Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.108182
Revised: May 24, 2025
Accepted: July 23, 2025
Published online: August 27, 2025
Processing time: 142 Days and 10 Hours
To improve understanding of the multifaceted nature of metabolic dysfunction-associated steatotic liver disease (MASLD), the American Association for the Study of Liver Diseases, in collaboration with the European Association for the Study of the Liver and the Latin American Association for the Study of the Liver, proposed a broader and more flexible definition, highlighting the role of underlying metabolic dysfunction. MASLD represents the most common chronic liver disease worldwide; however, the impact of the disease goes beyond its epidemiological aspects. Currently, the impact on patients and healthcare syst
Core Tip: Metabolic dysfunction-associated steatotic liver disease is a complex disease with multifactorial causes. The individual's dietary pattern contributes significantly to the progression of this disease. Epigenetics, modulated by diet, is a key process helps explain the development as well as new therapeutic targets for the disease.
- Citation: Rodriguez S, Dahlem MLF, Rossoni C, Marroni NP, Marroni CA, Fernandes SA. Nutrients as epigenetic modulators in metabolic dysfunction-associated steatotic liver disease. World J Hepatol 2025; 17(8): 108182
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/108182.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.108182
According to global estimates, approximately 30% of the population is affected by metabolic dysfunction-associated steatotic liver disease (MASLD), although this percentage is likely underestimated due to underdiagnosis and underreporting, particularly in adult and juvenile populations[1]. The global prevalence of MASLD varies significantly across regions: 31.2% in North America and Australia, 28.0% in the Asia-Pacific region, 25.1% in Western Europe, 33.1% in Southeast Asia, 29.7% in East Asia, 33.8% in South Asia, 44.4% in Latin America, and 36.5% in the Middle East and North Africa[2]. These data underscore the global burden of MASLD, highlighting the critical need for effective diagnosis and targeted interventions.
The impact of MASLD extends beyond epidemiological statistics. Data from the Global Burden of Disease study reveal an accelerated increase in healthcare and economic costs associated with MASLD in recent decades[3,4]. In 2021, the disability-adjusted life years attributable to MASLD were estimated at 3.67 million (95%CI: 2.90-4.61) globally, more than double the figures reported three decades ago. Of these, men accounted for 51.1% (1.87 million), while women represented 48.9% (1.79 million)[5-8].
Reflecting its complexity, non-alcoholic fatty liver disease (NAFLD) has been reclassified as MASLD. This change not only aims to eliminate the stigmatizing terms "non-alcoholic" and "fatty" but also to capture the broader metabolic dysfunction associated with its pathogenesis[1]. MASLD encompasses a spectrum of hepatic alterations secondary to metabolic imbalances, ranging from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), with varying degrees of fibrosis and an elevated risk of cirrhosis and hepatocellular carcinoma if left untreated[6-8].
MASLD is strongly linked to both hepatic complications—such as cirrhosis, clinically significant portal hypertension and hepatocellular carcinoma—and extrahepatic conditions, including diabetes, obesity, dyslipidemia, chronic kidney disease, and cardiometabolic disorders[9,10]. Consequently, MASLD has become the most prevalent chronic liver disease globally and the leading cause of liver-related morbidity and mortality[6].
Understanding the complex pathophysiology and clinical burden of MASLD is essential for designing effective strategies aimed at identifying high-risk populations and preventing disease progression. This approach also facilitates insights into environmental triggers, as well as genetic and epigenetic factors, which play pivotal roles in the deve
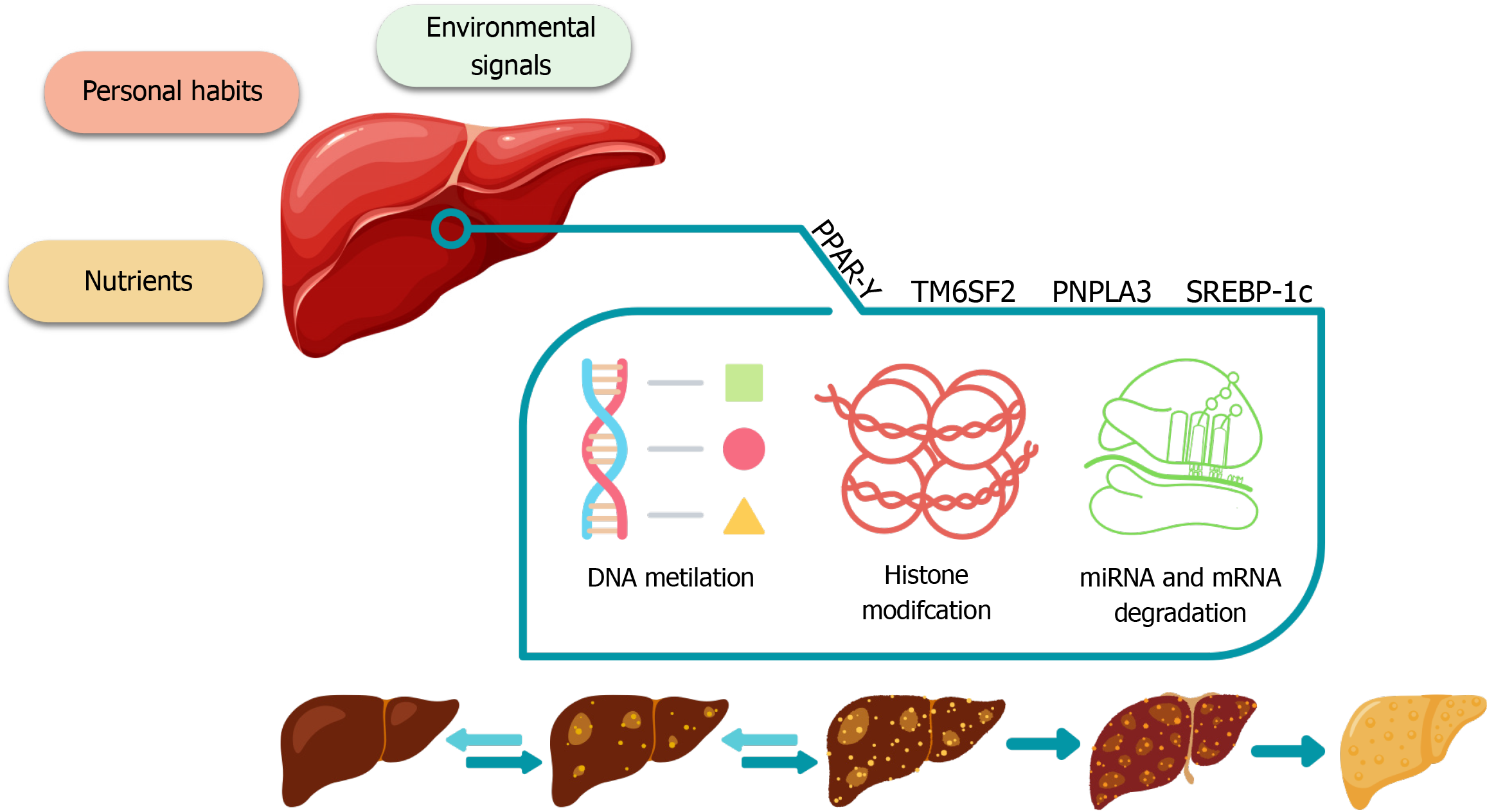
Epigenetics involves altering gene expression in response to environmental stimuli and is strongly influenced by an individual's daily habits, pathologies, aging and sex. These changes in gene expression involve mechanisms such as DNA methylation, histone modifications, and microRNAs, which collectively regulate the activation or silencing of specific genomic regions and influence protein synthesis. Such epigenetic mechanisms operate in an intricate and interconnected manner, forming a regulatory network that shapes gene activity in response to environmental cues—modulating expression without altering the DNA sequence[11].
Epigenetic mechanisms can be activated and modified through the individual's genetic inheritance or by environmental factors, such as diet[12], which is a potent epigenetic modulador in MASLD.
The main epigenetic mechanisms operate on chromatin, histones and non-histone proteins. Histones are responsible for DNA condensation, which directly influences the regulation of protein synthesis. Regions of active expression (euchromatin) are less condensed and thus more accessible to the transcriptional machinery, facilitating gene expression. In contrast, highly condensed regions (heterochromatin) limit accessibility and are transcriptionally silent. These factors can be influenced by histone methylation, which involves adding methyl groups to the tails of these proteins, an epigenetic modification that regulates active and inactive gene expression zones[13,14].
Furthermore, microRNAs can also regulate gene expression. Post-transcriptionally, they can bind to specific regions of mRNA, leading to mRNA degradation or inhibition of mRNA translation, resulting in reduced protein synthesis. Specific microRNAs are associated with MASLD, influencing cell differentiation, apoptosis, and lipid metabolism. Certain microRNAs can be overexpressed or under expressed, impacting the expression of genes related to lipid metabolism and liver inflammation[13-18].
Regardless of the etiology, the progression of chronic liver damage and inflammation to cirrhosis and/or cancer is highly variable between individuals[16]. The disease course and rate of progression are often unpredictable. The degree of progression varies, and the molecular basis for this variability still needs deeper understanding[16]. It is well established that there are numerous non-genetic factors that influence the progression of liver disease[19-21]. The study of epigenetics is, in essence, the investigation of how non-genetic factors act upon the genome to influence gene expression and phenotype[13,16].
As previously mentioned, these epigenetic mechanisms can be activated and modified by environmental stimuli, including an individual's diet. Therefore, epigenetics plays a crucial role in liver diseases, as the liver's normal function critically depends on maintaining the properties of its constituent cells. Epigenetic modifications may be linked to specific pathologies such as cirrhosis or cancer. Impaired liver function results in a loss of homeostasis that prevents constituent cells from maintaining their phenotypic characteristics in the face of changes in the microenvironment[16] (Figure 1).
The most significant ways in which epigenetics plays a role in liver disease are associated with modifications in lipid metabolism and hepatic inflammation. DNA methylation, histone modifications, and microRNA alterations can exacerbate the progression of MASLD[13-16].
The main genes involved in epigenetic changes regarding liver diseases are[13]: (1) PNPLA3 (adiponutrin): The expression of this gene can be influenced by epigenetic modifications, which affect the metabolism of lipids in the liver, with some variants being strongly associated with MASLD; (2) TM6SF2: This gene undergoes epigenetic variations associated with MASLD and is involved in hepatic lipid transport; and (3) SREBP-1c and PPAR-γ: These genes, involved in lipid metabolism, may have their expression influenced by epigenetic modifications, contributing to fat accumulation in the liver in MASLD.
In addition, the dysregulation of SIRT1 plays a crucial role in the progression of MASLD. SIRT1, a NAD+-dependent deacetylase, regulates hepatic lipid metabolism by deacetylating PPARs, particularly PPARα, thereby promoting fatty acid oxidation and reducing hepatic steatosis. A decrease in SIRT1 expression or activity, often observed in conditions like high-fat diets, results in the hypermethylation of PPARα target genes, leading to its inactivation and subsequent lipid accumulation in the liver[22].
Furthermore, persistent activation of JNK1 can induce the degradation of the SIRT1 protein, exacerbating hepatic steatosis. This mechanism involves the phosphorylation of SIRT1 by JNK1, promoting its proteasomal degradation, which disrupts lipid homeostasis and contributes to MASLD progression[23].
Therefore, the interaction between SIRT1, PPARs, and JNK1 constitutes a critical axis in the regulation of hepatic lipid metabolism. The dysfunction of SIRT1, either through direct inactivation or JNK1-mediated degradation, leads to PPAR inactivation and activation of inflammatory pathways, thereby accentuating steatosis and advancing MASLD progression[23,24].
In chronic viral hepatitis B and C, modifications in the host genome can influence the immune response, chronic inflammation, and progression to cirrhosis and hepatocellular carcinoma. In cirrhosis and hepatocellular carcinoma, aberrant DNA methylation and histone modifications occur, contributing to dysregulation of gene expression associated with uncontrolled cell growth and resistance to apoptosis[13-17,25].
In hereditary hemochromatosis, epigenetic changes may affect gene expression in iron homeostasis. In primary biliary cholangitis and primary sclerosing cholangitis, there is involvement in regulating autoimmune responses that influence chronic liver inflammation. In metabolic diseases and type 2 diabetes mellitus, epigenetic modifications are associated with insulin resistance and genes involved in lipid metabolism[13-17,25].
Interestingly, the majority of genes dysregulated in livers of alcoholic steatohepatitis and MASH patients have been reported to be identical[26]. Little is known about the effects of a diet containing high fat and alcohol on the aging process at agenetic level regarding changes in transcriptional and epigenetic profiles[25].
MASLD is highly associated with obesity, diabetes, hypertension, hypercholesterolemia, and cardiovascular disease, and drugs used to treat cardiometabolic conditions are frequently used in MASLD patients. However, most of these drugs were not originally studied in the MASLD population, and these patients could be at risk of altered drug response by identifying changes in the hepatic expression of genes that mediate drug disposition (pharmacogenes) across histological MASLD severity[25]. The research results demonstrate that expression levels of several pharmacogenes are significantly associated with the histological severity of MASLD. Notably, CYP2C19 was severely downregulated – by 46% in MASH, 58% in high NAS, and 43% in severe fibrosis[12]. This supports the development of personalized medicine approaches for drugs sensitive to metabolism by the CYP2C19 enzyme[25].
Epigenetic mechanisms play a crucial role in the development and progression of liver diseases. Understanding these mechanisms offers potential avenues for therapeutic intervention and ongoing research in this field promises to yield novel strategies for preventing and treating hepatic disorders[14,15,25].
Regardless of pharmacological and/or surgical intervention, the individual's dietary pattern must be adjusted according to their specific needs. Indeed, dietary therapy, whether applied alone or in combination with other treatments, has emerged as a pivotal strategy for the preventive management and remission of MASLD. However, for nutritional intervention to be effective and targeted, the pathophysiological mechanisms, epigenetic modifications, and nutrigenomic factors underlying MASLD must be prioritized as the foundation for establishing an appropriate dietary plan. Understanding these molecular and metabolic pathways allows for the development of precision nutrition strategies that address the unique metabolic profiles of individuals, thereby enhancing therapeutic outcomes and reducing disease progression[27,28].
Nutrigenomics represents a promising avenue for understanding the interplay between dietary intake, genetic predisposition, and MASLD progression. This field encompasses both nutrigenetics, which examines how genetic variations affect dietary response, and nutri-epigenetics, which explores how nutrients influence gene expression through mechanisms such as DNA methylation and histone modification[24]. These epigenetic alterations are critical in modulating hepatic lipid metabolism, inflammation, and fibrosis, thereby influencing the overall pathophysiology of MASLD[27].
Genetic polymorphisms, such as PNPLA3 I148M, TM6SF2, and MBOAT7, have been strongly associated with increased hepatic fat deposition and heightened risk of fibrosis in MASLD. The PNPLA3 I148M variant, particularly prevalent in Hispanic populations, is linked to enhanced lipid accumulation in hepatocytes and elevated liver enzyme levels. Moreover, these individuals tend to exhibit greater sensitivity to high-carbohydrate diets and processed meats, which exacerbate lipid deposition and inflammatory responses[29]. This heightened sensitivity to specific dietary components underscores the critical need for personalized nutritional strategies tailored to genetic profiles.
Nutri-epigenetics provides a deeper understanding of MASLD by demonstrating how dietary components can modulate epigenetic marks and regulate gene expression without altering the DNA sequence[30,31].
One-carbon metabolism plays a pivotal role in the regulation of gene expression through epigenetic modifications. This metabolic pathway is primarily mediated by S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH). SAM donates methyl groups necessary for DNA and histone methylation, which are crucial for maintaining cellular homeostasis and gene regulation. In hepatocytes, SAM is synthesized from methionine by the enzyme methionine adenosyltransferase. After donating its methyl group in these reactions, SAM is converted into SAH, which is then hydrolyzed into adenosine and homocysteine by SAH hydrolase[32].
The balance between SAM and SAH is critical for proper methylation activity. Elevated levels of SAH act as potent inhibitors of methyltransferases, leading to global DNA hypomethylation and altered histone modifications. This imbalance has been linked to increased lipid accumulation and fibrosis in MASLD[32].
To sustain efficient one-carbon metabolism, certain micronutrients are essential. Methionine and choline are directly involved in SAM synthesis, while folate and betaine contribute to the remethylation of homocysteine back to methionine, ensuring the continuation of methylation reactions[31].
Deficiencies in these nutrients disrupt methyl group availability, leading to epigenetic alterations that accelerate MASLD progression. Consequently, dietary interventions that restore one-carbon metabolism emerge as promising therapeutic avenues to mitigate liver damage and fibrosis in MASLD[33].
The integration of Precision Nutrition into MASLD management builds on genetic and epigenetic insights, enabling the identification of distinct endotypes—such as those defined by PNPLA3, TM6SF2, and MBOAT7 variants. These subgroups of patients, characterized by unique molecular profiles, display differential responses to dietary interventions[29].
For example, carriers of the PNPLA3 I148M variant are particularly sensitive to high-carbohydrate diets and processed meats, which worsen hepatic fat deposition and inflammation. On the other hand, dietary strategies that emphasize carbohydrate restriction and increased intake of polyunsaturated fatty acids (PUFAs) have proven beneficial in reducing liver damage and fibrosis among these genetically predisposed individuals[34].
These findings underscore the importance of precision nutrition, where dietary strategies are designed not only to prevent disease progression but also to optimize therapeutic outcomes based on individual molecular profiles. This targeted nutritional approach represents a paradigm shift in MASLD management, offering a path toward more effective and personalized dietary interventions[35].
Methionine, an essential amino acid metabolized in the liver, actively participates in DNA methylation processes and histone modification[36].
Dietary intake, protein breakdown, and homocysteine remethylation are sources of methionine[37]. Methionine is converted into SAM through a reaction catalyzed by methionine adenosyl transferase. SAM methylates RNA, DNA, and histone metabolites. After methylation reactions, it transforms into SAH and homocysteine to restart the cycle[38].
Studies have shown that diets deficient in methionine and choline inhibit the enzyme stearoyl-CoA desaturase, leading to increased accumulation of intrahepatic lipids, inducing MASLD[39,40]. Animal-derived foods with significant methionine content include beef, lamb, tuna, salmon, and shrimp. Meanwhile, plant-based sources include beans, soy, cashews, tofu, lentils, wheat germ, and spirulina[41,42].
Recent evidence suggests that methionine restriction (MR) could alleviate obesity and improve insulin sensitivity by modulating the circadian rhythm of lipid and bile acid metabolism. MR activates the AMPK/PGC-1α signaling pathway, which enhances the expression of FGF21 and restores the cyclical fluctuations of lipidolysis and bile acid synthesis genes. This modulation helps reduce hepatic lipid accumulation and improves systemic metabolic homeostasis, suggesting a protective role of MR against MASLD progression[36].
Additionally, MR has been linked to improved hepatic circadian clock function, which is crucial for metabolic regulation, reducing the impact of high-fat diets on hepatic steatosis and insulin resistance. These findings suggest that nutritional modulation through methionine control could be a potential strategy for managing MASLD[36].
Folate, also known as vitamin B9, is a water-soluble vitamin that participates in DNA and RNA methylation processes. Folate undergoes oxidation to generate betaine in the liver, contributing to methionine biosynthesis. In other tissues, folate exists as N5-methyltetrahydrofolate, which is necessary for the remethylation of homocysteine[43].
Changes in folate or methionine metabolism bi-directionally affect choline metabolism homeostasis. Folate deficiency in hepatic tissue leads to mitochondrial and endoplasmic reticulum dysfunction due to increased oxidative stress. Homocysteine remethylation through betaine and choline supplementation instead of folate modifies the upregulation of gene expression related to the accumulation and reduction of hepatic lipid export via very low-density lipoprotein secretion[43,44]. Foods rich in folate include spinach, avocado, peas, Brussels sprouts, fermented foods, legumes, rice, corn, wheat, potatoes, and cassava[45].
Furthermore, folate deficiency has been associated with disrupted one-carbon metabolism, which increases levels of homocysteine and decreases methionine synthesis. This imbalance contributes to hepatic steatosis and fibrosis development in MASLD by impairing very low density lipoproteins (VLDL) secretion and promoting lipid accumulation in the liver[38].
Interestingly, studies have highlighted that low folate levels can exacerbate oxidative stress and mitochondrial dysfunction, which are critical factors in MASLD pathogenesis[38].
Choline participates in phospholipid synthesis within the mitochondria. It oxidizes and transforms into betaine or metabolizes into phosphocholine[46].
In the liver, dietary choline follows two metabolic pathways. First, choline oxidizes and transforms into betaine in a reaction catalyzed by choline dehydrogenase and betaine aldehyde dehydrogenase, eventually converting into methionine. In the second pathway, catalyzed by choline kinase, choline transforms into phosphocholine, a precursor of lecithin[47].
Evidence suggests that choline deficiency reduces SAM activity while increasing SAH activity, inhibiting methyltransferase activity. Consequently, this reduces phosphatidylcholine synthesis, leading to abnormal export of triglycerides in the form of very low-density lipoprotein and increasing lipid accumulation in the liver, promoting MASLD development[37,39].
Choline deficiency has been directly linked to disrupted SAM synthesis and increased SAH, resulting in impaired phosphatidylcholine synthesis. This disruption leads to abnormal VLDL-mediated triglyceride export, culminating in hepatic lipid accumulation and contributing to MASLD pathogenesis[38].
Sources of choline include beef, pork, eggs, salmon, beans, nuts, almonds, broccoli, red potatoes, and white rice. Additionally, de novo synthesis represents an important source of choline through the sequential methylation of phosphatidylethanolamine using methyl groups provided by SAM. Water-soluble molecules reach the liver directly, while lipid-soluble forms do so through lymphatic circulation within chylomicrons[48].
It is an amino acid present in the liver, kidneys, and testicles. In both the mitochondria and cytoplasm, choline oxidizes into betaine aldehyde in a reaction catalyzed by mitochondrial choline oxidase. Subsequently, betaine aldehyde dehydrogenase catalyzes the transformation into betaine. Hence, betaine serves as a methyl group donor for the remethylation of homocysteine[49].
A case-control study assessed DNA methylation levels in hepatic tissue from 47 patients with MASLD and 18 controls[50]. The results demonstrated reduced methylation levels, along with significantly higher serum concentrations of homocysteine and a considerably lower betaine/choline ratio, suggesting a negative correlation between homocysteine concentration and MASLD severity.
Among foods with higher betaine content are wheat, spinach, shellfish, sugar beet, whole grains, shrimp, and all foods containing choline[51].
Betaine supplementation has been shown to improve hepatic lipid metabolism by enhancing VLDL export and reducing hepatic steatosis. This effect is partially attributed to its role as a methyl donor in the remethylation of homo
Probiotics (PROs) are live microorganisms that can be consumed through fermented foods or supplements, and which, when administered in adequate amounts, confer a health benefit on the host. Among foods rich in PROs, there are yogurt, kefir, kimchi, sauerkraut, pickles, aged cheese and fermented soy products, such as miso and tempeh.
The gut microbiota plays a critical role in modulating host metabolic pathways, impacting the development and progression of MASLD. Recent findings highlight the role of the gut microbiota as a key player in the host's nutrigenomic landscape. Microbial-derived metabolites, such as short-chain fatty acids (SCFAs), modulate gene expression through histone deacetylase inhibition and DNA methylation, bridging the interaction between dietary inputs and the host epigenome[52].
This crosstalk influences hepatic lipid metabolism, insulin sensitivity, and inflammatory responses, underscoring the relevance of the gut-liver axis in MASLD management.
Nutrigenomic interactions extend to the modulation of lipid metabolism genes by specific microbial populations. For instance, Akkermansia muciniphila has been associated with improved lipid profiles and reduced liver fat accumulation, mediated by enhanced bile acid signaling and gut barrier integrity[53].
Moreover, PROs and prebiotics have demonstrated potential in restoring microbial balance and reducing endotoxemia, thereby mitigating hepatic inflammation and steatosis[54].
These findings suggest that integrating microbiome modulation with nutrigenomic strategies could enhance therapeutic outcomes in MASLD. Targeted dietary interventions, including synbiotic formulations, may optimize microbial composition, reduce hepatic fat deposition, and improve systemic metabolic responses. Thus, the gut microbiota emerges as a pivotal modulator of metabolic reprogramming, with significant implications for personalized nutrition and precision medicine in MASLD[52].
Evidence indicates that PROs effectively improve intestinal permeability and the translocation of bacterial endotoxins by reducing duodenal inhibitor of kappa B protein levels and restoring duodenal tight junction protein levels, which translates into an effective reduction of inflammation and hepatic steatosis[55-59].
When gut microbiota dysbiosis occurs, it is associated with increased intestinal permeability, allowing the translocation of bacterial endotoxins such as lipopolysaccharides into the bloodstream. This process triggers systemic inflammation and contributes to the progression of MASLD. PROs help restore microbial balance by promoting the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium, which are known to enhance mucosal barrier function and reduce endotoxemia[38].
In addition to PROs, prebiotics are also part of the complex mechanism for modulating the intestinal microbiota. They are fermentable carbohydrates that selectively modulate the composition and activity of the microbiota[60], reducing hepatic lipogenesis and blood triglyceride levels[61,62].
Prebiotics serve as substrates for beneficial gut bacteria, promoting the production of SCFAs such as butyrate, acetate, and propionate. These SCFAs play a protective role in MASLD by enhancing intestinal barrier integrity, modulating local and systemic inflammation, and regulating lipid metabolism in the liver[36].
A clinical trial demonstrated that PROs improve serum levels of liver enzymes, lipids and insulin resistance in patients with MASLD[63]. Conversely, Escouto et al[64], in a randomized clinical trial (RCT) involving a six-month intervention with PROs in 48 patients diagnosed with MASLD via liver biopsy, reported that PRO administration did not lead to significant alterations in the gut microbiota composition of these patients. The authors further emphasized that intestinal dysbiosis is associated not only with liver disease but also with its common comorbidities, underscoring the need for a personalized nutritional approach to effectively address these interconnected conditions.
However, there is still a need for further clinical trials regarding the subject due to the numerous metabolic implications that the use of PROs may have in patients with MASLD[65].
In addition to macronutrients such as fatty acids, bioactive dietary compounds—such as resveratrol, curcumin, sulforaphane, and polyphenols—demonstrate significant epigenetic regulatory potential. These molecules modulate histone acetylation, microRNA expression, and chromatin remodeling, influencing hepatic lipid metabolism, inflammation, and oxidative stress[66,67].
For instance, the consumption of resveratrol-rich foods, such as grapes, has been demonstrated to activate SIRT1, thereby enhancing mitochondrial function and attenuating oxidative stress within hepatocytes[67]. Curcumin and sulforaphane, in turn, modulate the Nrf2 pathway, improving antioxidant responses and attenuating hepatic steatosis[68]. Polyphenols, widely found in berries and green tea, also exhibit anti-inflammatory effects through the suppression of NF-kB signaling, which is crucial in MASLD pathogenesis[69].
Collectively, the integration of bioactive compounds with nutrigenomic and epigenetic insights may represent a promising avenue for personalized dietary strategies in MASLD management. Importantly, the personalized nature of these interventions must be emphasized, as the same bioactive compounds that exhibit anti-inflammatory benefits may, in certain individuals, induce hepatotoxicity. Therefore, tailoring these strategies to individual genetic and metabolic profiles is essential to maximize therapeutic efficacy while minimizing potential adverse effects[36].
Nutritional intervention aims to reduce insulin resistance and hepatic transaminase levels and, consequently, the promote remission of liver disease by improving dietary patterns and reducing body weight[70].
Although it may seem like a modest weight reduction, the loss of around 7% to 10% of body weight promotes a significant outcome in the course of liver disease, reflected by the activity of liver enzymes and hepatic steatosis evaluated histologically, and inflammation with less impact on fibrosis[71].
In fibrosis, reducing body weight by > 10% demonstrates improvements[72].
A reduction in body weight/body fat is possible through good dietary practices, among which the Mediterranean diet (MedD) is strongly recommended for preventing and treating MASLD[72].
Corroborating Bischoff et al[72], the 2023 guideline of the American Society for Liver Studies also recommends MedD for treating MASLD based on the concept of lifestyle medicine. Aside from the MedD, 150 minutes/week of resistance exercise to no alcohol consumption, no smoking and good quality of sleep are also recommended[17].
MedD is rich in monounsaturated fatty acids (MUFAs), such as olive oil, nuts and low consumption of red meat. Furthermore, it must be low in carbohydrates (40%), based on whole unprocessed cereals (For example, oats, brown rice, rice flour). The consumption of plants, fruits, and foods rich in omega 3, such as fish and low-fat dairy products, is also part of MedD. This dietary composition meets the nutritional needs for managing patients with steatotic liver and associated comorbidities[72-74].
In contrast, Xu et al[75], through a Mendelian randomization study, demonstrated that MUFAs appear to be more lipogenic but less apoptotic compared to saturated fatty acids (SFAs). Their findings revealed that MUFAs do not play a central role in the lipid-mediated mechanisms of liver damage when compared to SFAs. The underlying epigenetic mechanism is attributed to the insufficient activation of PPAR-γ by oleic acid (OA), a common MUFA, while simultaneously promoting a more effective increase in SREBP-1 expression than SFAs[76].
This mechanistic pathway allows hepatic cells to survive while accumulating large amounts of fat, leading to progressive steatosis without the cell death that would typically limit this process. This combination of enhanced lipogenesis, reduced fat oxidation, and lower cellular toxicity creates optimal conditions for the development and progression of hepatic steatosis, thus challenging the traditionally accepted protective role of the MedD in liver health[77-79].
These findings raise questions regarding the quality and origin of dietary fatty acids. While MUFAs derived from olive oil are enriched with phenolic compounds and antioxidants that modulate inflammation and oxidative stress[80], processed sources of MUFAs, such as refined oils, may not provide the same protective effects. Additionally, saturated fatty acids (SFAs) play a dual role in MASLD pathogenesis. Although associated with lipid accumulation and hepatic inflammation, the modulation of SFAs types and their balance with PUFAs may influence these negative outcomes[81].
This discussion underscores the necessity for a personalized approach to dietary interventions for MASLD, taking into account the genetic profile and quality of ingested lipids. Dietary pattern reviews should evaluate not only the quantity of fats but also their sources and epigenetic interactions within hepatic tissue[72,79,82].
Oxidative damage to macromolecules resulting from the imbalance between pro-oxidant and antioxidant molecules, known as oxidative stress, plays a significant role in the pathophysiology of MASLD[83].
Diets high in fat elevate lipid peroxidation in the liver, leading to a consequential alteration in the redox state due to the increased production of reactive oxygen species (ROS) - including singlet oxygen molecules, superoxide anions, hydrogen peroxide, and hydroxyl radicals[84].
The heightened presence of ROS in hepatic tissue results in ATP and nicotinamide adenine dinucleotide depletion, DNA and cellular membrane damage, and protein degradation, as well as an augmented release of pro-inflammatory cytokines, which are critical factors in the progression from MASLD to MASH[85-87].
Evidence has demonstrated that chromatin remodeling enzymes are activated during the alteration of the redox state, modulating crucial pro-inflammatory responses—particularly, the adaptation to hypoxia through desumoylation and SIRT1—in the progression of the disease[88].
In the liver, SIRT1 inactivation diminishes PPARα signaling, inducing hepatic steatosis. Furthermore, the alteration in redox state activates hepatic JNK1, which reduces SIRT1 Levels, accentuating hepatic steatosis. Thus, both the genetic control of inflammatory responses and the epigenetic causes of oxidative stress/inflammation contribute to MASLD[89-91].
Antioxidants: Antioxidants are molecules that prevent oxidative damage by neutralizing ROS and reactive nitrogen species, which are highly reactive free radicals capable of causing cellular and molecular damage[92,93].
These reactive species are naturally produced during metabolic processes such as mitochondrial respiration and β-oxidation of fatty acids. However, their excessive accumulation, often induced by high-fat diets and metabolic dysfunctions, leads to oxidative stress—a key driver in the progression of MASLD[92,93].
The use of antioxidant therapy in MASLD has been explored to mitigate oxidative damage and restore redox balance. Vitamin E, for example, is widely studied for its lipid-soluble antioxidant properties, preventing lipid peroxidation in cellular membranes. Clinical trials, such as the PIVENS study, demonstrated that vitamin E supplementation improves liver histology in non-diabetic patients with MASH by reducing inflammation and oxidative stress markers[94].
Vitamin E: Vitamin E exhibits epigenetic potential due to its anti-inflammatory and antioxidant properties. The hydroxyl group of the tocochromatol ring blocks free radicals and ROS by donating a hydrogen molecule. Vitamin E has been an option in the treatment of MASLD as well as MASH[95-97].
The main isoforms of tocopherol are α, β, γ, and δ, with the δ-isoform being the molecule with the least enzymatic and antioxidant capacity[98].
Among the most important trials regarding vitamin E in patients with MASLD is the PIVENS trial, which compared pioglitazone vs vitamin E vs Placebo for treating non-diabetic patients with MASH[94].
The authors concluded that both medications improve steatosis, lobular inflammation, and hepatocellular ballooning. However, regarding fibrosis, vitamin E was associated with clinical improvement[94].
The TONIC study evaluated vitamin E vs metformin in the treatment of children with MASH. Both interventions improved hepatocellular ballooning and the MASLD activity score. However, there was no improvement in serum aminotransferase levels[99].
A systematic review with 1317 patients from 15 randomized controlled clinical trials concluded that vitamin E improves the biochemical and histological parameters of adult and pediatric patients, demonstrating a significant and inversely proportional relationship between the dose of Vitamin E and normalization of aminotransferases[100].
In adult patients, improved serum aminotransferase levels, fibrosis and MASLD Score were observed in early and late follow-up. However, there are also questions about its safety profile[100].
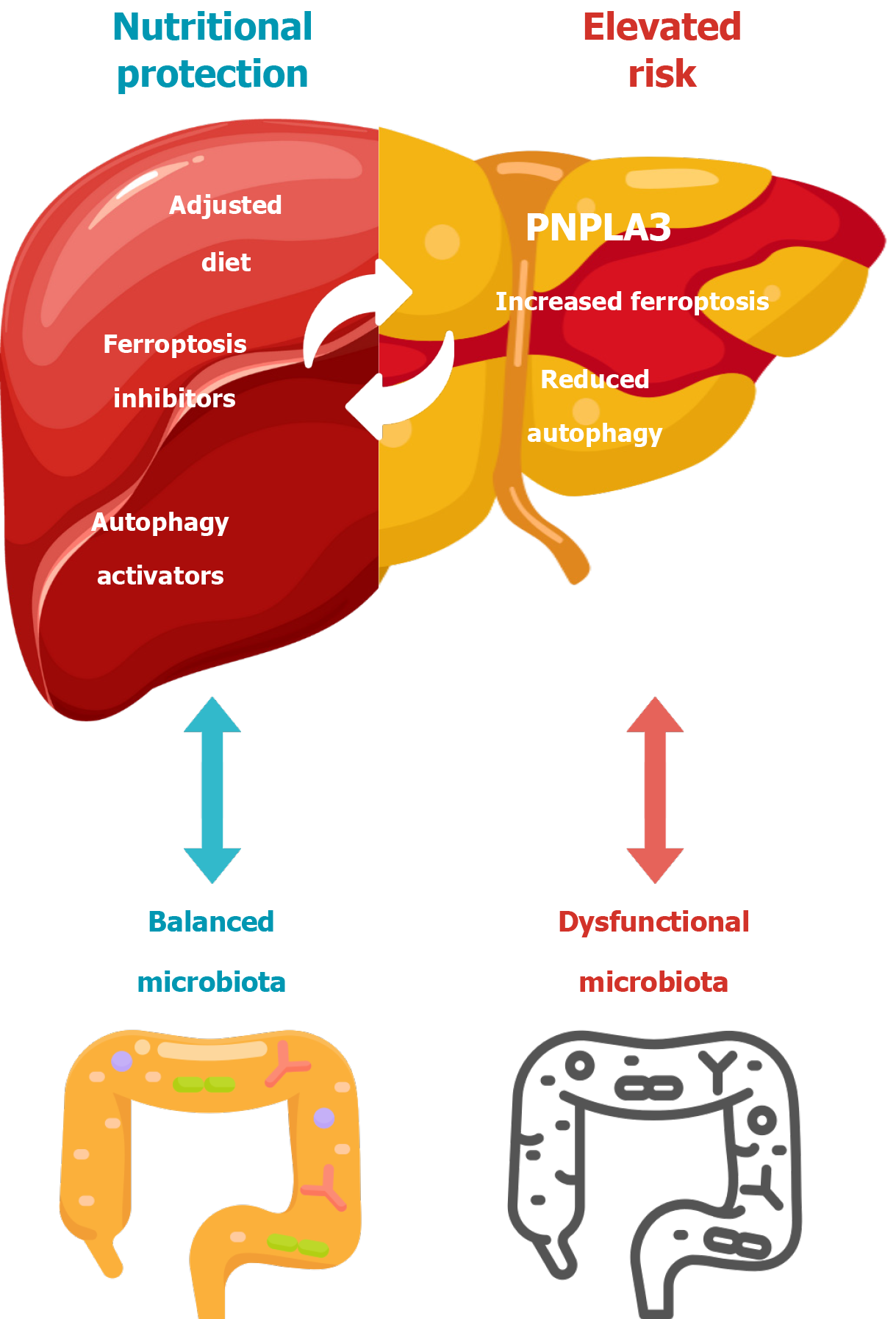
Additionally, there are other mechanisms that trigger metabolites significantly influencing the progression of MASLD. Among them, we can highlight ferroptosis, is a regulated form of cell death characterized by iron-dependent lipid peroxidation, contributing to cellular damage and inflammation in hepatic tissues. In the context of MASLD, increased oxidative stress and lipid peroxidation can trigger ferroptosis, exacerbating liver damage and fibrosis. Emerging research suggests that inhibiting ferroptosis can mitigate these pathological changes by preserving hepatocyte integrity and preventing lipid peroxidation[101].
Historically, the interplay between metabolic regulation and liver fibrosis has been primarily attributed to hepatocytes, given their central role in hepatic metabolic processes. However, emerging insights from single-cell RNA sequencing and proteomic analyses have unveiled the remarkable heterogeneity and plasticity of hepatic macrophages. These studies reveal distinct macrophage phenotypes that are intricately involved in the initiation, perpetuation, and resolution of liver injury, challenging the traditional paradigm that solely centers on hepatocyte-driven fibrosis[101,102].
Equally pivotal is the activation of hepatic stellate cells (HSCs), which undergo a profound phenotypic shift into a myofibroblast-like state upon liver injury. This transdifferentiation process is not merely a passive cellular adaptation; it is a highly energy-intensive transformation driven by comprehensive metabolic reprogramming. Activated HSCs display augmented glycolysis, enhanced lipid droplet mobilization, intensified β-oxidation, and upregulated glutaminolysis, underscoring the critical metabolic shifts that sustain their fibrogenic activity[103].
These findings underscore the influence of the metabolic microenvironment—including nutrient availability, gut-derived metabolites, and microbiota composition—in modulating cellular metabolism and the metabolic stress response. Consequently, targeting the metabolic reprogramming of immune cells, stromal cells, and HSCs emerges as a compelling therapeutic strategy to disrupt the fibrotic cascade and mitigate liver damage. Such an approach not only addresses the cellular drivers of fibrosis but also aligns with emerging concepts of metabolic flexibility and immunometabolism in chronic liver disease[101].
Metabolic reprogramming represents a strategic advancement in the treatment of MASLD. Alterations in specific metabolic pathways can modulate the hepatic microenvironment, reducing cellular damage and inflammation. Among the critical mechanisms involved, ferroptosis inhibitors and autophagy activators stand out as promising therapeutic targets[101].
Therapeutic agents targeting ferroptosis pathways, such as iron chelators and lipid peroxidation inhibitors, have demonstrated potential in experimental models, reducing steatosis and hepatic inflammation. For instance, UAMC-3203 demonstrated efficacy in murine models of MASLD by reducing ALT levels and hepatic steatosis, although with limited effects on ballooning, lobular inflammation, and fibrosis[104].
Furthermore, inhibitors such as Fer-1 and Lip-1 have been shown to reduce lipid peroxidation and the expression of pro-inflammatory cytokines, including IL-1β and TNF-α, in experimental settings[104].
This suggests a promising therapeutic avenue for MASLD management by attenuating ferroptotic cell death, thereby preventing further progression of hepatic injury[101].
It is well established that diet has a direct connection with the activation of the pro-inflammatory cascade, con
Autophagy plays a crucial role in maintaining cellular homeostasis through the degradation and recycling of damaged cellular components, including lipid droplets. In MASLD, impaired autophagy contributes to excessive lipid accumulation and mitochondrial dysfunction, exacerbating liver steatosis and inflammation. The activation of autophagy has been shown to ameliorate these effects by enhancing lipid turnover and reducing hepatocellular stress[105,106].
The Fanlian Huazhuo formula, for example, has been found to regulate autophagy through the AMPKα/SREBP-1C signaling pathway, reducing lipid accumulation and oxidative stress in NAFLD models. This mechanism highlights the therapeutic potential of autophagy activators in improving lipid homeostasis and preventing the progression of MASLD[105,106].
In summary, the relationship between PNPLA3 mutations, autophagy activation, ferroptosis inhibition, and microbiota balance is pivotal in MASLD progression and therapeutic intervention. The illustrated model (Figure 2) visually represents these interactions, highlighting how nutritional strategies, ferroptosis inhibitors, and autophagy activators can mitigate hepatic damage induced by dysfunctional microbiota and PNPLA3-associated risk. A balanced microbiota contributes to reduced inflammation and oxidative stress, while targeted interventions like adjusted diets and specific molecular inhibitors optimize cellular resilience and lipid turnover in hepatic tissues[101,105,106].
The progression of MASLD/MASH to liver fibrosis, cirrhosis, and hepatocellular carcinoma remains a critical concern, especially when early diagnosis and effective treatment are not achieved. Addressing this challenge requires innovative therapeutic approaches that target the underlying pathophysiological mechanisms. Hepatic fibrosis, recognized as a reversible stage before the onset of cirrhosis, is driven by a multifaceted interplay among hepatic cell populations—both resident and non-resident—and significant metabolic adaptations that promote a chronic pro-inflammatory and pro-fibrogenic microenvironment. Key cellular mediators, such as macrophages, lymphoid cells, and HSCs, orchestrate this fibrotic response[101].
Future therapeutic strategies should focus on modulating critical pathways implicated in fibrosis, including endoplasmic reticulum stress, autophagy, ferroptosis, and nuclear receptor signaling. Targeting these mechanisms holds promise for disrupting endothelial activation, immune cell recruitment, and excessive extracellular matrix deposition. As our understanding of these pathways deepens, novel interventions aimed at halting or even reversing fibrosis progression may become feasible, offering renewed hope for MASLD/MASH patients[101].
The evaluation of therapeutic strategies for MASLD has been extensively explored through well-structured clinical trials and comprehensive meta-analyses. These studies provide high-level evidence on the efficacy and safety of pharmacological and non-pharmacological interventions, supporting clinical decision-making in MASLD management. Among the most pivotal clinical trials, the PIVENS trial and TONIC study assessed the impact of vitamin E vs Pioglitazone vs placebo in non-diabetic patients with MASLD, as previously mentioned[107].
The FLINT trial introduced obeticholic acid as a therapeutic candidate, revealing its capacity to reduce fibrosis progression in patients with MASLD. The trial reported improvements in liver biochemistry and histological markers of fibrosis, positioning obeticholic acid as a promising agent for advanced stages of MASLD[96].
Meta-analyses have further consolidated findings from multiple clinical trials, offering stronger statistical power and more robust conclusions. A comprehensive meta-analysis published in The Lancet Gastroenterology & Hepatology (2023) synthesized data from 15 RCTs, affirming the role of lifestyle interventions, dietary modifications, and targeted pharmacological treatments in attenuating fibrosis and improving metabolic parameters in MASLD patients[108].
Moreover, ongoing clinical trials, such as the MAESTRO-NASH study, are evaluating the efficacy of resmetirom, a selective thyroid hormone receptor-β agonist, in reducing hepatic fat and fibrosis, showing promising preliminary results[109].
These findings underscore the evolving landscape of MASLD therapeutic strategies, guided by robust clinical evidence and continuous evaluation.
In addition to the studies previously discussed, a notable European meta-analysis conducted in 2022 evaluated the efficacy of dietary and lifestyle interventions in patients with MASLD and NASH. The combined analysis demonstrated a significant reduction in disease progression risk through interventions based on dietary and behavioral modifications, thereby reinforcing the importance of such approaches in the clinical management of the condition. This evidence broadens the spectrum of available non-pharmacological therapies, which is particularly relevant for populations with limited access to specific pharmacological treatments[77].
As previously discussed, the study by Xu et al[75] raised critical questions regarding current dietary recommendations on oil consumption and fatty acid saturation profiles, further emphasizing the necessity for personalized nutritional strategies in the management of MASLD.
Finally, the results of the ESSENCE clinical trial, published in 2025, introduced new perspectives for the treatment of MASLD. This multicenter phase 3 study evaluated the efficacy of semaglutide, a GLP-1 receptor agonist, in patients with MASH and stage 2 or 3 hepatic fibrosis. The interim analysis demonstrated that weekly semaglutide administration (2.4 mg) resulted in significant improvements in steatohepatitis and fibrosis compared to placebo, in addition to contributing to weight loss. These findings underscore the therapeutic potential of semaglutide not only in glycemic control and weight reduction but also in modulating hepatic and metabolic markers in MASLD patients[110].
Collectively, the incorporation of these complementary studies strengthens the evidence base guiding therapeutic strategies for MASLD (Table 1). The methodological diversity—encompassing RCTs, meta-analyses, and genetic studies—reflects the complexity of disease management and signals the growing importance of personalized approaches integrating genetic, metabolic, and behavioral factors.
| Study | Intervention | Population | Key findings | Ref. |
| PIVENS trial | Vitamin E, pioglitazone vs placebo | Non-diabetic MASLD patients | Vitamin E improved liver histology | Chalasani et al[107], 2009 |
| TONIC study | Vitamin E and metformin | Pediatric MASLD patients | Improved hepatocellular ballooning and MASLD activity score; no improvement in ALT levels | Lavine et al[99], 2011 |
| FLINT trial | Obeticholic acid | MASLD patients | Reduced fibrosis progression | Gao et al[91], 2011 |
| European meta-analysis | Dietary and lifestyle modifications | MASLD and MASH patients | Significant risk reduction with dietary intervention | Buzzetti et al[77], 2021 |
| Lancet meta-analysis | Lifestyle, pharmacological Interventions | MASLD patients (meta-analysis of 15 RCTs) | Validated efficacy of lifestyle and pharmacological interventions | Mózes et al[108], 2023 |
| MAESTRO-NASH trial | Resmetirom (thyroid hormone receptor-β agonist) | MASLD with liver fibrosis (phase 3 RCT) | Promising reduction of hepatic fat and fibrosis | Harrison et al[109], 2024 |
| - | MUFA vs SFA intake | MASLD patients with genetic stratification | MUFA may contribute to disease progression | Xu et al[75], 2024 |
| Semaglutide trial (NEJM) | Semaglutide (GLP-1 receptor agonist) | MASH patients with compensated cirrhosis (phase 3 RCT) | Significant improvements in steatohepatitis and fibrosis | Sanyal et al[110], 2025 |
The implementation of risk prediction models in MASLD represents a significant step forward in early diagnosis and personalized management. These models utilize clinical, genetic, and biochemical markers to stratify patients according to their risk of fibrosis progression and adverse liver-related outcomes. Among the most widely adopted, the fibrosis-4 index (FIB-4) and the NAFLD fibrosis score have demonstrated robust predictive capabilities for advanced fibrosis, leveraging parameters such as age, platelet count, and liver enzyme levels[111].
Advanced imaging technologies like magnetic resonance elastography and transient elastography further enhance diagnostic precision, enabling non-invasive assessment of hepatic stiffness and fibrosis staging. These methods have shown high correlation with histological findings, providing a reliable alternative to liver biopsy[111].
Moreover, the integration of omics data, including transcriptomics and metabolomics, allows for more accurate risk stratification by identifying molecular signatures associated with disease progression[112].
Machine learning approaches are now emerging as transformative tools in MASLD risk prediction. Algorithms trained on large datasets have been able to identify complex interactions between genetic polymorphisms, dietary habits, and metabolic markers, facilitating early intervention strategies. For instance, deep learning models have achieved high sensitivity and specificity in detecting advanced fibrosis from routine clinical data, surpassing traditional scoring methods[113].
As previously emphasized, the clinical and economic burden of MASLD/MASH on healthcare systems is profound, underscoring the critical need for effective strategies in early detection and targeted intervention. In this context, Artificial Intelligence (AI)—leveraging sophisticated algorithms trained on vast datasets—has emerged as a transformative tool in hepatology, enhancing diagnostic accuracy and enabling proactive prevention strategies guided by precision medicine[114,115].
Recent advances have also explored the potential of blood-based machine learning models to improve non-invasive diagnostics in MASLD. As demonstrated by Fan et al[116] AI algorithms that utilize routine blood biomarkers can accurately classify and predict disease severity, offering a scalable and cost-effective solution for early risk stratification. This approach is particularly advantageous in primary care settings, where advanced imaging technologies may not be readily available. The integration of AI-driven blood biomarker analysis complements existing tools such as FIB-4 and APRI scoring by providing additional layers of clinical decision support, facilitating timely referral and targeted intervention in high-risk patients[116].
The evidence presented here highlights the key role of diet in the epigenetic regulation and modulation of MASLD, emphasizing its potential as a dynamic and reversible process. Thus, dietary interventions represent promising therapeutic strategies to improve the prognosis and treatment of MASLD, regardless of the stage of fibrosis. The development of predictive tools, as well as the assessment of diagnostic and therapeutic efficacy based on epigenetic mechanisms and dietary modulation, is poised to drive advances in precision medicine and nutrition for the treatment of MASLD. A key component of this precision-based approach is the understanding of genetic variations, such as the PNPLA3 I148M polymorphism, which remains one of the most significant genetic risk factors for MASLD progression to date. Despite these promising perspectives, further clinical trials are warranted to elucidate the impact of specific diets, nutritional supplements, and epigenetic markers on MASLD progression and therapeutic outcomes. Enhanced clinical evidence will be crucial for translating these insights into personalized treatment protocols that effectively mitigate liver injury and disease progression.
| 1. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1305] [Article Influence: 652.5] [Reference Citation Analysis (1)] |
| 2. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1443] [Article Influence: 721.5] [Reference Citation Analysis (2)] |
| 3. | Wang D, Xu Y, Zhu Z, Li Y, Li X, Li Y, Shen H, Wu W, Liu Y, Han C. Changes in the global, regional, and national burdens of NAFLD from 1990 to 2019: A systematic analysis of the global burden of disease study 2019. Front Nutr. 2022;9:1047129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 4. | Ge X, Zheng L, Wang M, Du Y, Jiang J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990-2017: a population-based observational study. BMJ Open. 2020;10:e036663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Zhang H, Zhou XD, Shapiro MD, Lip GYH, Tilg H, Valenti L, Somers VK, Byrne CD, Targher G, Yang W, Viveiros O, Opio CK, Mantzoros CS, Ryan JD, Kok KYY, Jumaev NA, Perera N, Robertson AG, Abu-Abeid A, Misra A, Wong YJ, Ruiz-Úcar E, Ospanov O, Kızılkaya MC, Luo F, Méndez-Sánchez N, Zuluaga M, Lonardo A, Al Momani H, Toro-Huamanchumo CJ, Adams L, Al-Busafi SA, Sharara AI, Chan WK, Abbas SI, Sookoian S, Treeprasertsuk S, Ocama P, Alswat K, Kong AP, Ataya K, Lim-Loo MC, Oviedo RJ, Szepietowski O, Fouad Y, Zhang H, Abdelbaki TN, Katsouras CS, Prasad A, Thaher O, Ali A, Molina GA, Sung KC, Chen QF, Lesmana CRA, Zheng MH. Global burden of metabolic diseases, 1990-2021. Metabolism. 2024;160:155999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 6. | Kaya E, Yilmaz Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J Clin Transl Hepatol. 2022;10:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 244] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 8. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 375] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 9. | Lee CH, Lui DT, Lam KS. Non-alcoholic fatty liver disease and type 2 diabetes: An update. J Diabetes Investig. 2022;13:930-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Zhou XD, Targher G, Byrne CD, Somers V, Kim SU, Chahal CAA, Wong VW, Cai J, Shapiro MD, Eslam M, Steg PG, Sung KC, Misra A, Li JJ, Brotons C, Huang Y, Papatheodoridis GV, Sun A, Yilmaz Y, Chan WK, Huang H, Méndez-Sánchez N, Alqahtani SA, Cortez-Pinto H, Lip GYH, de Knegt RJ, Ocama P, Romero-Gomez M, Fudim M, Sebastiani G, Son JW, Ryan JD, Ikonomidis I, Treeprasertsuk S, Pastori D, Lupsor-Platon M, Tilg H, Ghazinyan H, Boursier J, Hamaguchi M, Nguyen MH, Fan JG, Goh GB, Al Mahtab M, Hamid S, Perera N, George J, Zheng MH. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int. 2023;17:773-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 11. | Zhang L, Lu Q, Chang C. Epigenetics in Health and Disease. Adv Exp Med Biol. 2020;1253:3-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 12. | Dongiovanni P, Valenti L. A Nutrigenomic Approach to Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2017;18:1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Isac T, Isac S, Rababoc R, Cotorogea M, Iliescu L. Epigenetics in inflammatory liver diseases: A clinical perspective (Review). Exp Ther Med. 2022;23:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Wu YL, Lin ZJ, Li CC, Lin X, Shan SK, Guo B, Zheng MH, Li F, Yuan LQ, Li ZH. Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduct Target Ther. 2023;8:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 173] [Reference Citation Analysis (0)] |
| 15. | Mann DA. Epigenetics in liver disease. Hepatology. 2014;60:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Hardy T, Mann DA. Epigenetics in liver disease: from biology to therapeutics. Gut. 2016;65:1895-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Zhang X, Asllanaj E, Amiri M, Portilla-Fernandez E, Bramer WM, Nano J, Voortman T, Pan Q, Ghanbari M. Deciphering the role of epigenetic modifications in fatty liver disease: A systematic review. Eur J Clin Invest. 2021;51:e13479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, Garrett ME, Ashley-Koch A, Suzuki A, Tillmann HL, Hauser MA, Diehl AM. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 19. | Kuchay MS, Misra A. From non-alcoholic fatty liver disease (NAFLD) to metabolic-associated fatty liver disease (MAFLD): A journey over 40 years. Diabetes Metab Syndr. 2020;14:695-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Stover PJ, Caudill MA. Genetic and epigenetic contributions to human nutrition and health: managing genome-diet interactions. J Am Diet Assoc. 2008;108:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Meroni M, Longo M, Erconi V, Valenti L, Gatti S, Fracanzani AL, Dongiovanni P. mir-101-3p Downregulation Promotes Fibrogenesis by Facilitating Hepatic Stellate Cell Transdifferentiation During Insulin Resistance. Nutrients. 2019;11:2597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Kosgei VJ, Coelho D, Guéant-Rodriguez RM, Guéant JL. Sirt1-PPARS Cross-Talk in Complex Metabolic Diseases and Inherited Disorders of the One Carbon Metabolism. Cells. 2020;9:1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, Tie J, Hu D. Regulation of SIRT1 and Its Roles in Inflammation. Front Immunol. 2022;13:831168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 287] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 24. | Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2983] [Cited by in RCA: 3181] [Article Influence: 167.4] [Reference Citation Analysis (1)] |
| 25. | Powell NR, Liang T, Ipe J, Cao S, Skaar TC, Desta Z, Qian HR, Ebert PJ, Chen Y, Thomas MK, Chalasani N. Clinically important alterations in pharmacogene expression in histologically severe nonalcoholic fatty liver disease. Nat Commun. 2023;14:1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Odena G, Chen J, Lozano JJ, Altamirano J, Rodrigo-Torres D, Affo S, Morales-Ibanez O, Matsushita H, Zou J, Dumitru R, Caballeria J, Gines P, Arroyo V, You M, Rautou PE, Valla D, Crews F, Seki E, Sancho-Bru P, Bataller R. LPS-TLR4 Pathway Mediates Ductular Cell Expansion in Alcoholic Hepatitis. Sci Rep. 2016;6:35610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Maji RK, Czepukojc B, Scherer M, Tierling S, Cadenas C, Gianmoena K, Gasparoni N, Nordström K, Gasparoni G, Laggai S, Yang X, Sinha A, Ebert P, Falk-Paulsen M, Kinkley S, Hoppstädter J, Chung HR, Rosenstiel P, Hengstler JG, Walter J, Schulz MH, Kessler SM, Kiemer AK. Alterations in the hepatocyte epigenetic landscape in steatosis. Epigenetics Chromatin. 2023;16:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 574] [Article Influence: 191.3] [Reference Citation Analysis (1)] |
| 29. | Jegodzinski L, Rudolph L, Castven D, Sayk F, Rout AK, Föh B, Hölzen L, Meyhöfer S, Schenk A, Weber SN, Rau M, Meyhöfer SM, Schattenberg JM, Krawczyk M, Geier A, Mallagaray A, Günther UL, Marquardt JU. PNPLA3 I148M variant links to adverse metabolic traits in MASLD during fasting and feeding☆. JHEP Rep. 2025;7:101450. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Ferguson JF, Allayee H, Gerszten RE, Ideraabdullah F, Kris-Etherton PM, Ordovás JM, Rimm EB, Wang TJ, Bennett BJ; American Heart Association Council on Functional Genomics and Translational Biology, Council on Epidemiology and Prevention, and Stroke Council. Nutrigenomics, the Microbiome, and Gene-Environment Interactions: New Directions in Cardiovascular Disease Research, Prevention, and Treatment: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet. 2016;9:291-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011;3:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 32. | Wang JJ, Chen XY, Zhang YR, Shen Y, Zhu ML, Zhang J, Zhang JJ. Role of genetic variants and DNA methylation of lipid metabolism-related genes in metabolic dysfunction-associated steatotic liver disease. Front Physiol. 2025;16:1562848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Barchetta I, Zampieri M, Cimini FA, Dule S, Sentinelli F, Passarella G, Oldani A, Karpach K, Bacalini MG, Baroni MG, Reale A, Cavallo MG. Association Between Active DNA Demethylation and Liver Fibrosis in Individuals with Metabolic-Associated Steatotic Liver Disease (MASLD). Int J Mol Sci. 2025;26:1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Nobili V, Liccardo D, Bedogni G, Salvatori G, Gnani D, Bersani I, Alisi A, Valenti L, Raponi M. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at-risk adolescents. Genes Nutr. 2014;9:392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Reytor-gonzález C, Simancas-racines D, Campuzano-donoso M, Castano Jimenez J, Román-galeano NM, Sarno G, Frias-toral E. Harnessing nutrition to combat MASLD: a comprehensive guide to food-based therapeutic strategies. Food Agr Immunol. 2025;36:2135. [DOI] [Full Text] |
| 36. | Rivera-Aguirre J, López-Sánchez GN, Chávez-Tapia NC, Uribe M, Nuño-Lámbarri N. Metabolic-associated Fatty Liver Disease Regulation through Nutri Epigenetic Methylation. Mini Rev Med Chem. 2023;23:1680-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Amenyah SD, Hughes CF, Ward M, Rosborough S, Deane J, Thursby SJ, Walsh CP, Kok DE, Strain JJ, McNulty H, Lees-Murdock DJ. Influence of nutrients involved in one-carbon metabolism on DNA methylation in adults-a systematic review and meta-analysis. Nutr Rev. 2020;78:647-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Radziejewska A, Muzsik A, Milagro FI, Martínez JA, Chmurzynska A. One-Carbon Metabolism and Nonalcoholic Fatty Liver Disease: The Crosstalk between Nutrients, Microbiota, and Genetics. Lifestyle Genom. 2020;13:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Aissa AF, Tryndyak V, de Conti A, Melnyk S, Gomes TD, Bianchi ML, James SJ, Beland FA, Antunes LM, Pogribny IP. Effect of methionine-deficient and methionine-supplemented diets on the hepatic one-carbon and lipid metabolism in mice. Mol Nutr Food Res. 2014;58:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006;47:2280-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Wang L, Ren B, Zhang Q, Chu C, Zhao Z, Wu J, Zhao W, Liu Z, Liu X. Methionine restriction alleviates high-fat diet-induced obesity: Involvement of diurnal metabolism of lipids and bile acids. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Rafii M, Pencharz PB, Ball RO, Tomlinson C, Elango R, Courtney-Martin G. Bioavailable Methionine Assessed Using the Indicator Amino Acid Oxidation Method Is Greater When Cooked Chickpeas and Steamed Rice Are Combined in Healthy Young Men. J Nutr. 2020;150:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Ganz AB, Shields K, Fomin VG, Lopez YS, Mohan S, Lovesky J, Chuang JC, Ganti A, Carrier B, Yan J, Taeswuan S, Cohen VV, Swersky CC, Stover JA, Vitiello GA, Malysheva OV, Mudrak E, Caudill MA. Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis. FASEB J. 2016;30:3321-3333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Christensen KE, Wu Q, Wang X, Deng L, Caudill MA, Rozen R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J Nutr. 2010;140:1736-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Ebara S. Nutritional role of folate. Congenit Anom (Kyoto). 2017;57:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Porter RK, Scott JM, Brand MD. Choline transport into rat liver mitochondria. Characterization and kinetics of a specific transporter. J Biol Chem. 1992;267:14637-14646. [PubMed] |
| 47. | Wong ER, Thompson W. Choline oxidation and labile methyl groups in normal and choline-deficient rat liver. Biochim Biophys Acta. 1972;260:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients. 2018;10:1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 49. | Ueland PM, Holm PI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med. 2005;43:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 50. | Lai Z, Chen J, Ding C, Wong K, Chen X, Pu L, Huang Q, Chen X, Cheng Z, Liu Y, Tan X, Zhu H, Wang L. Association of Hepatic Global DNA Methylation and Serum One-Carbon Metabolites with Histological Severity in Patients with NAFLD. Obesity (Silver Spring). 2020;28:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 543] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 52. | Olalekan SO, Bakare OO, Osonuga IO, Faponle AS, Adegbesan BO, Ezima EN. Gut microbiota-derived metabolites: implications for metabolic syndrome and therapeutic interventions. Egypt J Intern Med. 2024;36:72. [DOI] [Full Text] |
| 53. | Niu H, Zhou M, Zogona D, Xing Z, Wu T, Chen R, Cui D, Liang F, Xu X. Akkermansia muciniphila: a potential candidate for ameliorating metabolic diseases. Front Immunol. 2024;15:1370658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 54. | Pan Y, Yang Y, Wu J, Zhou H, Yang C. Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 2024;24:283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 55. | Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, Yeung DK, Law PT, Kwan HS, Yu J, Sung JJ, Chan HL. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8:e62885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 56. | Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 57. | Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 58. | Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, Russo R, D'Agostino G, Di Costanzo M, Canani RB, Calignano A, Meli R. Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem. 2014;25:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Ritze Y, Bárdos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, Bischoff SC. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 60. | Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 3196] [Article Influence: 399.5] [Reference Citation Analysis (0)] |
| 61. | Delzenne NM, Kok N. Effects of fructans-type prebiotics on lipid metabolism. Am J Clin Nutr. 2001;73:456S-458S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Letexier D, Diraison F, Beylot M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am J Clin Nutr. 2003;77:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a Probiotic and Metformin on Liver Aminotransferases in Non-alcoholic Steatohepatitis: A Double Blind Randomized Clinical Trial. Int J Prev Med. 2013;4:531-537. [PubMed] |
| 64. | Escouto GS, Port GZ, Tovo CV, Fernandes SA, Peres A, Dorneles GP, Houde VP, Varin TV, Pilon G, Marette A, Buss C. Probiotic Supplementation, Hepatic Fibrosis, and the Microbiota Profile in Patients with Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. J Nutr. 2023;153:1984-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Tarantino G, Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol. 2015;10:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | de Toro-Martín J, Arsenault BJ, Després JP, Vohl MC. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients. 2017;9:913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 67. | El-Sayed A, Aleya L, Kamel M. Epigenetics and the role of nutraceuticals in health and disease. Environ Sci Pollut Res Int. 2023;30:28480-28505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Li J, Xie S, Teng W. Sulforaphane Attenuates Nonalcoholic Fatty Liver Disease by Inhibiting Hepatic Steatosis and Apoptosis. Nutrients. 2021;14:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 69. | Mamun AA, Shao C, Geng P, Wang S, Xiao J. Polyphenols Targeting NF-κB Pathway in Neurological Disorders: What We Know So Far? Int J Biol Sci. 2024;20:1332-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 70. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (3)] |
| 71. | Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, Jebb SA, Aveyard P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 72. | Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Plauth M, Burgos Peláez R, Rivera Irigoin R. [ESPEN Practical Guideline: clinical nutrition in liver disease]. Nutr Hosp. 2022;39:434-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 73. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1168] [Article Influence: 584.0] [Reference Citation Analysis (1)] |
| 74. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3181] [Article Influence: 353.4] [Reference Citation Analysis (4)] |
| 75. | Xu F, Albadry M, Döding A, Chen X, Dirsch O, Schulze-Späte U, Dahmen U. The effects of saturated and unsaturated fatty acids on MASLD: a Mendelian randomization analysis and in vivo experiment. Eur J Nutr. 2024;64:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 76. | Njei B, Osta E, Njei N, Al-Ajlouni YA, Lim JK. An explainable machine learning model for prediction of high-risk nonalcoholic steatohepatitis. Sci Rep. 2024;14:8589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 77. | Buzzetti E, Linden A, Best LM, Madden AM, Roberts D, Chase TJG, Freeman SC, Cooper NJ, Sutton AJ, Fritche D, Milne EJ, Wright K, Pavlov CS, Davidson BR, Tsochatzis E, Gurusamy KS. Lifestyle modifications for nonalcohol-related fatty liver disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;6:CD013156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Haigh L, Kirk C, El Gendy K, Gallacher J, Errington L, Mathers JC, Anstee QM. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin Nutr. 2022;41:1913-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 79. | Kosmalski M, Mokros Ł. Non-Alcoholic Fatty Liver Disease in Everyday Clinical Practice: From Diagnosis to Therapy. Life (Basel). 2025;15:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 80. | Tian C, Huang R, Xiang M. SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol Res. 2024;203:107155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 81. | Huang L, Luo Y, Zhang L, Wu M, Hu L. Machine learning-based disease risk stratification and prediction of metabolic dysfunction-associated fatty liver disease using vibration-controlled transient elastography: Result from NHANES 2021-2023. BMC Gastroenterol. 2025;25:255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J Obes Metab Syndr. 2023;32:197-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 284] [Article Influence: 142.0] [Reference Citation Analysis (1)] |
| 83. | Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082-8091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 910] [Cited by in RCA: 794] [Article Influence: 72.2] [Reference Citation Analysis (8)] |
| 84. | Chandimali N, Bak SG, Park EH, Lim HJ, Won YS, Kim EK, Park SI, Lee SJ. Free radicals and their impact on health and antioxidant defenses: a review. Cell Death Discov. 2025;11:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 85. | Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135-G1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 86. | Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 87. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 734] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 88. | Webster BR, Lu Z, Sack MN, Scott I. The role of sirtuins in modulating redox stressors. Free Radic Biol Med. 2012;52:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 90. | Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 912] [Cited by in RCA: 883] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 91. | Gao Z, Zhang J, Kheterpal I, Kennedy N, Davis RJ, Ye J. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem. 2011;286:22227-22234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 92. | Svobodová G, Horní M, Velecká E, Boušová I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: a review. Arch Toxicol. 2025;99:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 93. | Clare K, Dillon JF, Brennan PN. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis of MAFLD. J Clin Transl Hepatol. 2022;10:939-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 94. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2471] [Article Influence: 164.7] [Reference Citation Analysis (2)] |
| 95. | Miyazawa T, Burdeos GC, Itaya M, Nakagawa K, Miyazawa T. Vitamin E: Regulatory Redox Interactions. IUBMB Life. 2019;71:430-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 96. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 485] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 97. | Hoofnagle JH, Van Natta ML, Kleiner DE, Clark JM, Kowdley KV, Loomba R, Neuschwander-Tetri BA, Sanyal AJ, Tonascia J; Non-alcoholic Steatohepatitis Clinical Research Network (NASH CRN). Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 98. | Abraham A, Kattoor AJ, Saldeen T, Mehta JL. Vitamin E and its anticancer effects. Crit Rev Food Sci Nutr. 2019;59:2831-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 99. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Ünalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 849] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 100. | Abdel-Maboud M, Menshawy A, Menshawy E, Emara A, Alshandidy M, Eid M. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Therap Adv Gastroenterol. 2020;13:1756284820974917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Horn P, Tacke F. Metabolic reprogramming in liver fibrosis. Cell Metab. 2024;36:1439-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 90] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 102. | Guilliams M, Scott CL. Liver macrophages in health and disease. Immunity. 2022;55:1515-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 210] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 103. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1998] [Article Influence: 249.8] [Reference Citation Analysis (0)] |
| 104. | Peleman C, Hellemans S, Veeckmans G, Arras W, Zheng H, Koeken I, Van San E, Hassannia B, Walravens M, Kayirangwa E, Beyene NT, Van Herck MA, De Vos WH, Pintelon I, van Nassauw L, Oosterlinck B, Smet A, Vits L, Dirinck E, Verrijken A, De Man J, Van Eyck A, Kwanten WJ, Vonghia L, Driessen A, Augustyns K, Toyokuni S, De Winter B, Van Steenkiste C, Francque S, Vanden Berghe T. Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease. Cell Death Differ. 2024;31:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 105. | Raza S, Rajak S, Yen PM, Sinha RA. Autophagy and hepatic lipid metabolism: mechanistic insight and therapeutic potential for MASLD. NPJ Metab Health Dis. 2024;2:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 106. | Niu MY, Dong GT, Li Y, Luo Q, Cao L, Wang XM, Wang QW, Wang YT, Zhang Z, Zhong XW, Dai WB, Li LY. Fanlian Huazhuo Formula alleviates high-fat diet-induced non-alcoholic fatty liver disease by modulating autophagy and lipid synthesis signaling pathway. World J Gastroenterol. 2024;30:3584-3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 107. | Chalasani NP, Sanyal AJ, Kowdley KV, Robuck PR, Hoofnagle J, Kleiner DE, Unalp A, Tonascia J; NASH CRN Research Group. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30:88-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 108. | Mózes FE, Lee JA, Vali Y, Alzoubi O, Staufer K, Trauner M, Paternostro R, Stauber RE, Holleboom AG, van Dijk AM, Mak AL, Boursier J, de Saint Loup M, Shima T, Bugianesi E, Gaia S, Armandi A, Shalimar, Lupșor-Platon M, Wong VW, Li G, Wong GL, Cobbold J, Karlas T, Wiegand J, Sebastiani G, Tsochatzis E, Liguori A, Yoneda M, Nakajima A, Hagström H, Akbari C, Hirooka M, Chan WK, Mahadeva S, Rajaram R, Zheng MH, George J, Eslam M, Petta S, Pennisi G, Viganò M, Ridolfo S, Aithal GP, Palaniyappan N, Lee DH, Ekstedt M, Nasr P, Cassinotto C, de Lédinghen V, Berzigotti A, Mendoza YP, Noureddin M, Truong E, Fournier-Poizat C, Geier A, Martic M, Tuthill T, Anstee QM, Harrison SA, Bossuyt PM, Pavlides M; LITMUS investigators. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 109. | Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, Anstee QM, Abdelmalek MF, Younossi Z, Baum SJ, Francque S, Charlton MR, Newsome PN, Lanthier N, Schiefke I, Mangia A, Pericàs JM, Patil R, Sanyal AJ, Noureddin M, Bansal MB, Alkhouri N, Castera L, Rudraraju M, Ratziu V; MAESTRO-NASH Investigators. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. 2024;390:497-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 731] [Article Influence: 731.0] [Reference Citation Analysis (0)] |
| 110. | Sanyal AJ, Newsome PN, Kliers I, Østergaard LH, Long MT, Kjær MS, Cali AMG, Bugianesi E, Rinella ME, Roden M, Ratziu V; ESSENCE Study Group. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N Engl J Med. 2025;392:2089-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 34] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 111. | Sterling RK, Duarte-Rojo A, Patel K, Asrani SK, Alsawas M, Dranoff JA, Fiel MI, Murad MH, Leung DH, Levine D, Taddei TH, Taouli B, Rockey DC. AASLD Practice Guideline on imaging-based noninvasive liver disease assessment of hepatic fibrosis and steatosis. Hepatology. 2025;81:672-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 112. | Raverdy V, Tavaglione F, Chatelain E, Lassailly G, De Vincentis A, Vespasiani-Gentilucci U, Qadri SF, Caiazzo R, Verkindt H, Saponaro C, Kerr-Conte J, Baud G, Marciniak C, Chetboun M, Oukhouya-Daoud N, Blanck S, Vandel J, Olsson L, Chakaroun R, Gnemmi V, Leteurtre E, Lefebvre P, Haas JT, Yki-Järvinen H, Francque S, Staels B, Le Roux CW, Tremaroli V, Mathurin P, Marot G, Romeo S, Pattou F. Data-driven cluster analysis identifies distinct types of metabolic dysfunction-associated steatotic liver disease. Nat Med. 2024;30:3624-3633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 113. | Li C, Wang Y, Bai R, Zhao Z, Li W, Zhang Q, Zhang C, Yang W, Liu Q, Su N, Lu Y, Yin X, Wang F, Gu C, Yang A, Luo B, Zhou M, Shen L, Pan C, Wang Z, Wu Q, Yin J, Hou Y, Shi Y. Development of fully automated models for staging liver fibrosis using non-contrast MRI and artificial intelligence: a retrospective multicenter study. EClinicalMedicine. 2024;77:102881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 114. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2038] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 115. | Ampuero J, Pais R, Aller R, Gallego-Durán R, Crespo J, García-Monzón C, Boursier J, Vilar E, Petta S, Zheng MH, Escudero D, Calleja JL, Aspichueta P, Diago M, Rosales JM, Caballería J, Gómez-Camarero J, Lo Iacono O, Benlloch S, Albillos A, Turnes J, Banales JM, Ratziu V, Romero-Gómez M; HEPAmet Registry. Development and Validation of Hepamet Fibrosis Scoring System-A Simple, Noninvasive Test to Identify Patients With Nonalcoholic Fatty Liver Disease With Advanced Fibrosis. Clin Gastroenterol Hepatol. 2020;18:216-225.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 116. | Fan R, Yu N, Li G, Arshad T, Liu WY, Wong GL, Liang X, Chen Y, Jin XZ, Leung HH, Chen J, Wang XD, Yip TC, Sanyal AJ, Sun J, Wong VW, Zheng MH, Hou J. Machine-learning model comprising five clinical indices and liver stiffness measurement can accurately identify MASLD-related liver fibrosis. Liver Int. 2024;44:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |