Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.108533
Revised: June 16, 2025
Accepted: July 24, 2025
Published online: August 27, 2025
Processing time: 132 Days and 19.9 Hours
In randomized controlled trials (RCTs), the placebo arm has often been ignored as the attention tends to be focused on the treatment arm. We undertook a meta-analysis based on the data from the placebo arm in RCTs of hepatocellular carci
To systematically evaluate the response rates, survival status and AEs in the placebo arms of RCTs for HCC.
A systematic search was performed on PubMed, Ovid MEDLINE, Embase and Cochrane Library to identify relevant trials evaluating the efficacy of drugs for the treatment of HCC, published until December 31, 2023. Statistical analysis was performed using R statistical software (version 4.3.2).
A total of 18 RCTs, involving 2390 patients, met the criteria for inclusion in the meta-analysis. The pooled overall disease control rate and objective response rate in the placebo group were 38% [95% confidence interval (CI): 33%-42%] and 1% (95%CI: 1%-2%), respectively. Overall survival and progression-free survival in the placebo group were 7.9 months (95%CI: 7.6-8.31 months) and 1.9 months (95%CI: 1.6-2.1 months), respectively. The incidence of grade 3 or 4 AEs was 37% (95%CI: 30%-43%). Additionally, the incidence of interruptions or dose reductions due to AEs was 20% (95%CI: 13%-27%), while the incidence of treatment discontinuation due to AEs was 9% (95%CI: 6%-12%).
Over one-third of advanced HCC patients exhibit therapy-free disease control, with placebo-arm AEs observed. These findings guide single-arm trials design and enhance patient acceptance of anticancer therapies.
Core Tip: While the therapeutic arm dominates hepatocellular carcinoma clinical trials, the placebo comparator serves as a critical but underutilized window into tumor biology. The natural course of hepatocellular carcinoma under placebo conditions remains poorly quantified, masking potential spontaneous stabilization mechanisms. Furthermore, placebo-associated adverse events in randomized controlled trials remain poorly characterized. Our meta-analysis addresses these gaps through systematically evaluated response rates, survival status and adverse events across placebo arms of hepatocellular carcinoma randomized controlled trials.
- Citation: Chen WY, Chen Q, Wang CC, Zhang CY, Chen SK, Meng ZQ, Han P, Dong S, Chen QW. Response rates, survival status and adverse events of placebo in randomized control trials for hepatocellular carcinoma: A meta-analysis. World J Hepatol 2025; 17(8): 108533
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/108533.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.108533
Primary liver cancer was the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide in 2022[1], with China accounting for 45.3% of new cases worldwide[2]. China bears a substantial burden of hepatocellular carcinoma (HCC) due to high prevalence of chronic hepatitis B infection, making it a significant public health concern nationwide[3]. Over the past decade, several clinical trials on new drugs for HCC have been conducted, including several single-arm trials (SATs) that have received approval from the United States Food and Drug Administration (FDA).
In the study of HCC, people are always concerned about the therapeutic effect of anticancer drugs, but ignore the results of the placebo arm in randomized controlled trials (RCTs). Is tumor regression solely attributable to the effect of medication? Or rather, can placebos also lead to stabilization or even regression of HCC? Previous studies have shown that solid tumors may also occasionally regress even without treatment, either spontaneously or due to placebo effects[4,5].
Therefore, we performed this analysis to reveal the response rates, survival status and adverse events (AEs) of placebo in RCTs of anticancer medicine in HCC. Spontaneous regression, stable disease and AEs may represent natural process during the disease course rather than resulting from drugs. Additionally, data on disease control rate (DCR), objective response rate (ORR), overall survival (OS), progression-free survival (PFS) and AEs in the placebo-controlled group can provide insights into future treatment strategies and serve as references for designing clinical trials, especially for SATs seeking accelerated approval for market launch.
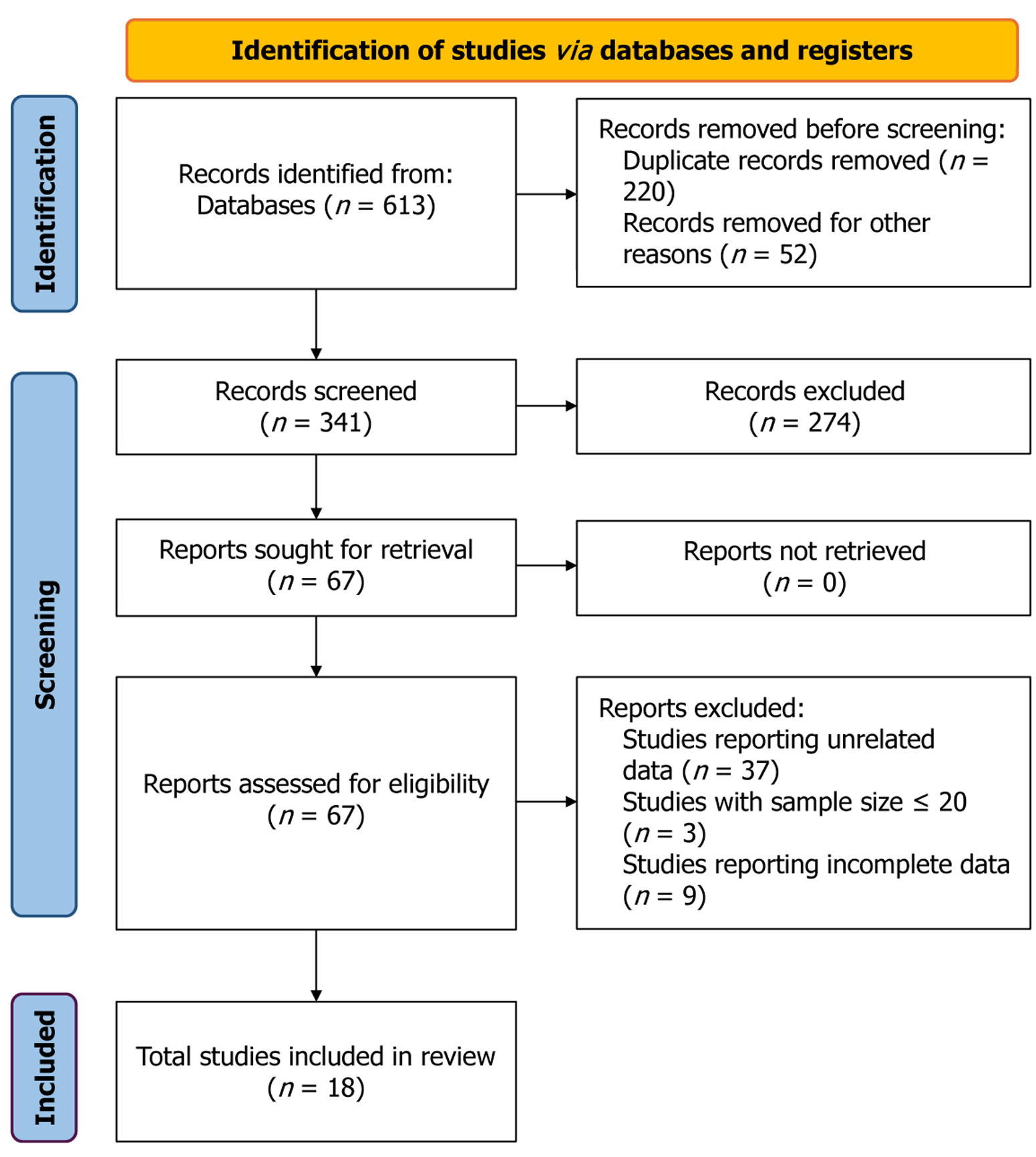
This systematic review and meta-analysis adhered to the guidelines outlined in PRISMA[6] (Supplementary material 1) and AMSTAR[7] (Supplementary material 2). We systematically searched PubMed, Ovid MEDLINE, Embase and Cochrane Library to identify all RCTs evaluating anticancer drugs in advanced HCC patients, with publication cutoff date of December 31, 2023 and language restriction to English. The detailed search strategies for each database are presented in Supplementary material 3. Eventually 18 studies were included (Figure 1). The inclusion criteria were as follows: (1) Assessed advanced HCC in adult only; (2) Exclusion of (neo)adjuvant therapy; (3) Exclusion of local therapy such as surgery, radiation or transarterial chemoembolization; (4) RCTs with a sample size > 20 participants in each arm; (5) Inclusion of a placebo arm either placebo monotherapy or placebo combined with best supportive care without anti
After independently screening titles and abstracts by the two authors, the full text of potentially relevant studies was downloaded, and relevant data were independently extracted from the published reports by the same authors. Subsequently, this process was validated separately by two other authors, and any discrepancy was resolved through mutual negotiation.
We collected the following relevant trial characteristics: Study name, year of publication, lines of treatment, treatment type, number of patients randomized into each trial arm, tumor response [including DCR, ORR, complete response (CR), partial response (PR) and stable disease (SD)], OS and PFS, and AEs. ORR was defined as the sum of CR (complete disappearance of lesions) and PR (reduction in the sum of diameters of target lesions by ≥ 30%). DCR was defined as the sum of CR, PR and SD (< 30% reduction and < 20% increase in the sum of diameters of target lesions). This study did not involve any individual-level data; therefore, ethical approval was not applicable.
The statistical analysis was performed using R statistical software (version 4.3.2). The meta-analysis of rates was performed using a random effects model based on the inverse-variance approach to accommodate clinical heterogeneity. Rates following a normal distribution were directly calculated using the PRAW method, and studies that did not conform to a normal distribution and reported zero patient values used the Freeman–Tukey double arcsine transformation[10]. Confidence intervals (CIs) for each study were determined using the Clopper–Pearson method[11]. Statistical heterogeneity among the included studies was assessed using the DerSimonian–Laird method estimate for tau-squared (τ2, which quantifies the variance of effect sizes for the data using the Cochrane’s Q statistic[12]). CIs for τ and τ2 were derived using the Jackson method[13]. Statistical heterogeneity was considered statistically significant if P < 0.10, and quantified using the I2 statistic, where I2 < 25% indicated no heterogeneity, I2 = 25%-50% represented moderate heterogeneity, and I2 > 50% indicated considerable heterogeneity[14]. The median meta-analysis was performed using the Weighted Median of Medians to estimate the median. CIs for each study were determined using the sign test[15].
Forest plots for DCR, ORR, CR, PR, SD, median OS and median PFS were generated to assess the efficacy in the placebo arm in HCC RCTs. Forest plots of AEs were generated to assess the incidence of AEs in the placebo arm in HCC RCTs. Prespecified subgroup analyses were conducted to explore potential sources of statistical heterogeneity. Potential publication bias was assessed by Egger test, with statistical significance set at P < 0.05[16].
Out of the initially identified 1287 studies, 18 RCTs were found to meet the inclusion criteria, involving 2390 patients in the placebo arm (Figure 1). Targeted therapy was the predominant treatment modality (83%). Only three trials (17%) were performed in naive patients, while the remaining trials focused on second-line or later therapies. Approximately 22% of the trials were classified as Phase 2, with the remainder being Phase 3. The placebo arm comprised 2390 patients, whose tumor response rates (DCR and ORR) and median OS were evaluable. However, the number of evaluable patients for median PFS and AEs varied, with some missing. The details of the risk of bias assessment can be obtained for all studies in Supplementary material 4.
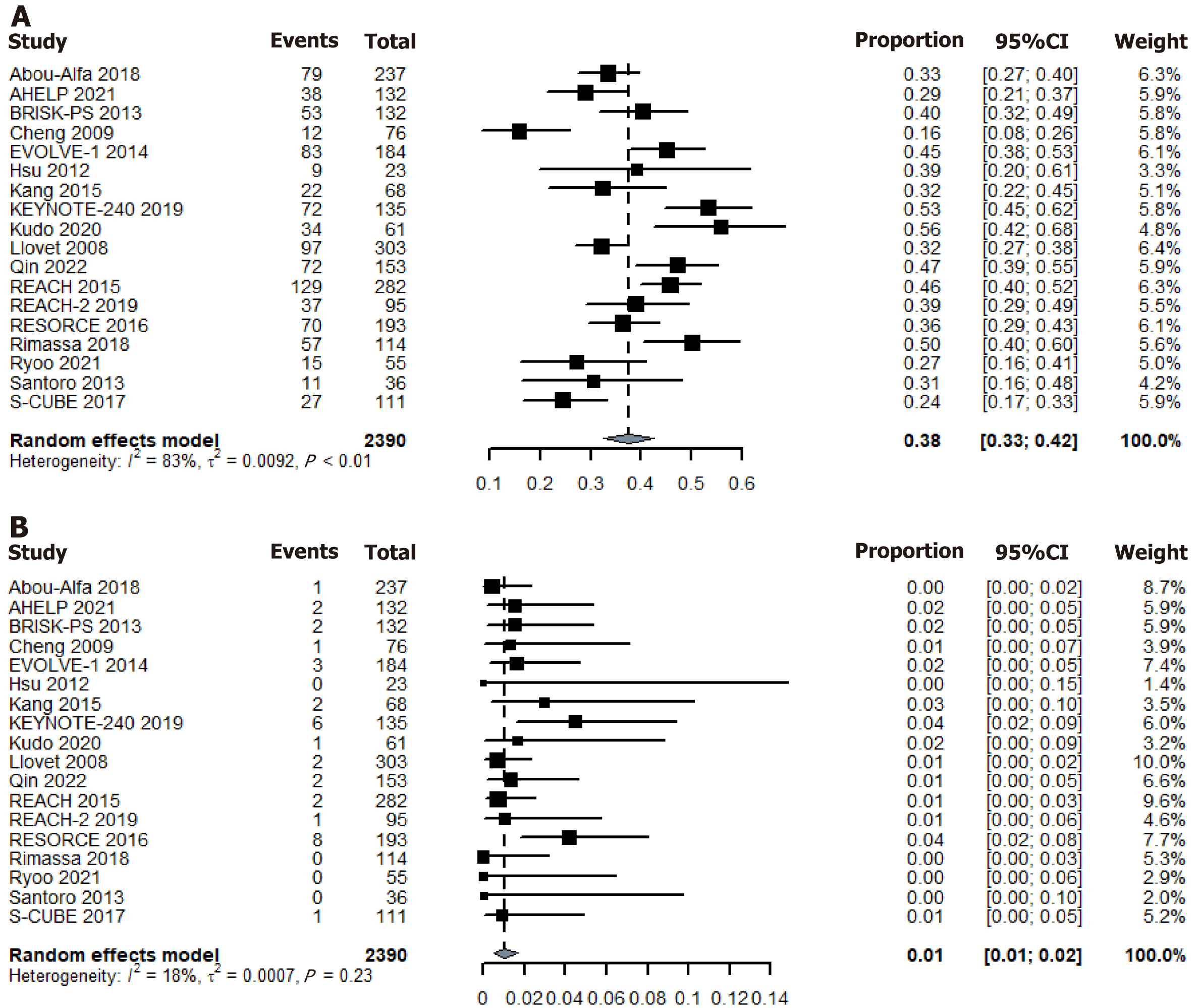
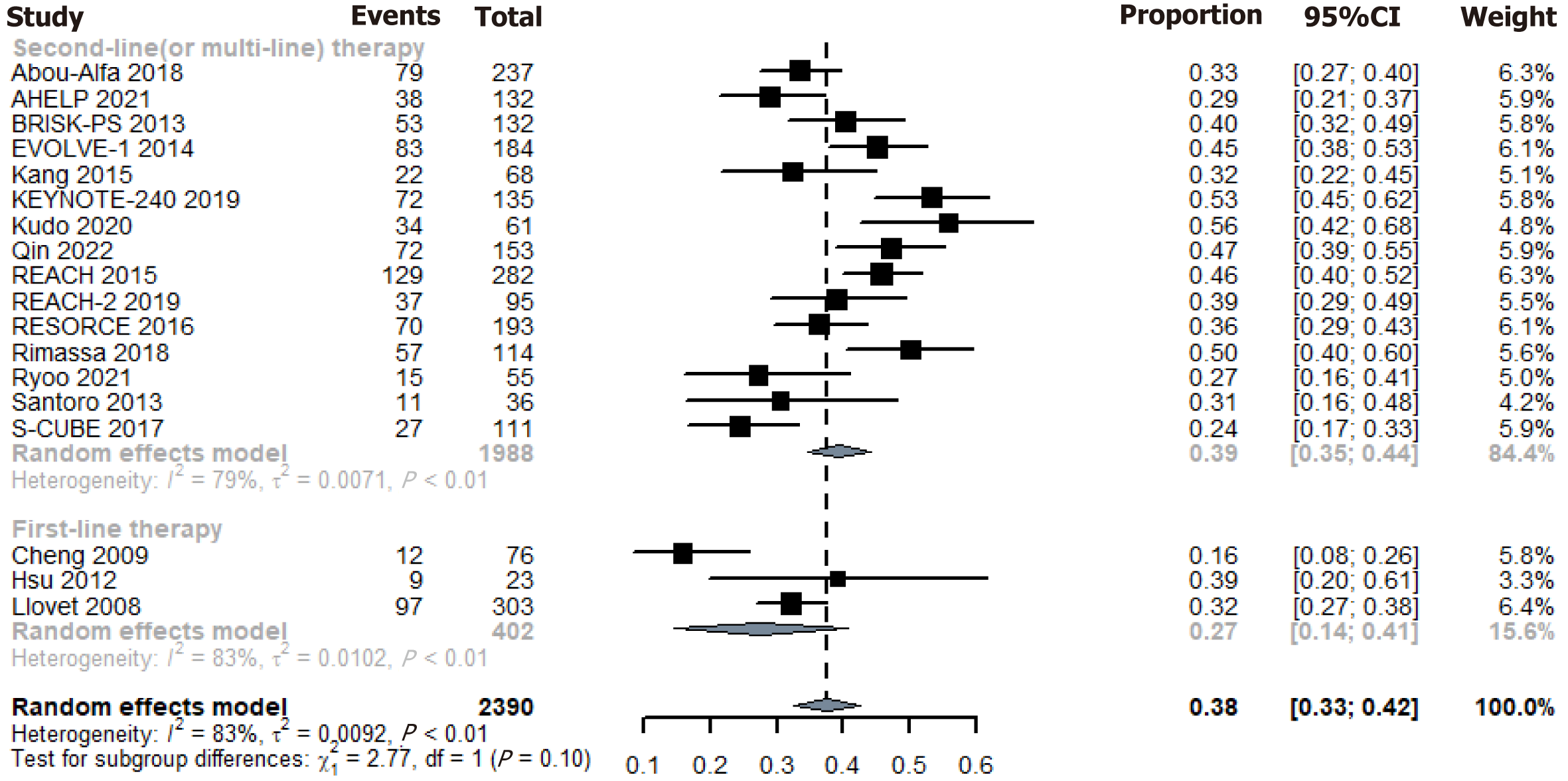
In the RCT placebo arm, 917 of the evaluable patients attained disease control, resulting in an overall DCR of 38.4%. Using the random-effects model, the pooled DCR for placebo was 38% (95%CI: 33%-42%) (Figure 2). Additionally, 34 patients achieved an objective response, leading to a pooled ORR of 1.2%. Using the random-effects model, the pooled ORR for the placebo was 1% (95%CI: 1%-2%) (Figure 2). Please refer to the Supplementary material 5 for other tumor response data.
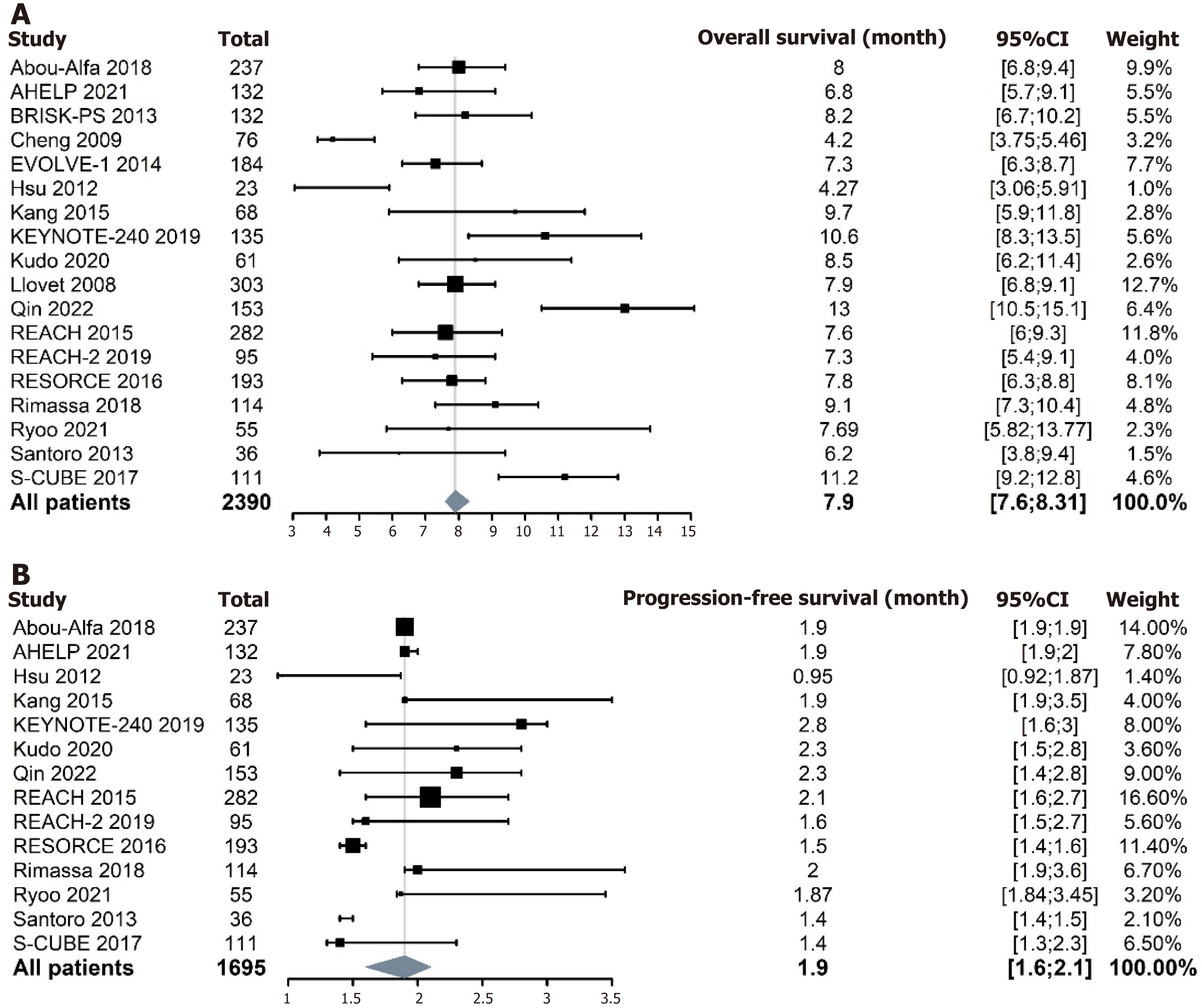
Among the evaluable patients in the RCT placebo arm, the pooled median OS was 7.9 months (95%CI: 7.6-8.31 months) (Figure 3). Some studies did not report median PFS data. The pooled median PFS among evaluable patients in the RCT placebo arm was 1.9 months (95%CI: 1.6-2.1 months) (Figure 3). Given that median OS and PFS were continuous variables analyzed using the weighted median of medians, this analytical approach did not generate conventional heterogeneity metrics (e.g., I2 statistics or Cochran's Q).
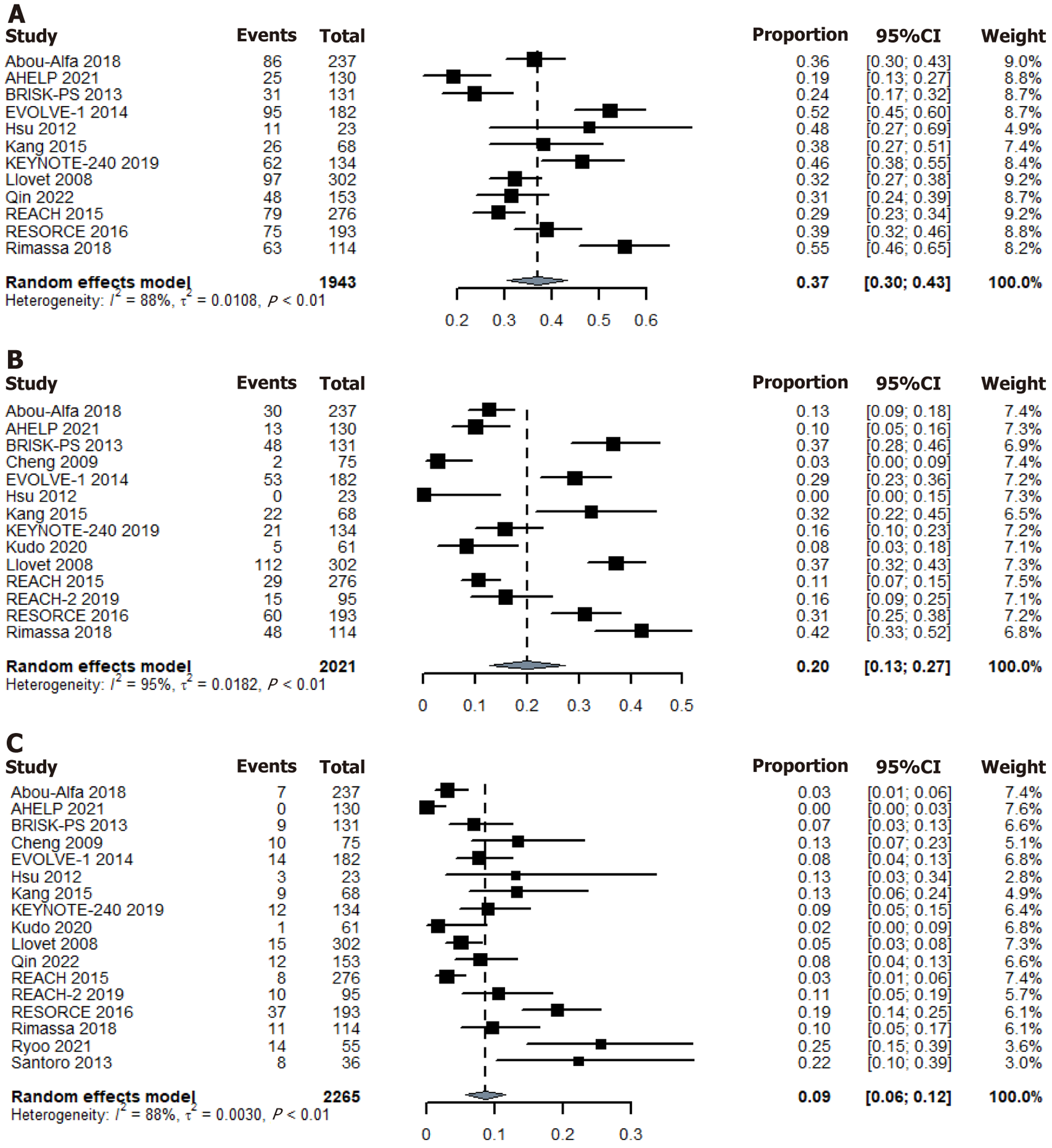
AE data were not detailed in some studies. Among the evaluable patients in the RCT placebo arm, AEs were reported in 1735 patients. Using a random-effects model, the pooled incidence of Grade 3 or 4 AEs was 37% (95%CI: 30%-43%) (Figure 4). A total of 458 patients experienced interruptions or dose reductions due to AEs, with a pooled incidence of 20% (95%CI: 13%-27%) (Figure 4). AEs requiring treatment discontinuation occurred in 180 patients, with a pooled incidence of 9% (95%CI: 6%-12%) (Figure 4). Please refer to the Supplementary material 5 for other AE data.
Significant heterogeneity was observed in placebo DCR (I2 = 83%, Q = 100.01, P < 0.0001); therefore, subgroup analyses were conducted based on the treatment lines (Figure 5). These analyses revealed a significant difference in placebo DCR between the first-line treatment (27%) and the second-line or later therapies (39%) (between groups χ2 = 2.77, P = 0.096). Baseline functional status heterogeneity may further contribute to outcome variability. Given the unavailability of patient-level data across included studies, we conducted surrogate subgroup analyses by stratifying trials into categories based on aggregate functional status metrics: (1) Favorable vs unfavorable prognostic profiles; and (2) High-burden vs low-burden cohorts. No significant heterogeneity in placebo DCR was observed across subgroups stratified by Child–Pugh classification, Barcelona Clinic Liver Cancer staging, Eastern Cooperative Oncology Group performance status, macrovascular invasion status, extrahepatic disease, or HBV infection (Supplementary material 5). Consistently, subgroup analyses of ORR and AEs also demonstrated no significant variations.
Potential publication bias was estimated with Egger's test. All studies yielded a score of P = 0.82, indicating a symmetric distribution across all studies and suggesting no publication bias. However, the funnel plot indicates that some of the studies were in significant regions, indicating possible heterogeneity. Please refer to the Supplementary material 5 for other data.
In cancer RCTs, oncologists and scientists focus more attention on the treatment arm, whereas the placebo arm is often ignored as a "stepping stone" for the therapeutic drugs. In contrast to previous studies, our study shifted this focus to the placebo arm, which has been neglected in recent decades. This systematic review and meta-analysis included 18 RCTs involving 2390 HCC patients. It marks the first study dedicated to exploring the survival status and AEs of placebo in RCTs for HCC.
This meta-analysis revealed that 38% of patients who were not treated with anticancer drugs achieved disease control, with median OS and median PFS of 7.9 and 1.9 months, respectively. Whether a high DCR equates to clinical benefit warrants careful consideration. Implementation of high-quality patient-reported outcome studies may provide critical insights by evaluating treatment benefits through the dimension of patient quality of life[17]. The median OS of 7.9 months in the placebo arm is comparable to the 6.8-months median OS reported by Cabibbo et al[18] in their natural course study of HCC. The observed difference reflects how stringent eligibility criteria in modern clinical trials (e.g., preserved liver function) limit the generalization of placebo arm survival data to unselected real-world cohorts. Nevertheless, these data remain clinically relevant for patients with adequate hepatic reserve, particularly those classified as Child–Pugh class A. Subgroup analysis indicated a higher DCR with second-line or later therapies compared to first-line treatment. This may stem from only three small-sample first-line treatment trials being included in our study. There is a possibility that some patients are sensitive to anticancer therapies, leading to intolerance rather than disease progression with first-line treatments. The observed efficacy in RCTs involving second-line or later therapies may partially stem from prior therapeutic interventions. Discrepancies may also stem from heterogeneous baseline functional status across study cohorts. However, the lack of individual patient data in source publications precluded stratified analysis to verify this hypothesis.
In our study, a large portion of patients exhibited stable disease during the follow-up, leading to a high DCR. Fur
This finding also suggests that even among patients not receiving any antitumor therapy (including chemotherapy, targeted agents, or immunotherapy) there may be a 1% chance of PR. This cannot be solely attributable to an incidental anecdote, as studies conducted by Bruix et al[20] and Finn et al[21] have reported ORRs of up to 4% in the placebo arm, which is higher than our study. Spontaneous tumor regression has been documented in various types of tumors as well. For example, approximately 20% of patients with desmoid tumors have experienced spontaneous remission[4]. Addi
Our study demonstrates that AEs can occur in the placebo arm, as mentioned in some previous studies[22-24]. Fatigue, abdominal pain and decreased appetite were the most common AEs reported in the placebo arm, primarily consisting of subjective symptoms. In contrast, the treatment group also experienced rashes and objective abnormalities such as increased alanine aminotransferase or aspartate aminotransferase and hypertension. This indicates that AEs manifest not only in advanced HCC patients undergoing antitumor therapies, but also in those abstaining from conventional anti
In advanced cancer treatment, the combined approach of conventional anticancer treatments and complementary and alternative medicine (CAM) is commonly applied[27,28]. Certainly, the concern over potential side effects of traditional treatments may prompt some patients to exclusively use CAM in search of a more comfortable treatment experience, but this approach often results in a worse prognosis. The study by Greenlee et al[29] suggests that patients using dietary supplements such as herbs were more likely to refuse chemotherapy. Also, a retrospective observational study suggests that patients receiving CAM are more likely to refuse conventional anticancer therapies and have a higher risk of death[30]. Our findings can raise patients’ consciousness that both conventional anticancer therapies and placebo treatment can incur AEs, and they should not solely avoid conventional anticancer treatment due to concerns about their potential side effects. In addition, it can also remind patients that the remission of cancer is not necessarily the result of anticancer drugs, but also the natural course of the disease. Clinical responses observed with CAM monotherapy may reflect nonspecific effects rather than direct antitumor activity. Current evidence does not support substituting CAM for conventional anticancer therapies.
In recent years, a few anti-HCC drugs have received accelerated approval from the FDA through SATs[31,32], primarily relying on ORR; a reasonable predictor of survival improvement[33]. However, whether a drug can be approved for market access is not only determined by ORR, but also by factors such as OS, PFS and whether the AEs are within acceptable ranges. Simultaneously, SATs may be prone to biases due to various factors, such as lack of randomization, potential confounding variables, or the absence of a control group for comparison. Here, we provide various indicators in the placebo-controlled group, including OS, as well as data on DCR, ORR and AEs, as reference for future SAT design. The rapidly changing drug development landscapes and the high failure rates in Phase 3 trials both underscore the need for more efficient and robust Phase 2 trial design. For trials that fail to demonstrate outcomes superior to those achieved in the placebo arm, proceeding to Phase 3 should be cautious, thereby avoiding unnecessary effort in labor, time cost and financial resources. The DCR, ORR, OS and PFS in the placebo-controlled group can also provide insights into the natural course of advanced HCC patients with adequate hepatic reserve, which is obscure to outline in the real-world data, leading to better understanding and providing references for designing future treatment strategies. By understanding the survival and efficacy data in the placebo arm, improvements can be made in sample size calculation, endpoint selection, follow-up time and other aspects of clinical trials, thus ensuring that the studies have sufficient statistical power and scientific reliability.
Possible publication bias and heterogeneity among included studies were potential limitations of our research. Publication bias towards negative studies may have affected our results by excluding studies with higher placebo response rates or longer survival durations. The lack of available supplemental data in some trials may limit our interpretation of the results. Therefore, it is uncertain whether all AEs are related to the placebo, and the correlation between clinicopathological variables such as age, gender, stage, Child–Pugh score, and indicators including DCR, ORR, OS, PFS and AEs could not be derived. Additionally, the inclusion of a relatively high proportion of second-line or later therapies in studies may lead to residual effects or AEs from previous treatments that can potentially influence the assessment of efficacy and AEs in subsequent research.
The findings of this meta-analysis suggest that 38% of patients with advanced HCC can experience a period of disease control without conventional anticancer treatments. In RCTs, AEs may also occur in the placebo group. These findings can serve as a valuable reference for future SATs, while also preventing patients from being misled by erroneous information, thereby contributing to a higher acceptance of conventional anticancer treatments.
Special thanks to Jin-Ming Yu for statistical support. Lastly, we appreciate all participating colleagues for making this research possible.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8165] [Article Influence: 8165.0] [Reference Citation Analysis (2)] |
| 2. | Jiang L, Zhao N, Xu M, Pei J, Lin Y, Yao Q, Hu M, Zhu C. Incidence trends of primary liver cancer in different geographical regions of China from 1978 to 2012 and projections to 2032: An age-period-cohort analysis. Int J Cancer. 2024;154:465-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Chen T, Zhang Y, Liu J, Rao Z, Wang M, Shen H, Zeng S. Trends in liver cancer mortality in China from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13:e074348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Gounder MM, Mahoney MR, Van Tine BA, Ravi V, Attia S, Deshpande HA, Gupta AA, Milhem MM, Conry RM, Movva S, Pishvaian MJ, Riedel RF, Sabagh T, Tap WD, Horvat N, Basch E, Schwartz LH, Maki RG, Agaram NP, Lefkowitz RA, Mazaheri Y, Yamashita R, Wright JJ, Dueck AC, Schwartz GK. Sorafenib for Advanced and Refractory Desmoid Tumors. N Engl J Med. 2018;379:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 311] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 5. | Saad F, Fizazi K, Jinga V, Efstathiou E, Fong PC, Hart LL, Jones R, McDermott R, Wirth M, Suzuki K, MacLean DB, Wang L, Akaza H, Nelson J, Scher HI, Dreicer R, Webb IJ, de Wit R; ELM-PC 4 investigators. Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): a double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol. 2015;16:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 4614] [Article Influence: 1153.5] [Reference Citation Analysis (0)] |
| 7. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 5704] [Article Influence: 713.0] [Reference Citation Analysis (0)] |
| 8. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13078] [Article Influence: 523.1] [Reference Citation Analysis (0)] |
| 9. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21616] [Article Influence: 1351.0] [Reference Citation Analysis (1)] |
| 10. | Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci Rep. 2020;3:e178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 11. | Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404-413. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2852] [Cited by in RCA: 2870] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 12. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30422] [Article Influence: 780.1] [Reference Citation Analysis (0)] |
| 13. | rdrr.io. metaprop: Meta-analysis of single proportions. Accessed April 11, 2024. Available from: https://rdrr.io/cran/meta/man/metaprop.html. |
| 14. | Ryan R. Heterogeneity and subgroup analyses in Cochrane Consumers and Communication Group reviews: planning the analysis at protocol stage. Accessed April 11, 2024. Available from: https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/heterogeneity_subgroup_analyses_revising_december_1st_2016.pdf. |
| 15. | McGrath S, Zhao X, Ozturk O, Katzenschlager S, Steele R, Benedetti A. metamedian: An R package for meta-analyzing studies reporting medians. Res Synth Methods. 2024;15:332-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 16. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40548] [Article Influence: 1448.1] [Reference Citation Analysis (2)] |
| 17. | Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, Li D, Mulla S, Verret W, Xu DZ, Hernandez S, Ding B, Liu J, Huang C, Lim HY, Cheng AL, Ducreux M. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 18. | Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M, Brancatelli G, Cammà C, Craxì A, Di Marco V. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol. 2012;4:256-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 20. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2714] [Article Influence: 339.3] [Reference Citation Analysis (0)] |
| 21. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1341] [Article Influence: 268.2] [Reference Citation Analysis (0)] |
| 22. | Mitsikostas DD, Chalarakis NG, Mantonakis LI, Delicha EM, Sfikakis PP. Nocebo in fibromyalgia: meta-analysis of placebo-controlled clinical trials and implications for practice. Eur J Neurol. 2012;19:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Chacón MR, Enrico DH, Burton J, Waisberg FD, Videla VM. Incidence of Placebo Adverse Events in Randomized Clinical Trials of Targeted and Immunotherapy Cancer Drugs in the Adjuvant Setting: A Systematic Review and Meta-analysis. JAMA Netw Open. 2018;1:e185617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Llavero-Valero M, Guillén-Grima F, Zafon C, Galofré JC. The placebo effect in thyroid cancer: a meta-analysis. Eur J Endocrinol. 2016;174:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | de la Cruz M, Hui D, Parsons HA, Bruera E. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer. 2010;116:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Mitsikostas DD. Nocebo in headaches: implications for clinical practice and trial design. Curr Neurol Neurosci Rep. 2012;12:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Shankar A, Saini D, Roy S, Bharati SJ, Mishra S, Singh P. Role of Complementary and Alternative Medicine in the Management of Cancer Cachexia. Asia Pac J Oncol Nurs. 2021;8:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Paul M, Davey B, Senf B, Stoll C, Münstedt K, Mücke R, Micke O, Prott FJ, Buentzel J, Hübner J. Patients with advanced cancer and their usage of complementary and alternative medicine. J Cancer Res Clin Oncol. 2013;139:1515-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Greenlee H, Neugut AI, Falci L, Hillyer GC, Buono D, Mandelblatt JS, Roh JM, Ergas IJ, Kwan ML, Lee M, Tsai WY, Shi Z, Lamerato L, Kushi LH, Hershman DL. Association Between Complementary and Alternative Medicine Use and Breast Cancer Chemotherapy Initiation: The Breast Cancer Quality of Care (BQUAL) Study. JAMA Oncol. 2016;2:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Johnson SB, Park HS, Gross CP, Yu JB. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients With Curable Cancers. JAMA Oncol. 2018;4:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 31. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1898] [Article Influence: 271.1] [Reference Citation Analysis (0)] |
| 32. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 970] [Article Influence: 194.0] [Reference Citation Analysis (0)] |
| 33. | United States Food and Drug Administration. Guidance for industry expedited programs for serious conditions drugs and biologics. Accessed April 11, 2024. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-serious-conditions-drugs-and-biologics. |