard deviations below the average for young adults. Osteopenia is defined as a T-score between -1 and -2.5. For individuals aged less than 50 years, the Z-score is recommended to compare BMD with age-, race-, and sex-matched controls[76]. WHO also created the Fracture Risk Assessment Tool (FRAX®) to assess individual risk based on clinical factors and BMD at the femoral neck. FRAX estimates the 10-year probability of hip and major osteoporotic fractures. However, presence of fragility fracture needs immediate treatment, without the need for BMD measurement. These cut offs are derived from general osteoporosis guidelines, where CLD patients are underrepresented. Dedicated studies to determine treatment threshold for CLD patients are lacking.
In cirrhotic patients with ascites, paracentesis should be done before BMD measurements as fluid can falsely lower lumbar spine BMD values during a DXA scan[77]. In 2003, the American Gastroenterological Association (AGA) released gui
Given the high prevalence of HO, it is imperative that all patients with CLD undergo a comprehensive assessment of bone health. This should include the measurement of vitamin D and blood calcium levels. Additionally, thyroid and gonadal functions should be evaluated to exclude other potential causes of osteoporosis.
Bone turnover markers (BTMs) are derivatives of the bone remodelling process detected in the blood and urine of patients with bone disorders. The most commonly utilized markers of osteogenesis include osteocalcin, alkaline phosphatase (bone specific), and procollagen type 1 carboxyterminal propeptide. Resorption markers include urinary pyridinoline, deoxypyridinoline, type 1 collagen amino-terminal telopeptide, and hydroxyproline. Till date, there is no consensus regarding their use in clinical practice in CLD patients, However, they can be used to monitor therapy when antiresorptive therapy is initiated.
It is essential to recognise that liver dysfunction can influence serum concentrations of BTMs, revealing an increased degradation of bone matrix. Consequently, the evaluation of BTMs provides limited conclusions for individuals with CLD[79].
Limited guidelines are available for screening for osteoporosis in patients with CLD. A higher prevalence of osteopenia and osteoporosis has been observed in individuals with PBC compared to age and sex-matched controls[2]. In general, evaluation of BMD should be conducted in cirrhotic patients and in patients with cholestatic liver disorders, those on long-term corticosteroid therapy, and as part of the liver transplantation evaluation[80]. In conditions associated with rapid bone loss, like in cholestatic patients with multiple risk factor for osteoporosis, and in whom high-dose corticosteroid treatment has been started recently, it is advisable to repeat DXA in a shorter interval of around one year. For patients with advanced cirrhosis, the same schedule is also recommended[80]. In individuals within normal BMD, DXA may be repeated after 2-3 years, as is suggested in the non-cirrhotic population. A flow chart for diagnosis and treatment of bone disease in CLD patients is provided in Figure 3.
General measures: The current treatment of osteoporosis in CLD mainly focuses on alleviation of risk factors, treatment of primary liver disease, and optimization of nutritional status. Caloric intake recommendations vary between 35-50 kcal/kg/day, with daily protein intake of 1.2-1.5 g/kg actual body weight, depending on the severity of malnutrition[81]. Supplements of calcium (1000–1500 mg/day) and vitamin D (400–800 IU/day) or the dose required to preserve normal levels should be provided to all CLD patients as primary prevention[80,82]. However, the role of calcium and vitamin D supplementation in preventing HO has not been established[83] and clinical trials addressing this issue are needed. Vitamin D levels should be assessed in all patients with CLD, specifically in those with advanced disease[84], non-alcoholic fatty liver and cholestatic diseases[85]. In absence of any specific recommendations in patients with CLD barring those with chronic cholestasis, it seems rational to supplement all patients with CLD with 25(OH)D levels < 20 ng/mL with oral vitamin D until a serum 25(OH)D level of above 30 ng/mL is achieved[80,86]. Additionally, vitamin D levels should be monitored annually in perimenopausal and postmenopausal women with PBC.
Specific antiosteoporotic therapy: There is no universal agreement regarding the appropriate time to initiate treatment, however, people with established osteoporosis, and consequently with fragility fractures, should receive treatment to reduce the risk of future fractures. PBC patients with a T-score below -1.5 have a high risk of hip and vertebral fractures, supporting this T-score as a guide in practice for starting specific therapy in these patients[87] (Figure 3). It is logical to consider specific treatment in these patients and in all osteoporosis patients prior to transplantation. Although commonly recommended as first-line treatment for osteoporosis[2], effectiveness of bisphosphonates (BPs) in CLD is not as clear because of the limited number of studies with small sample size and short-term follow-up. Studies on in CLD have mostly been done in patients with PBC[88]. In particular, alendronate and ibandronate have been found to be effective in improving bone mass in PBC patients[56]. In a study of post-menopausal women with PBC and osteoporosis, iban
Current understanding of PBC-related osteoporosis indicates that it results from decreased bone formation, which may explain the attenuated effect of traditional antiresorptive agents. In this context, anabolic agents like parathyroid hormone (PTH) analogues, teriparatide or abaloparatide, may be more efficacious than BPs. In one study involving rats that underwent biliary ductal ligation, use of recombinant human PTH 1-34 showed a significant improvement in BMD compared to untreated controls[93], but has not been specifically evaluated in humans with CLD.
Figure 4 illustrating contributing factors to the development of osteoporosis in CLD, probable preventive strategies, and treatment of osteoporosis.
Hormone replacement: Initially, concerns over worsening of cholestasis restricted estrogen use in CLD but randomized controlled trials found no deterioration in liver disease[94,95]. Hormone replacement therapy (HRT) may be effective in increasing BMD in PBC, however no improvement in fracture risk has been demonstrated and adverse effects limit the use[96]. Considering available agents with less side effects, HRT is not recommended for treatment of osteoporosis in CLD patients without primary hypogonadism[1]. It seems sensible to screen for and treat hypogonadism with testo
Liver-directed therapies: Effectiveness of therapies aimed at the primary liver disease depends on the aetiology. Tre
HO remains a highly prevalent, yet underrecognized complication of CLD. Its unique pathogenesis, spanning dis
We thank Dr. Somnath Mondal, PhD, Associate Director, IPD Analytics, Bhubaneswar for preparing the illustrations for this article.
| 1. | Danford CJ, Trivedi HD, Bonder A. Bone Health in Patients With Liver Diseases. J Clin Densitom. 2020;23:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Guañabens N, Parés A. Management of osteoporosis in liver disease. Clin Res Hepatol Gastroenterol. 2011;35:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Crosbie OM, Freaney R, McKenna MJ, Hegarty JE. Bone density, vitamin D status, and disordered bone remodeling in end-stage chronic liver disease. Calcif Tissue Int. 1999;64:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Gasser RW. Cholestasis and metabolic bone disease - a clinical review. Wien Med Wochenschr. 2008;158:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 6. | Nakchbandi IA, van der Merwe SW. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Weiss KH, Van de Moortele M, Gotthardt DN, Pfeiffenberger J, Seessle J, Ullrich E, Gielen E, Borghs H, Adriaens E, Stremmel W, Meersseman W, Boonen S, Cassiman D. Bone demineralisation in a large cohort of Wilson disease patients. J Inherit Metab Dis. 2015;38:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Manrai M, Wangmo S, Mishra Y, Premdeep H, Srivastava S, Dawra S, Pakhetra R. The HOPeFUL Study: Hepatic Osteodystrophy Prevalence and Contributory Factors Unveiling Study. J Mar Med Soc. 2025;. [DOI] [Full Text] |
| 9. | Lee SH, Yun JM, Kim SH, Seo YG, Min H, Chung E, Bae YS, Ryou IS, Cho B. Association between bone mineral density and nonalcoholic fatty liver disease in Korean adults. J Endocrinol Invest. 2016;39:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | López-Larramona G, Lucendo AJ, González-Delgado L. Alcoholic liver disease and changes in bone mineral density. Rev Esp Enferm Dig. 2013;105:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Liang J, Meng WD, Yang JM, Li SL, Zhong MN, Hou XX, Wang R, Long YY, Bao LX, Bao M. The association between liver cirrhosis and fracture risk: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2018;89:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Shirinezhad A, Eshlaghi FM, Salabat D, Azarboo A, Ardakani ZF, Esmaeili S, Hoveidaei AH, Ghaseminejad-Raeini A. Prevalent osteoporosis and fracture risk in patients with hepatic cirrhosis: a systematic review and meta-analysis. BMC Gastroenterol. 2025;25:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Haagsma EB, Thijn CJ, Post JG, Slooff MJ, Gips CH. Bone disease after orthotopic liver transplantation. J Hepatol. 1988;6:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Kim MJ, Shim MS, Kim MK, Lee Y, Shin YG, Chung CH, Kwon SO. Effect of chronic alcohol ingestion on bone mineral density in males without liver cirrhosis. Korean J Intern Med. 2003;18:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, Wiese M, Moessner J. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol. 2005;11:1843-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (7)] |
| 16. | Seki A, Ikeda F, Miyatake H, Takaguchi K, Hayashi S, Osawa T, Fujioka SI, Tanaka R, Ando M, Seki H, Iwasaki Y, Yamamoto K, Okada H. Risk of secondary osteoporosis due to lobular cholestasis in non-cirrhotic primary biliary cholangitis. J Gastroenterol Hepatol. 2017;32:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Ehnert S, Aspera-Werz RH, Ruoß M, Dooley S, Hengstler JG, Nadalin S, Relja B, Badke A, Nussler AK. Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development. Int J Mol Sci. 2019;20:2555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Gao H, Peng X, Li N, Gou L, Xu T, Wang Y, Qin J, Liang H, Ma P, Li S, Wu J, Qin X, Xue B. Emerging role of liver-bone axis in osteoporosis. J Orthop Translat. 2024;48:217-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Stellon AJ, Webb A, Compston J, Williams R. Low bone turnover state in primary biliary cirrhosis. Hepatology. 1987;7:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 89] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85:1356-1362. [PubMed] |
| 21. | Guañabens N, Parés A. Osteoporosis in chronic liver disease. Liver Int. 2018;38:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Guañabens N, Parés A, Alvarez L, Martínez de Osaba MJ, Monegal A, Peris P, Ballesta AM, Rodés J. Collagen-related markers of bone turnover reflect the severity of liver fibrosis in patients with primary biliary cirrhosis. J Bone Miner Res. 1998;13:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Liu Z, Han T, Werner H, Rosen CJ, Schaffler MB, Yakar S. Reduced Serum IGF-1 Associated With Hepatic Osteodystrophy Is a Main Determinant of Low Cortical but Not Trabecular Bone Mass. J Bone Miner Res. 2018;33:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Marek B, Kajdaniuk D, Niedziołka D, Borgiel-Marek H, Nowak M, Siemińska L, Ostrowska Z, Głogowska-Szeląg J, Piecha T, Otremba Ł, Holona K, Kazimierczak A, Wierzbicka-Chmiel J, Kos-Kudła B. Growth hormone/insulin-like growth factor-1 axis, calciotropic hormones and bone mineral density in young patients with chronic viral hepatitis. Endokrynol Pol. 2015;66:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 27. | Marini F, Giusti F, Palmini G, Brandi ML. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos Int. 2023;34:213-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 28. | Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801-3811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Guañabens N, Ruiz-Gaspà S, Gifre L, Miquel R, Peris P, Monegal A, Dubrueil M, Arias A, Parés A. Sclerostin Expression in Bile Ducts of Patients With Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J Bone Miner Res. 2016;31:1725-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Rhee Y, Kim WJ, Han KJ, Lim SK, Kim SH. Effect of liver dysfunction on circulating sclerostin. J Bone Miner Metab. 2014;32:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Jadzic J, Milovanovic PD, Cvetkovic D, Zivkovic V, Nikolic S, Tomanovic N, Djuric MP, Djonic D. The altered osteocytic expression of connexin 43 and sclerostin in human cadaveric donors with alcoholic liver cirrhosis: Potential treatment targets. J Anat. 2022;240:1162-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95:2581-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Ruiz-Gaspà S, Martinez-Ferrer A, Guañabens N, Dubreuil M, Peris P, Enjuanes A, Martinez de Osaba MJ, Alvarez L, Monegal A, Combalia A, Parés A. Effects of bilirubin and sera from jaundiced patients on osteoblasts: contribution to the development of osteoporosis in liver diseases. Hepatology. 2011;54:2104-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Guichelaar MM, Malinchoc M, Sibonga JD, Clarke BL, Hay JE. Bone histomorphometric changes after liver transplantation for chronic cholestatic liver disease. J Bone Miner Res. 2003;18:2190-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Hodgson SF, Dickson ER, Eastell R, Eriksen EF, Bryant SC, Riggs BL. Rates of cancellous bone remodeling and turnover in osteopenia associated with primary biliary cirrhosis. Bone. 1993;14:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | McCaughan GW, Feller RB. Osteoporosis in chronic liver disease: pathogenesis, risk factors, and management. Dig Dis. 1994;12:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Moschen AR, Kaser A, Stadlmann S, Millonig G, Kaser S, Mühllechner P, Habior A, Graziadei I, Vogel W, Tilg H. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J Hepatol. 2005;43:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom. 2013;16:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Phillips JR, Angulo P, Petterson T, Lindor KD. Fat-soluble vitamin levels in patients with primary biliary cirrhosis. Am J Gastroenterol. 2001;96:2745-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Nussler AK, Wildemann B, Freude T, Litzka C, Soldo P, Friess H, Hammad S, Hengstler JG, Braun KF, Trak-Smayra V, Godoy P, Ehnert S. Chronic CCl4 intoxication causes liver and bone damage similar to the human pathology of hepatic osteodystrophy: a mouse model to analyse the liver-bone axis. Arch Toxicol. 2014;88:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Hochrath K, Ehnert S, Ackert-Bicknell CL, Lau Y, Schmid A, Krawczyk M, Hengstler JG, Dunn J, Hiththetiya K, Rathkolb B, Micklich K, Hans W, Fuchs H, Gailus-Durner V, Wolf E, de Angelis MH, Dooley S, Paigen B, Wildemann B, Lammert F, Nüssler AK. Modeling hepatic osteodystrophy in Abcb4 deficient mice. Bone. 2013;55:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Jamil Z, Arif S, Khan A, Durrani AA, Yaqoob N. Vitamin D Deficiency and Its Relationship with Child-Pugh Class in Patients with Chronic Liver Disease. J Clin Transl Hepatol. 2018;6:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Zhao XY, Li J, Wang JH, Habib S, Wei W, Sun SJ, Strobel HW, Jia JD. Vitamin D serum level is associated with Child-Pugh score and metabolic enzyme imbalances, but not viral load in chronic hepatitis B patients. Medicine (Baltimore). 2016;95:e3926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 44. | Iruzubieta P, Terán Á, Crespo J, Fábrega E. Vitamin D deficiency in chronic liver disease. World J Hepatol. 2014;6:901-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 45. | Santos LA, Romeiro FG. Diagnosis and Management of Cirrhosis-Related Osteoporosis. Biomed Res Int. 2016;2016:1423462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Diamond T, Stiel D, Posen S. Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or other factors? Ann Intern Med. 1989;110:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Foresta C, Schipilliti M, Ciarleglio FA, Lenzi A, D'Amico D. Male hypogonadism in cirrhosis and after liver transplantation. J Endocrinol Invest. 2008;31:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Springer JE, Cole DE, Rubin LA, Cauch-Dudek K, Harewood L, Evrovski J, Peltekova VD, Heathcote EJ. Vitamin D-receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology. 2000;118:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Parés A, Guañabens N, Alvarez L, De Osaba MJ, Oriola J, Pons F, Caballería L, Monegal A, Salvador G, Jo J, Peris P, Rivera F, Ballesta AM, Rodés J. Collagen type Ialpha1 and vitamin D receptor gene polymorphisms and bone mass in primary biliary cirrhosis. Hepatology. 2001;33:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Lakatos PL, Bajnok E, Tornai I, Folhoffer A, Horvath A, Lakatos P, Habior A, Szalay F. Insulin-like growth factor I gene microsatellite repeat, collagen type Ialpha1 gene Sp1 polymorphism, and bone disease in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2004;16:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Tang R, Wei Y, Li Z, Chen H, Miao Q, Bian Z, Zhang H, Wang Q, Wang Z, Lian M, Yang F, Jiang X, Yang Y, Li E, Seldin MF, Gershwin ME, Liao W, Shi Y, Ma X. A Common Variant in CLDN14 is Associated with Primary Biliary Cirrhosis and Bone Mineral Density. Sci Rep. 2016;6:19877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Zhao J, Lei H, Wang T, Xiong X. Liver-bone crosstalk in non-alcoholic fatty liver disease: Clinical implications and underlying pathophysiology. Front Endocrinol (Lausanne). 2023;14:1161402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 53. | Soylu AR, Tuglu C, Arikan E, Yetisyigit T, Kunduracılar H, Koker IH, Unsal G, Tezel AH, Umit H, Berkarda S. The role of serum cytokines in the pathogenesis of hepatic osteodystrophy in male cirrhotic patients. Gastroenterol Res Pract. 2012;2012:425079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Sampson HW. Alcohol, osteoporosis, and bone regulating hormones. Alcohol Clin Exp Res. 1997;21:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Maran A, Zhang M, Spelsberg TC, Turner RT. The dose-response effects of ethanol on the human fetal osteoblastic cell line. J Bone Miner Res. 2001;16:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Santori C, Ceccanti M, Diacinti D, Attilia ML, Toppo L, D'Erasmo E, Romagnoli E, Mascia ML, Cipriani C, Prastaro A, Carnevale V, Minisola S. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Yamasaki K, Hagiwara H. Excess iron inhibits osteoblast metabolism. Toxicol Lett. 2009;191:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood. 2010;116:2582-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 59. | Chen X, Li M, Yan J, Liu T, Pan G, Yang H, Pei M, He F. Alcohol Induces Cellular Senescence and Impairs Osteogenic Potential in Bone Marrow-Derived Mesenchymal Stem Cells. Alcohol Alcohol. 2017;52:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Pan XY, Wang L, You HM, Cheng M, Yang Y, Huang C, Li J. Alternative activation of macrophages by prostacyclin synthase ameliorates alcohol induced liver injury. Lab Invest. 2021;101:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Röjdmark S, Brismar K. Decreased IGF-I bioavailability after ethanol abuse in alcoholics: partial restitution after short-term abstinence. J Endocrinol Invest. 2001;24:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 63. | Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A, Singer MV, Nakchbandi IA. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res. 2008;23:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int J Mol Sci. 2022;23:10705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 65. | Schmidt T, Schwinge D, Rolvien T, Jeschke A, Schmidt C, Neven M, Butscheidt S, Kriz M, Kunzmann L, Mussawy H, Hubert J, Hawellek T, Rüther W, Oheim R, Barvencik F, Lohse AW, Schramm C, Schinke T, Amling M. Th17 cell frequency is associated with low bone mass in primary sclerosing cholangitis. J Hepatol. 2019;70:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Zaidi M, Yuen T, Iqbal J. Reverse cholesterol transport and hepatic osteodystrophy. Cell Metab. 2022;34:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Yao Y, Miao X, Zhu D, Li D, Zhang Y, Song C, Liu K. Insulin-like growth factor-1 and non-alcoholic fatty liver disease: a systemic review and meta-analysis. Endocrine. 2019;65:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Filip R, Radzki RP, Bieńko M. Novel insights into the relationship between nonalcoholic fatty liver disease and osteoporosis. Clin Interv Aging. 2018;13:1879-1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Gonzalez-Calvin JL, Gallego-Rojo F, Fernandez-Perez R, Casado-Caballero F, Ruiz-Escolano E, Olivares EG. Osteoporosis, mineral metabolism, and serum soluble tumor necrosis factor receptor p55 in viral cirrhosis. J Clin Endocrinol Metab. 2004;89:4325-4330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Byrne DD, Newcomb CW, Carbonari DM, Nezamzadeh MS, Leidl KB, Herlim M, Yang YX, Hennessy S, Kostman JR, Leonard MB, Localio AR, Lo Re V 3rd. Risk of hip fracture associated with untreated and treated chronic hepatitis B virus infection. J Hepatol. 2014;61:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 72. | Dalla Grana E, Rigo F, Lanzafame M, Lattuada E, Suardi S, Mottes M, Valenti MT, Dalle Carbonare L. Relationship Between Vertebral Fractures, Bone Mineral Density, and Osteometabolic Profile in HIV and Hepatitis B and C-Infected Patients Treated With ART. Front Endocrinol (Lausanne). 2019;10:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Bozkaya G, Nart A, Uslu A, Onman T, Aykas A, Doğan M, Karaca B. Impact of calcineurin inhibitors on bone metabolism in primary kidney transplant patients. Transplant Proc. 2008;40:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Lee J, Kim JH, Kim K, Jin HM, Lee KB, Chung DJ, Kim N. Ribavirin enhances osteoclast formation through osteoblasts via up-regulation of TRANCE/RANKL. Mol Cell Biochem. 2007;296:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Solís-Herruzo JA, Castellano G, Fernández I, Muñoz R, Hawkins F. Decreased bone mineral density after therapy with alpha interferon in combination with ribavirin for chronic hepatitis C. J Hepatol. 2000;33:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 485] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 77. | Guañabens N, Monegal A, Muxi A, Martinez-Ferrer A, Reyes R, Caballería J, Del Río L, Peris P, Pons F, Parés A. Patients with cirrhosis and ascites have false values of bone density: implications for the diagnosis of osteoporosis. Osteoporos Int. 2012;23:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Leslie WD, Bernstein CN, Leboff MS; American Gastroenterological Association Clinical Practice Commitee. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 79. | Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 80. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (2)] |
| 81. | Jeong HM, Kim DJ. Bone Diseases in Patients with Chronic Liver Disease. Int J Mol Sci. 2019;20:4270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 82. | Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 83. | Dasarathy J, Varghese R, Feldman A, Khiyami A, McCullough AJ, Dasarathy S. Patients with Nonalcoholic Fatty Liver Disease Have a Low Response Rate to Vitamin D Supplementation. J Nutr. 2017;147:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Stokes CS, Volmer DA, Grünhage F, Lammert F. Vitamin D in chronic liver disease. Liver Int. 2013;33:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 85. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1203] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 86. | Ravaioli F, Pivetti A, Di Marco L, Chrysanthi C, Frassanito G, Pambianco M, Sicuro C, Gualandi N, Guasconi T, Pecchini M, Colecchia A. Role of Vitamin D in Liver Disease and Complications of Advanced Chronic Liver Disease. Int J Mol Sci. 2022;23:9016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Guañabens N, Cerdá D, Monegal A, Pons F, Caballería L, Peris P, Parés A. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138:2348-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Rudic JS, Giljaca V, Krstic MN, Bjelakovic G, Gluud C. Bisphosphonates for osteoporosis in primary biliary cirrhosis. Cochrane Database Syst Rev. 2011;CD009144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Guañabens N, Monegal A, Cerdá D, Muxí Á, Gifre L, Peris P, Parés A. Randomized trial comparing monthly ibandronate and weekly alendronate for osteoporosis in patients with primary biliary cirrhosis. Hepatology. 2013;58:2070-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Raj H, Kamalanathan S, Sahoo J, Mohan P, Nagarajan K, Reddy SVB, Durgia H, Palui R. Effect of Zoledronic Acid in Hepatic Osteodystrophy: A Double-Blinded Placebo-Controlled Trial. Indian J Endocrinol Metab. 2023;27:552-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Levy C, Harnois DM, Angulo P, Jorgensen R, Lindor KD. Raloxifene improves bone mass in osteopenic women with primary biliary cirrhosis: results of a pilot study. Liver Int. 2005;25:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Saeki C, Saito M, Oikawa T, Nakano M, Torisu Y, Saruta M, Tsubota A. Effects of denosumab treatment in chronic liver disease patients with osteoporosis. World J Gastroenterol. 2020;26:4960-4971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 93. | Dresner-Pollak R, Gabet Y, Steimatzky A, Hamdani G, Bab I, Ackerman Z, Weinreb M. Human parathyroid hormone 1-34 prevents bone loss in experimental biliary cirrhosis in rats. Gastroenterology. 2008;134:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Boone RH, Cheung AM, Girlan LM, Heathcote EJ. Osteoporosis in primary biliary cirrhosis: a randomized trial of the efficacy and feasibility of estrogen/progestin. Dig Dis Sci. 2006;51:1103-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Ormarsdóttir S, Mallmin H, Naessén T, Petrén-Mallmin M, Broomé U, Hultcrantz R, Lööf L. An open, randomized, controlled study of transdermal hormone replacement therapy on the rate of bone loss in primary biliary cirrhosis. J Intern Med. 2004;256:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Barbu EC, Chițu-Tișu CE, Lazăr M, Olariu C, Bojincă M, Ionescu RA, Ion DA, Bădărău IA. Hepatic Osteodystrophy: A Global (Re)View of the Problem. Acta Clin Croat. 2017;56:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |