Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.107873
Revised: May 25, 2025
Accepted: July 2, 2025
Published online: August 27, 2025
Processing time: 138 Days and 5.3 Hours
Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality worldwide, necessitating innovative treatment strategies. Surgical resection and liver transplantation continue to be the gold standards for early-stage HCC; however, advances in imaging and minimally invasive techniques have improved patient selection and outcomes. Additionally, the emergence of targeted therapies and immunotherapy has transformed the treatment landscape for advanced HCC. This review highlights the efficacy of agents such as tyrosine kinase inhibitors, alongside emerging options like immune checkpoint inhibitors, which have shown promise in clinical trials. Furthermore, the role of locoregional therapies, including ablation in the setting of combined treatment, transarterial chemoembolization and transarterial radioembolization with flow catheters, cone-beam computed tomo
Core Tip: Hepatocellular carcinoma is the sixth most common cancer and is the fourth leading cause of cancer-related deaths worldwide. Its management can be challenging and relies on a multidisciplinary approach involving hepatobiliary surgeons, oncologists, hepatologists, endoscopists, interventional radiologists, radiotherapists and radiologists. This review investigates different treatments, with a special focus on most modern approaches.
- Citation: Cortese F, Anagnostopoulos F, Bazzocchi MV, Caringi S, Pisani AR, Renzulli M, Paraskevopoulos I, Laera L, Surgo A, Spiliopoulos S, Memeo R, Inchingolo R. Modern approach to hepatocellular carcinoma treatment. World J Hepatol 2025; 17(8): 107873
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/107873.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.107873
Hepatocellular carcinoma (HCC) is the predominant form of primary liver malignancies, comprising approximately 90% of all cases[1,2]. This is reflected in a 5-year survival rate of less than 20% and an incidence-to-mortality ratio of around 1:1[3,4]. On a global scale, HCC is ranked as the sixth most frequently diagnosed malignancy and a major cause of cancer-related mortality, with a marked predominance in male patients[2,5,6].
Several established risk factors contribute to the development of HCC, including chronic viral hepatitis B and C, metabolic disorders (such as diabetes mellitus, non- alcoholic fatty liver disease and non-alcoholic steatohepatitis), toxic exposures (including excessive alcohol consumption, nicotine abuse, and aflatoxins), hereditary conditions (such as hemochromatosis, α1-antitrypsin deficiency, tyrosinemia, and glycogen storage disease type 1a), and immune system-related disorders[7-9]. Regardless of the underlying cause, the progression to liver cirrhosis from chronic inflammatory damage is particularly concerning, as approximately one-third of these patients will develop HCC in their lifetime[1].
HCC’s inherent resistance to conventional treatment modalities, such as chemotherapy and radiotherapy, complicates its management[2]. However, advances in imaging techniques, minimally invasive procedures, and precision oncology are paving the way for innovative therapeutic strategies, aiming for a meaningful extension of overall and recurrence-free survival rates[3,10].
The Barcelona Clinic Liver Cancer (BCLC) model has emerged as the most validated therapeutic algorithm for HCC management, guiding tumor staging, prognosis, and treatment allocation based on incorporated evidence and expertise[6,11].
The role of surgical resection for HCC management has been heavily investigated in recent years. Several international organizations have developed comprehensive guidelines to standardize HCC treatment[12-14].
In summary, the current HCC resection guidelines highlight the importance of tailored treatment based on tumor characteristics. The most relevant factors included in the decision-making algorithm concern tumor characteristics (size and number of nodules, vascular invasion, and extra-hepatic spread), liver function [Child-Pugh, model for end lived disease (MELD)][15] and Eastern Cooperative Oncology Group performance status (ECOG PS)[16]. For major hepatectomies (≥ 3 segments), the volume and function of the future liver remnant (FLR) must also be analyzed[17]. The latter can be calculated by liver scintigraphy using 99mTc-labelled galactosyl human serum albumin[18] or 99mTc-labelled mebrofenin[19]. Computed tomography (CT) or magnetic resonance imaging (MRI) can calculate the volume, and it is necessary to have about 30% FLR in non-cirrhotic patients and at least 40% in cirrhotic patients[17].
For cirrhotic patients with HCC, resection is indicated as an alternative to liver transplantation (LT) when there is a single nodule with preserved liver function, absence of extra-hepatic spread, and ECOG PS 0. However, several studies[20-22] have shown that minimally invasive liver surgery (MILS), either laparoscopic or robotic, reduces post-operative liver failure risk, thus expanding the pool of patients with HCC who can benefit from liver resection (LR). In particular, the indications of robotic LRs (RLRs) have been expanded. Liu et al[23] demonstrated that the robotic system can be used in almost all types of LR, including minor hepatectomy, major hepatectomy, complex LR and donor hepatectomy. This is also due to the robotic system, which provides a 3D visualization of the surgery field, uses flexible robotic arms, and a tremor filter, breaking down some of the limitations of laparoscopic LR. However, lack of experience among practitioners and associated high costs limit the application of RLR[23]. However, the experience in RLR has been reported worldwide and its feasibility has gradually been accepted by clinical studies[24-26].
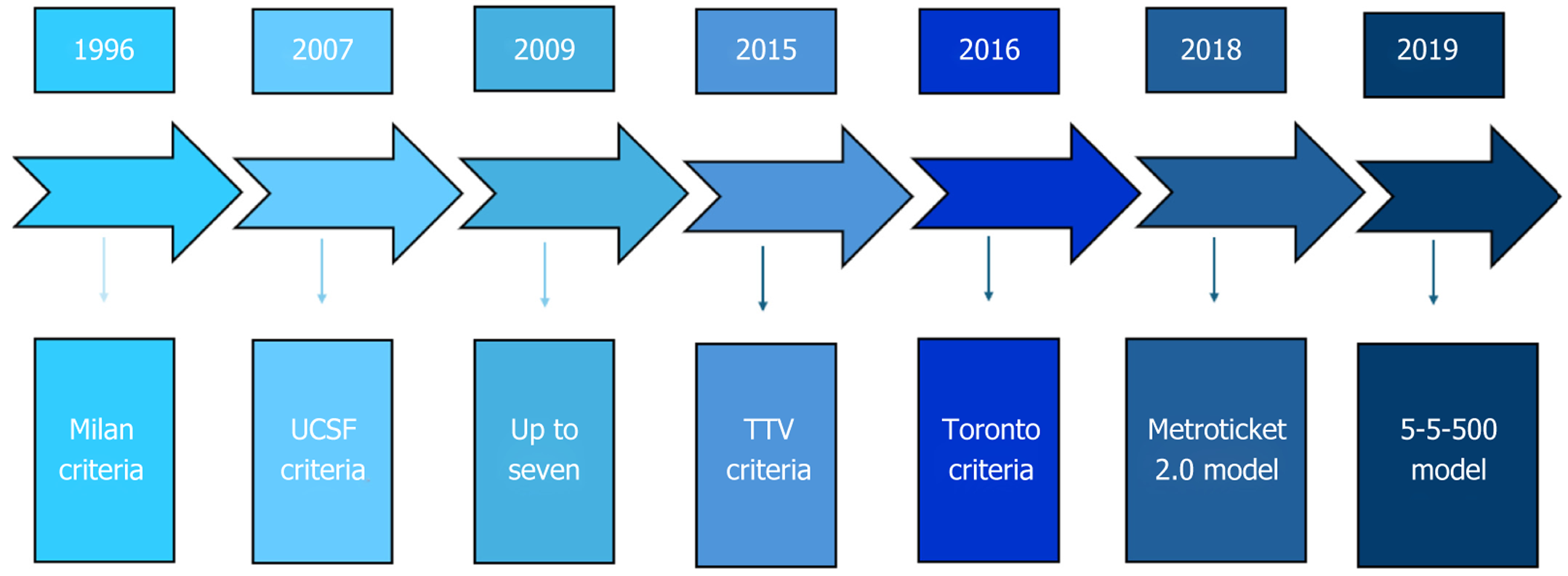
Unlike LRs, LT can treat both HCC and underlying cirrhosis. However, to be a candidate for LT, HCC must fulfill certain criteria. The first criteria to be developed were the Milan criteria (one lesion ≤ 5 cm, or three lesions ≤ 3 cm and no macrovascular invasion)[27]; these were subsequently not widely used because they were too restrictive. To date, the most widely used are the Up-to-seven criteria[28], the University of California-San Francisco criteria[29], the total tumor volume criteria[30], the Metroticket 2.0 model[31], the 5-5-500 model[32] and the Toronto criteria[33](Figure 1).
In addition, if the patient does not fit within transplantability criteria, downstaging therapy can be performed, e.g., performing a LR to bring the patient within the criteria[34,35]. The recent increase in indications for LT does not correspond to an increase in available donors, leading to HCC progression during the waiting list period; during active list maintenance checks, the patient may fall out of the transplantability criteria. For this reason, bridging therapy can be performed to reduce the risk of disease progression while the patient is on the waiting list[12].
However, approximately, one out of five patients are removed from the LT waitlist due to death or medical unsuitability[36] and this mortality continues to be partially driven by the discrepancy between organ supply and demand. The current model of organ allocation depends on the assessment of medical urgency for transplant and mortality on the waiting list using MELD-Na. Due to the paucity of organ supply, patients must often become very sick to receive a deceased donor organ. In the setting of scarce deceased donor organs, living donor LT (LDLT) represents an important alternative. Access to LDLT shortens the median waiting time and significantly decreases waitlist morbidity and mortality for all waitlisted patients[37,38]. However, the number of LDLTs has not increased much in recent years, and in the United States, they account for only 5% of LTs[36]. This is probably while donor risk is defined[39,40], there are still no adequately powered studies on the benefits of recipient survival. Howeverin their case-control study, Jackson et al[41] demonstrated how LDLT recipients gained an additional 13 to 17 Life-years compared with patients who never received an LDLT.
When comparing LR and LT, there are no unequivocal reports in the literature. Indeed, some studies have shown better survival in patients undergoing LT[42], while others have found that LRs are associated with better 5-year survival[43].
In conclusion, LT and LR, especially MILS, are curative surgical procedures. The difference lies in the fact that LR does not cure underlying liver disease, exposing the patient to a higher risk of recurrence. Conversely, LT is a more complex surgical procedure with significant operative and perioperative risks, in addition to the impact of immunosuppressive therapy. Although the criteria for inclusion on the waiting list for organ transplant are being broadened and new strategies are being developed to reduce waiting times such as bridging, downstaging and LDLT, the gap between patients on the waiting list for LT and available organs remains wide.
As reported by BCLC, locoregional therapies play a pivotal role in BCLC 0, A and B HCC[11]. In solitary HCC up to 2 cm, ablation should be considered as the first approach (evidence moderate; recommendation strong) if LT is not feasible because it is associated with similar survival outcomes to resection with fewer complications and similar life expectancy at a lower cost. Resection is favored beyond 2 cm, as ablation offers lower rate of complete responses and higher rate of local recurrences. Among ablation therapies, microwave ablation (MWA) is potentially the best option for HCC ≤ 4 cm given that it supplies more extensive tumor necrosis than radiofrequency ablation (RFA). Moreover, ablation can be used as bridging for LT candidates with expected waiting time > 6 months, as well as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE)[11,44].
If a patient with BCLC 0 or BCLC A is not a candidate for any first line treatment options, TACE can be considered as a strategy, according to the concept of treatment stage migration, recently introduced by the 2022 BCLC update. TACE is the first option in patients with BCLC B that do not meet the extended LT criteria and have preserved portal flow and defined tumor burden[11]. According to BCLC guidelines, TACE should be performed in a selective manner (evidence high; recommendation strong). Both conventional-TACE (cTACE) and drug-eluting bead TACE (DEB-TACE) have similar outcomes, but the latter has a more favorable pharmacokinetic profile and leads to less post-procedural pain[44].
Similarly to TACE, TARE is a valid option in BCLC 0 and BCLC A for single HCC up to 8 cm based on the LEGACY trial results[45,46].
Cone beam computed tomography (CBCT) is an imaging modality integrated into the angiography suite. It provides 3D images in addition to conventional 2D imaging such as fluoroscopy and conventional digital subtraction angiography (DSA)[47] (Figure 2). For transarterial procedures, CBCT can improve detection of both tumor and associated arteries to obtain safer and more efficient procedures than DSA alone, especially for small and hypovascular HCCs[48]. Moreover, patients receiving TACE with CBCT guidance have higher overall and progression-free survival (PFS) than those receiving TACE under DSA guidance alone[49].
Because intra-arterial dissemination of radioisotopes may lead to potential life-threatening adverse events, a meti
Thus, the use of CBCT is essential to optimize intra-arterial treatment and should be routinely used in the clinical practice of interventional radiology.
TARE, also known as selective internal radiotherapy (SIRT) or liver radioembolization (RE), has emerged as a significant endovascular treatment for HCC and has been endorsed by recent international guidelines[51,52] and supported by global practice patterns[53,54]. TARE is a minimally invasive procedure that involves the infusion of radioactive microspheres within the tumor via hepatic artery feeders and has been traditionally used as palliative treatment. How
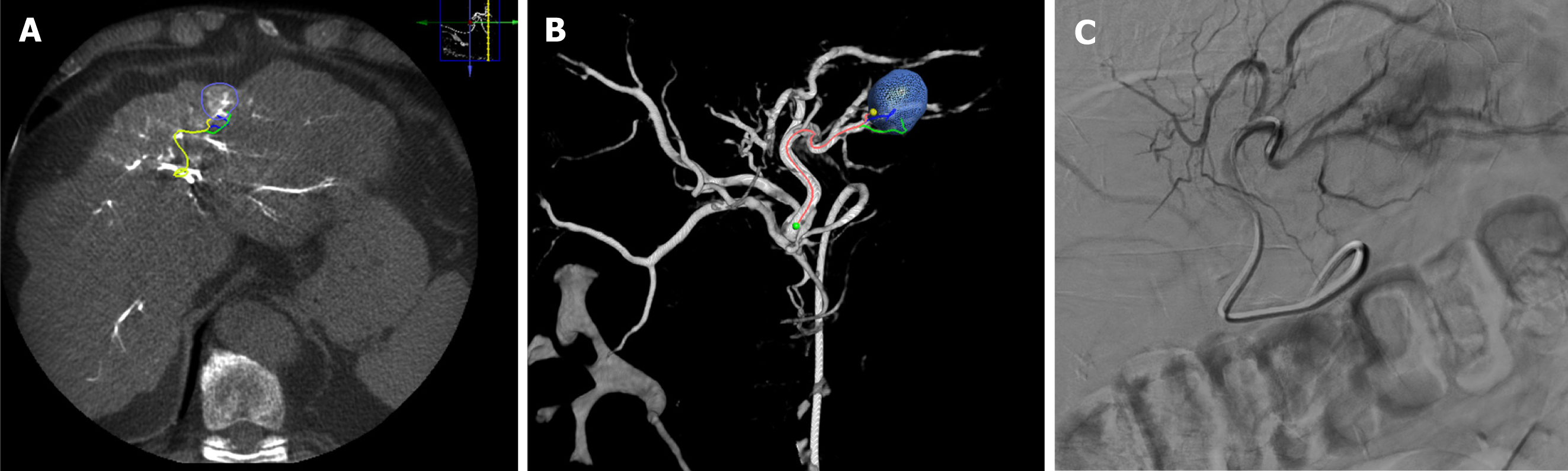
The concept of curative-intent TARE, also known as RE segmentectomy (Figure 3), was first introduced nearly 15 years ago[58] and involves catheterization of a segmental feeding artery to perform high-dose RE to selectively induce necrosis of a mass, including its relative liver segment (up to two Couinaud segments)[56,59]. The currently accepted threshold for ablative TARE using glass microspheres is ≥ 400 Gy and is based on recent pathological findings. Gabr et al[60] first suggested a higher threshold of ≥ 400 Gy for ablative targeted SIRT, as such doses demonstrated complete pathologic necrosis (CPN) without severe adverse events in 100% of the cases, compared to 65% complete necrosis using the previously proposed thresholds of 190 Gy. Subsequently, Montazeri et al[61] investigated the effect of treatment intensification on CPN in 75 HCC lesions following Y-90 glass microsphere radiation segmentectomy (RS). The authors reported that specific activity ≥ 327 Bq [area under the curve (AUC): 0.75, P < 0.001], dose ≥ 446 Gy (AUC: 0.69, P = 0.005), and treatment activity ≥ 2.55 Gbq (AUC: 0.71, P = 0.002) were predictive of CPN. Furthermore, a specific activity of ≥ 327 Bq was the sole independent predictor of CPN (P = 0.013).
Curative-intent TARE can be used in patients with: (1) Unresectable tumors: Ablative RE can be used to completely ablate the tumor and the adjacent liver segment, simulating a R0 surgical resection. According to the updated BCLC guidelines, it is indicated for unresectable/unablatable solitary ≤ 8 cm HCC lesions. It can also be used for tumor downstaging, allowing for subsequent safe and efficient surgical resection. Left liver lobe RE lobectomy can be performed with curative intent; (2) Bridge to transplantation: Ablative RE can be used to control tumor growth and improve liver function in patients with > 6 months on the waiting list for LT; and (3) Limited remnant liver volume right hepatectomy: RE lobectomy can be an alternative to portal vein embolization, as it can simultaneously induce remnant liver hyper
Based on updated evidence and expert opinions, the ideal candidates for TARE-segmentectomy are those presenting with ECOG 0-1, Child-Pugh score A cirrhosis without macrovascular invasion and an unresectable/unablatable solitary HCC tumor ≤ 8 cm in diameter. Using selective, liver-sparing approaches, ablative doses can be delivered only to the affected segment(s), while the surrounding normal liver parenchyma can be regenerated[62,63]. Moreover TARE-ablation can be performed in challenging or even inaccessible locations for percutaneous ablation, presenting high risk for in
Following the introduction of ablative SIRT in clinical practice, evidence indicating its safety and efficacy in selected HCC patients is constantly growing. In 2018, Lewandowski et al[65] published a retrospective series of 70 patients with early, solitary, ≤ 5 cm HCC who were treated with doses of > 190 Gy. The median overall survival (OS) was 6.7 years, with rates of 98%, 66% and 57% at 1-, 3- and 5-years, respectively.
Additional retrospective comparative findings suggested that ablative SIRT demonstrates equal safety and efficacy with established curative techniques such as MWA and open surgery. In a large retrospective analysis of 417 patients who underwent MWA and open surgery vs 235 patients who underwent TACE plus MWA for HCC lesions up to 3 cm, similar rates of complete response and OS were achieved[66]. Similarly, in a retrospective propensity score-matched (PSM) study comparing RS vs MWA (34 patients in each group; solitary HCC lesions < 4 cm), similar rates of liver toxicity, objective tumor response and OS were reported, while the targeted tumor mean PFS was superior in the TARE group (57.8 vs 38.6 months; P = 0.005)[67]. Impressively, in a retrospective multicenter study of 123 patients with solitary HCC ≤ 8 cm, ECOG status of 0-1, and absence of macrovascular invasion or extrahepatic disease, RS outcomes (57 patients) were compared with those of open surgical resection (66 patients). After adjusting for confounders, OS was similar in both groups, while severe adverse events (grade ≥ 3 per the Clavien-Dindo classification), were significantly higher in the surgical group (20% vs 0%; P < 0.001)[68].
In 2021, the LEGACY multicenter (three United States centers), single-arm, retrospective study reported excellent outcomes of segmental delivery of doses > 400 Gy (median dose 410 Gy) for solitary HCC ≤ 8 cm in 162 patients with Child-Pugh A cirrhosis and ECOG status 0-1. Remarkably, no cases of radiation-induced liver disease (RILD) were reported despite the high dose protocol. OS was 86.6% at 3-years and further improved to 92.8% for patients who underwent subsequent transplantation or resection. Local recurrence and severe adverse events possibly related to the procedure were both 5.6%. The authors reported that dose > 400 Gy achieved complete pathological necrosis and should be considered as the threshold dose for ablative effect using glass Y90 microspheres[50].
Moreover, the DOSISPHERE multicenter, randomized, controlled trial published in 2021 demonstrated the superiority of “personalized dosimetry” using > 205 Gy dose in tumor delivery vs “standard dosimetry” with 120 Gy lobar delivery in patients with unresectable advanced HCC with at least one lesion ≥ 7 cm (OS 26.6 months vs 10.6 months)[69].
Finally, in 2023 the RASER trial (prospective single-center, single-arm), investigated TARE segmentectomy in 29 patients with very early and early stage HCC. Initial target lesion complete response was 83% and partial response was 17%. Initial objective response was 100% and sustained complete response at 24 months was 90%[70].
Ongoing protocols are evaluating personalized dosimetry protocols to maximize RS safety and efficacy[62]. The DOORwaY90 prospective, multicenter, open-label, single arm study was designed to evaluate the safety and effectiveness of a personalized Y90 resin microsphere dosimetry approach as first-line treatment in 100 patients with unresectable and unablatable HCC. The Partition model dosimetry will be used to determine the optimal dose, and the target mean dose to tumor will be ≥ 150 Gy[71].
Recently, randomized data suggest a superior therapeutic effect using TARE (Table 1). In 2016, Salem et al[72] reported the results of the Premier randomized, phase 2, single-center study, of glass Y90 TARE with a median dose of 126 Gy vs cTACE. Although the study investigated doses below the currently suggested ablative threshold, TARE significantly prolonged time to progression. The 2022 TRACE phase II, single-center, prospective randomized, controlled trial, further supported the superiority in median OS of glass Y90 TARE using doses > 120 Gy vs doxorubicin DEB-TACE in selected patients with early or intermediate HCC, with similar rates of 30-day mortality and serious adverse events[73].
| Ref. | Main aim | Main findings |
| Salem et al[72] | Glass Y90 TARE (24 pt) vs cTACE (21 pt) | Time to progression: 26 vs 6.8 months (P = 0.0012) |
| TRACE[73] | Glass Y90 TARE (38 pt) vs DEB-TACE (34 pt) | Median OS: 30.2 months vs 15.6 months |
| Padia et al[74] | Segmental TARE (104 pt) vs TACE (138) | Overall complete response rate: 84% vs 58% (P < 0.001) |
| Median PFS: 564 days vs 271 days (P = 0.002, adjusted P > 0.001) | ||
| OS: 1198 days vs 1043 days (P = 0.35, adjusted P = 0.064) | ||
| Biederman et al[75] | Radiation segmentectomy (112 pt) vs TACE (55 pt) | Overall complete response rate: 92.1% vs 52.6% (P = 0.005) |
| Median time to secondary therapy: 812 days vs 161 days (P = 0.001) |
Further evidence on the superiority of RE segmentectomy over TACE was provided by two large retrospective PSM studies. The first, published in 2017 by Padia et al[74], investigated 104 patients with 132 tumors treated with 144 segmental RE procedures (target perfused tissue dose > 200 Gy) compared to 77 patients with 103 tumors treated with 138 chemoembolization procedures (76% DEB-TACE and 24% cTACE). Index and overall complete response rates were 92% and 84% for RE vs 74% and 58% for TACE (P = 0.001 and P < 0.001). Index tumor progression at 1 and 2 years was 8% and 15% for RE and 30% and 42% for TACE (P < 0.001). Median PFS and OS were 564 days and 1.198 days for RE vs 271 days and 1.043 days for TACE (PS-adjusted P = 0.002 and P = 0.35; censored by transplant PS-adjusted P < 0.001 and P = 0.064).
Subsequently, Biederman et al[75] reported the results of another retrospective PSM analysis of 112 patients who underwent RS vs 55 patients who underwent TACE for solitary HCC ≤ 3 cm without vascular invasion or metastasis. After PSM, the RS group showed better complete response and median time to secondary therapy, while OS was not significantly different.
Finally, although most studies investigating RS have used Y90 glass microspheres, recent retrospective data suggest that Y90 resins microspheres are equally effective. Villalobos et al[76] reported similar safety and efficacy outcomes in a retrospective study of 81 patients with HCC (20 in the resin cohort and 61 in the glass cohort). Notably, resin-based segmentectomy used lower mean tumor dose (TD) than glass-based segmentectomy (308 Gy ± 210 vs 794 Gy ± 523, P = 0.0002), while mean TD thresholds able to predict the objective and complete responses were 176 Gy and 247 Gy for resin-based segmentectomy and 290 Gy and 481 Gy for glass-based segmentectomy.
Moreover, in 2024, Sarwar et al[77] published a retrospective single-center series of 67 patients with HCC who underwent TARE segmentectomy with 90-Y resin microspheres. Doses were calculated using the single-compartment medical internal radiation dosimetry model and the median tumor absorbed dose was 232 Gy (IQR: 163-405 Gy). Median posttreatment OS was 26 months, while per-lesion and overall duration of response ≥ 1 year was 88% and 72%, res
Recent advances have significantly transformed the field of interventional radiology, enhancing precision, safety, and, consequently, patient outcomes. Among these innovations, the integration of 4D navigation systems with fusion imaging represents a cutting-edge platform designed to optimize precision and efficiency in interventional procedures. By merging 3D imaging with time-sequenced data, these systems provide dynamic, real-time visualizations of anatomical structures and procedural tools. This capability is invaluable for accurately guiding needles or catheters during complex interventions, such as tumor ablation or vascular procedures. For instance, they enable interventional radiologists to meticulously plan and monitor instrument trajectories, ensuring precise targeting and minimizing complication risks.
These novel techniques have a wide range of applications, including the treatment of HCC via both intra-arterial and percutaneous approaches. Specifically, the 4D navigation system offers a comprehensive one-room solution by integrating a CT scanner with a flat-panel angiography suite within the same interventional radiology room. The com
Several studies have demonstrated the utility of Angio-CT systems in image-guided procedures such as TACE. For instance, Piron et al[78] reported that radiation exposure during TACE varies significantly between high-end angiography suites, with lower total effective doses observed in Angio-CT suites compared to cone-beam CT suites. Creating a 3D roadmap from the celiac trunk at the beginning of the procedure provides a comprehensive visualization of liver vascularization, enabling faster tumor detection and facilitating super-selective catheterization. This approach reduces the number of required DSA runs and minimizes radiation exposure during fluoroscopy[78,79].
Emerging technologies include the integration of combined imaging modalities for the thermal ablation of hepatic tumors, offering effective solutions for treating lesions that are inaccessible with ultrasound (US)- or CT-guided approaches alone due to their localization or intrinsic characteristics. Ablation is a minimally invasive technique routinely performed as a standalone procedure or in combination with surgery[80]. For example, Hermida et al[81] proposed a method for the percutaneous thermal ablation of HCC located in the hepatic dome, a challenging region to access with US guidance, where lung interposition often compromises tumor visibility and accessibility. Their study demonstrated the efficacy and safety of CT-guided lesion ablation combined with CO2-induced artificial pneumothorax. Additional applications of fusion imaging in the thermal ablation of liver lesions include "lipiodol tagging," which involves selective intra-arterial lipiodol injection into tumors, thereby enhancing the visibility of lesions that are difficult to detect using US guidance alone[82]. For hypovascular lesions, which appear as hypoattenuating nodules on CT during the portal phase, CT-portography offers a viable solution. CT arterial portography or CT hepatic arteriography enables multiple contrast medium injections through the superior mesenteric artery, proving highly effective for identifying hypovascular lesions in challenging locations. This technique facilitates precise, real-time intraprocedural needle placement, significantly improving the accuracy and safety of ablation procedures[81,83].
In summary, Angio-CT systems significantly enhance procedural precision, minimize radiation exposure, and optimize the management of complex cases, making them particularly beneficial for training and supporting young interventional radiologists.
Among the emerging technologies in intra-arterial therapies, Balloon-Occluded TACE (B-TACE) has emerged as a pivotal advancement.
TACE is well-established as one of the primary treatments for intermediate-stage HCC and is currently recommended for patients with HCC in BCLC stages 0, A, and B[11,84]. It is also highlighted in the recent European Association for the Study of the Liver guidelines, where it is described as a "disease control" strategy rather than a definitive treatment[51].
This limitation arises from the lack of standardization in the technique: Various chemotherapeutic agents (such as Doxorubicin, Idarubicin, Farmorubicin, etc.) can be used, and different procedural approaches are available, including cTACE or DEB-TACE[85]. To date, no clear evidence has demonstrated the superiority of one chemotherapeutic agent over another or one embolization technique over another. In terms of response rate, Veloso Gomes et al[86] analyzed 580 patients treated with DEB-TACE, reporting a response rate of 60.14% after one or more procedures. Similarly, in a large study by Peng et al[87] among 699 patients who underwent one or multiple sessions of cTACE or DEB-TACE, a complete response rate of 22.3% was observed.
To overcome these limitations, it would be advisable to standardize this technique across all its phases, including the execution method, product type, drug selection, and embolization approach.
In this context, B-TACE has emerged as a promising technique. Its encouraging results suggest the potential to achieve at least partial standardization of the TACE procedure. B-TACE is a variant of the TACE procedure that employs a balloon microcatheter for arterial occlusion. First described in 2013[88], it is not yet included in clinical guidelines[51] and its specific indications remain undefined. However, B-TACE is generally characterized by the infusion of a chemotherapeutic agent and lipiodol emulsion, followed by gelfoam administered under the occlusion of feeding arteries by a balloon.
The placement of the microballoon (e.g., Occlusafe, Terumo®) proximal to all feeders maximizes therapeutic efficacy by temporarily occluding the feeding artery. This induces flow redistribution towards lower-resistance vascular territories and facilitates a pressure-gradient-driven chemoembolization. The drug-embolic mixture can be forcefully delivered into tumor vessels, ensuring higher drug accumulation, filling arterioportal microanastomoses, and achieving greater rates of complete tumor response. This approach has been particularly documented in cases where the balloon-occluded arterial stump pressure is 64 mmHg or lower and where significant collateral arteries are absent[89]. Additionally, the balloon minimizes the risk of leakage outside the target nodule, preventing backflow and non-target embolization[90].
B-TACE has demonstrated superior oncological outcomes compared to DEB-TACE in terms of response rate and longer time to progression, with no significant differences in adverse events, even in patients with larger tumors[91]. While there is no clear consensus on the optimal reference diameter for achieving the best outcomes with B-TACE, a 2021 study highlighted its superiority over cTACE for treating HCCs with diameters between 30-50 mm in a single session[92]. Similarly, in 2023, Chu et al[93] reported that B-TACE achieved superior local tumor progression control compared to cTACE in HCCs with diameters greater than 3 cm.
Recently, Lucatelli et al[94] performed a multicenter European study and confirmed the safety and efficacy of B-TACE, reporting a target complete response of 58.9% at 6 months with a mean lesion diameter of 37 mm. These findings are comparable to the response rate (60.14%) reported by Veloso Gomes et al[86].
Future directions include the potential standardization of this aspect of the procedure, which could pave the way for further studies aimed at standardizing additional procedural steps, ultimately ensuring greater consistency and reproducibility in interventional radiology practices for HCC management. Alongside B-TACE, advancements in interventional radiology, such as 4D navigation, fusion imaging, and Angio-CT, have significantly improved precision, safety, and oncological outcomes, heralding a promising future for minimally invasive therapies in HCC management.
Although the combined locoregional treatments are not included in the BCLC guidelines[11], they are widely applied by interventional radiologists in clinical practice in HCC treatment.
The most widespread strategy is based on the combination of the percutaneous approach, such as RFA or MWA, and the intra-arterial approach, such as TACE, especially in the treatment of HCC larger than 3 cm[95-97].
Given that ablation therapies are curative for very early and early HCC and are negatively affected by the heat sink effect and because the tumor necrosis rate of TACE for intermediate HCC is low and the recurrence rate is high, combined therapies could be effective than single approaches[98,99].
A common combination is TACE + RFA, with two variants: TACE followed by RFA or RFA followed by TACE, each with different rationales. However, it is not clear which is superior[98]. Moreover, there is no clear consensus about time interval between TACE and RFA. Iezzi et al[98] recommended a single-step “combined” approach to amplify the synergistic effects of RFA and TACE, leading to reduction of hospitalization days, decrease in patient discomfort and cost. Jiang et al[95] recently confirmed that TACE combined with RFA was more effective for HCC than TACE alone. For patients with a tumor larger than 3 cm, the combined treatment also achieved better results than RFA alone.
According to two meta-analyses, including 1736[100] and 1799[101] patients, combination therapy with TACE and MWA is better than TACE alone in HCCs > 3 cm regarding OS, PFS and treatment response.
In 2020, Iezzi et al[102] first described a new combined single-step therapy with balloon-occluded MWA followed by b-TACE in patients with unresectable single HCC. Selective catheterization of the target vascular segment was performed by advancing a 0.014-in hydrophilic guidewire and a 2.8Fr microcatheter with an occlusion-balloon on the tip; the 10 mm long micro-balloon is made of compliant polyurethane and has a diameter of 1-4 mm according to inflation volume. Thereafter, the balloon was inflated to occlude the flow and obtain an arterial stump pressure drop. Ablation was then performed using a14-G uncooled 2.450 MHz MWA system up to 40 W of applied power for 10 minutes with the balloon still inflated. After ablation, B-TACE was performed using 2 mL of 100-micron calibrated drug eluting-microspheres loaded with 50 mg doxorubicin mixed with 5 mL contrast medium and 3 mL distilled water. They treated five patients with good results. Technical success was obtained in all procedures and no residual tumor or local recurrence was registered at the 6-month CT follow-up. This combined approach has been strengthened by two multicenter studies, demonstrating a complete response, respectively, in 91, 3% at 1 month and in 85.7% at 3-6 months[103] and 79.4% a 1 month and 81.4% at 3-6 months[104]. The initial diameter of the lesion ≥ 5.0 cm has been proven to be as the sole independent prognostic factor for loss of complete response[104].
A recent and interesting meta-analysis demonstrated that for lesions > 5 cm, TACE + cryoablation (CRA) combination therapy has a significant decrease in alpha-fetoprotein (AFP) levels, a significant increase in CD4+ T cells and a significant decrease in CD8+ T cells compared to TACE alone, indicating that it is more beneficial for protecting or enhancing immune function. Moreover, TACE + CRA enhances the therapeutic efficacy and long-term survival rate, without increasing the risk of complications[105].
Systemic treatment of HCC has seen significant advances over the past decade. The introduction of tyrosine kinase inhibitors, immune checkpoint inhibitors (ICIs), and combination therapies has improved OS and quality of life for patients with advanced HCC. Systemic therapy is advised for patients with intermediate-stage disease who are either not candidates for or have not responded to local treatments (BCLC B), as well as for patients with advanced HCC and preserved liver function (BCLC C).
The treatment for HCC is performed using immune therapies (designed to trigger an immune response against the tumor or enhance an ongoing immune reaction) or targeted therapies (which involve drugs that focus on specific molecular pathways crucial for HCC cell proliferation). The use of systemic cytotoxic drugs (which indiscriminately kill dividing cells) has not been beneficial in clinical trials. Immune checkpoints include co-inhibitory molecules expressed by effector lymphocytes that prevent excessive activation. ICIs block this effect and rejuvenate effector cells. ICIs used in randomized clinical trials (RCTs) target PD-1 (nivolumab, pembrolizumab, sintilimab) or its ligand PD-L1 (atezolizumab, durvalumab, tislelizumab) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4, ipilimumab, tremelimumab). Vascular endothelial growth factor (VEGF) is produced within the tumor microenvironment by tumor or stromal cells. It promotes tumor growth by stimulating angiogenesis and also has several immune-modulating effects, such as inhibiting dendritic cell activity or the creation of immunosuppressive myeloid-derived stromal cells and regulatory T cells, among other mechanisms. Anti-VEGF drugs may counteract these effects by binding to circulating VEGF (bevacizumab), or by specifically blocking its cellular receptors like VEGFR-2 (rivoceranib and ramucirumab). Targeted treatments used for HCC include multi- tyrosine kinase inhibitors that share an anti-angiogenic effect through inhibition of VEGF and platelet-derived growth factor receptors and vary in their inhibition of molecules involved in different molecular pathways (such as RAF, FGFR, RET, KIT, TIE2, or MET). Due to their multiple mechanisms of action, the effects of multi-TKIs on the immune system are not completely understood.
Combining immunotherapies and TKIs forms the cornerstone of systemic therapy in HCC. Their benefit has been confirmed in large-scale RCTs with control arms that have evolved over time. When making treatment choices, direct cross-trial comparisons might lead to inaccurate conclusions, so physicians should carefully consider the patient's profile, any relative contraindications, and the availability of treatments, always discussing these factors with the patient and, once again, in multidisciplinary team setting[51,106].
Based on the findings of the global IMbrave 150[107] and HIMALAYA trials[108], combination therapies involving PD-1 or PD-L1 inhibitors should be considered the first-line standard of care for patients without contraindications to ICIs (and bevacizumab). There is no evidence suggesting that one option is preferable over the other. Sorafenib and lenvatinib remain first-line choices for these patients, and single-agent durvalumab and tislelizumab can also be considered.
The IMbrave150 trial, which assessed the combination of atezolizumab and bevacizumab (A-B) in patients with unresectable HCC, was the first Phase III study to show survival benefit for any treatment over sorafenib. The median OS was 19.2 months for the (A-B) group, compared to 13.4 months with sorafenib (HR: 0.66). Combination therapy achieved an objective response rate (ORR) of 30%, while sorafenib had an ORR of 11%, with (A-B) showing an 8% complete response rate vs < 1% for sorafenib. Additionally, there was a significant delay in the decline of quality-of-life measures in the (A-B) group compared to the sorafenib group[107].
HIMALAYA was the first study to demonstrate the effectiveness of dual ICI therapy. It compared durvalu
The global Phase III CheckMate-9DW study assessed nivolumab–ipilimumab vs lenvatinib (administered to 90% of patients) or sorafenib. The results showed that OS was improved with nivolumab–ipilimumab compared to TKIs (median OS of 23.7 months vs 20.6 months; HR: 0.79). TRAEs of any grade occurred in 84% of patients receiving nivolumab-ipilimumab and 91% of patients receiving TKI[110].
The Phase III RATIONALE-301 trial compared tislelizumab, a monoclonal antibody with strong affinity and specificity for PD-1, to sorafenib. The primary non-inferiority endpoint was achieved, with a median OS of 15.9 months for tislelizumab vs 14.1 months for sorafenib (HR: 0.85). The ORR was 14.3% for tislelizumab. Tislelizumab was associated with a lower incidence of TRAEs, leading to discontinuation[111].
Lenvatinib was compared to sorafenib as first-line treatment in the open-label, global, Phase III REFLECT trial. The primary endpoint was achieved, showing non-inferiority for lenvatinib with a median OS of 13.6 months, compared to 12.3 months for sorafenib (HR: 0.92). However, the secondary endpoints favored lenvatinib, with a higher ORR of 24% vs 9% (by mRECIST) and improved PFS of 7.4 months vs 3.7 months[112]. Lenvatinib was also studied in combination with TACE in the open-label LAUNCH trial in China, (median OS 17.8 months for TACE-lenvatinib, vs 11.5 months for lenvatinib)[113].
Sorafenib was the first systemic therapy to show a survival benefit in advanced HCC in a placebo-controlled Phase III trial[114]. A subsequent confirmatory trial in Asia, which primarily included patients with a history of HBV infection, yielded a similar HR favoring sorafenib[115].
The Phase III RESORCE trial compared regorafenib to placebo in patients who had progressed on sorafenib but had tolerated a dose of ≥ 400 mg daily for at least 20 out of the 28 days prior to discontinuation. Regorafenib showed improved OS with a median of 10.6 months compared to 7.8 months for placebo (HR: 0.63)[116]. Cabozantinib was compared to placebo in the global Phase III CELESTIAL trial involving patients who had received one or two prior treatments, including sorafenib. The median OS was better in the cabozantinib group (10.2 months vs 8.0 months with placebo; HR: 0.63). The ORR for cabozantinib was 4% by RECIST v1.1[117]. Cabozantinib was later included in the COSMIC-312 trial, which compared first-line cabozantinib to sorafenib, with cabozantinib being assessed as a secondary endpoint[118]. The final analysis showed a median PFS of 5.8 months for cabozantinib vs 4.3 months for sorafenib. However, due to the lack of data showing non-inferior survival, first-line cabozantinib is not currently recommended[119].
The Phase III REACH trial did not find that second-line ramucirumab was superior to placebo in advanced HCC, but a subgroup analysis indicated a potential benefit for patients with serum AFP ≥ 400 ng/mL[120]. The subsequent REACH-2 trial focused on patients with AFP ≥ 400 ng/mL. The results showed a significant improvement in OS with ramucirumab (median 8.5 months vs 7.3 months with placebo; HR: 0.71), although OS in both groups was relatively low, reflecting the poor prognosis associated with elevated AFP levels[121].
Among the local treatment strategies, external beam conformal radiotherapy was historically considered a palliative treatment, mainly linked to the risk of RILD[122,123].
Stereotactic body radiotherapy (SBRT) is a targeted radiation therapy that delivers precise high radiation doses to specific targets in a limited number of fractions in a non-invasive manner, minimizing exposure to surrounding healthy tissue[124-126].
In the last decade, several studies have demonstrated that SBRT is endorsed as a safe and effective locoregional treatment, especially for patients with inoperable localized or recurrent HCC. It can also serve as bridging or down
The reported rates of LC at 1 year and 2 years after SBRT range from 87% to 97% in this patient population[129-131]. Biologically effective dose assuming an α/β = 10 (BED10) ≥ 100 Gy is associated with significant LC[132]. A propensity score analysis of a retrospective single-institution cohort demonstrated durable LC rates of 97% at 1 year and 91% at 2 years for patients with one or two unresectable HCC tumors[133].
In the randomized multi-center phase 2 TRENDY trial, patients ineligible for RFA or additional surgery showed time to local recurrence of over 40 months following SBRT[134].
The RTOG 1112 study, a multi-center phase 3 randomized trial involving 177 patients with HCC who were unfit for transplantation, resection, RFA, or TACE, found that SBRT before Sorafenib resulted in median OS of 15.8 months, compared to 12.3 months for those who did not receive SBRT. PFS was significantly better with SBRT, showing 9.2 months vs 5.5 months. These patients had very advanced disease: 82% were classified as BCLC-C, 74% had MVI, 4% had metastases, and patients had at least five tumors[135].
Retrospective and prospective studies show that SBRT has low > G3 toxicity, ranging from 0% to 8%[133,134]. High quality-of-life scores were also reported, particularly regarding post-treatment improvements in pain[136].
An interesting topic could be the exploration of the immunomodulatory properties of SBRT combined with immunotherapy to improve local and systemic control of HCC. Preclinical studies are needed to understand the synergies between SBRT and immunotherapy, including optimal treatment volumes, dosages, predictive biomarkers, and the tumor microenvironment's role[137]. One example is Personalized Ultrahigh Fraction Adaptive Stereotactic Radiotherapy (PULSAR), which delivers high-dose radiation fractions weeks apart to allow for biological changes. When paired with immunotherapy, PULSAR may enhance anatomical adaptation and systemic response[138,139].
Deriving data about the use of SBRT as a bridge to transplant, recent studies have shown that SBRT could be considered in neoadjuvant treatment strategies for patients with locally advanced HCC[136,140]. A randomized controlled trial involving 164 patients with resectable HCC and tumor thrombus in the main trunk of the portal vein or its side branches compared two patient groups and found significant improvements in 12-month postoperative OS, HCC-related mortality, and rates of HCC recurrence[141].
The prospective phase 2 START-FIT study showed that a neoadjuvant regimen including TACE (day 1), followed by SBRT (day 28) and then Avelumab (14 days after SBRT), converted 55% of patients with initially unresectable, locally advanced HCC to curative treatment[142].
New therapeutic approaches are emerging in SBRT for HCC, such as stereotactic magnetic resonance-guided adaptive radiotherapy (SMART) and intensity-modulated stereotactic body proton therapy (SBPT).
SBRT targeting abdominal sites has limitations concerning the control of target motion and the daily reproducibility of organs at risk (OARs). Consequently, uncertainties during the planning and administration of SBRT restrict the safe delivery of ablative doses to tumors located near hepatobiliary and luminal OARs.
The SMART technique is useful in mitigating these uncertainties by employing on-table MRI from an MR linear accelerator for daily anatomical and positional adaptation, and movement management. This method enables a reduction in planning target volumes and allows for the assessment of dose escalation to improve LC[143]. Single-arm prospective and retrospective studies of SMART HCC, cholangiocarcinoma, and liver metastases have indicated a 2-year LC rate ranging from 73% to 100% and G3 toxicity ranging from 0% to 8% (no G4 toxicity was observed)[144].
Proton particles can help protect the uninvolved liver, reducing the risk of hepatic toxicity from radiotherapy and encouraging dose escalation[145]. Ongoing trials of SBPT for HCC (NCT04805788) are assessing the reduction of toxicity and the gain in oncologic outcomes.
Growing data on single-fraction SBRT of liver metastases reveal low toxicity and excellent control with doses of 18-40 Gy[146,147]. We await long-term results from larger series to ensure efficacy and safety.
Well-designed, multi-center RCTs are required to compare the safety and efficacy of ablative TARE with other curative-intent locoregional and surgical treatment options, as to provide solid data on its use in earlier-stage therapeutic lines. Additionally, defining the optimal dose for segmentectomy in various available TARE systems is imperative for further enhancing therapeutic efficacy[148]. Pressure-assisted microcatheters could also be used for more aggressive TACE/TARE but also as an occlusive tool to avoid non-selective delivery[94,149].
The combination of systemic and locoregional treatment modalities such as intraarterial treatments (TACE or TARE) plus immunotherapy strategies like checkpoint inhibitors or adoptive T-cell therapy[150], or between different locoregional treatments such as percutaneous ablation plus TACE or BTACE (B-TACE segmentectomy), have already provided optimistic initial results and are currently under further investigation[94,151].
The possibility of the abscopal effect following locoregional treatments is also a very promising concept under investigation. According to limited data, even subtherapeutic locoregional treatments for HCC could prime immune cells against tumor antigens to induce disease regression, but also enhance the effect of systemic immunotherapy against HCC. Such an effect could revolutionize treatment capabilities in poor prognosis patients with advanced and/or metastatic HCC[152-154]. More data are awaited soon regarding the synergistic effect or locoregional and systemic immunotherapy[155,156] or intra-lesional delivery of immunotherapy as sole treatment or combined with intraarterial therapy[157-159].
Personalized therapies are also proposed to improve outcomes. Recently, the molecular and immune classification of HCC distinguished two major molecular groups based on transcriptomic-based phenotypic classes (proliferation and non-proliferation class). The proliferation class is characterized by more aggressive tumors with poor histological differentiation, high vascular invasion and increased AFP[160]. Specific biomarkers such as circulating miRNAs and genomic profiling from ongoing translational studies could be used to guide personalized therapeutic protocols and select patients most likely to benefit from a given regimen. Tissue samples or biological fluids (blood, urine etc.) can undergo molecular analysis via omics-based methodologies to obtain molecular-based signatures for HCC recurrence. Omics signatures and clinical/pathological features could generate predictive systems and enable personalized treatment based on the mole
More recently, AI deep learning models have been proposed to assist in patient selection, lesion characterization and to optimize personalized treatment protocols[162]. AI could be used in the near future to improve patient selection decision-trees and issues on optimal dosimetry and accurate pre- and post-procedural imaging.
Future research on synergistic therapies and personalized treatment protocols should address numerous issues including the nature, timing and sequence of immunotherapy, and locoregional treatments to warrant the best clinical outcomes, as well as the biological and genetic biomarkers to recognize patients likely to benefit from such combined approaches.
Technological advancements in fusion imaging and 4D-CT systems should also be used to improve numerous parameters that affect the outcomes of locoregional treatments such as target lesion(s) identification, intraprocedural guidance, treatment delivery, and immediate outcome assessment, and at the same time reduce radiation exposure and contrast use[163].
The reduction of radiation exposure and technical accuracy could also be enhanced with the use of novel robotic systems designed for peripheral endovascular procedures. Robotic endovascular treatments are a promising and appealing technology and is expected to transform clinical practice in the next decade[164].
Improved, personalized dosimetry is also a major investigational concern for TARE procedures. Dosimetry using intraprocedural cone-beam CT, SPET-CT dosimetry calculating the perfused -and not necrotic- mass volume, as well as 99mTc-MAA-based dosimetry using dedicated software and AI options are anticipated to further increase the safety and effectiveness of TARE[165-167].
Future improvements in dosimetry using MRI-based dosimetry are also awaited for Ho-166 TARE[168].
Additional data highlighting specific characteristic and advantages of Holmium (MRI visibility, 166Ho scout dose possibility) and more recently developed TARE platforms such as imageable RE microspheres (radiopaque Eye90 microspheresTM), and Rhenium-188 [tungsten-188/rhenium-188 (W-188/Re-188) generator for onsite dose generation], will most likely increase their clinical penetration in the near future[169,170].
Finally, nanotechnology may offer new approaches to target HCC cells and can be employed for the optimization of intraarterial drug-delivery. Specifically, nanoparticles are currently under investigation as drug-carriers for local chemotherapy, or gene therapy agents. Nanoparticles functionalized with specific HCC-target ligands, could increase intratumoral drug accumulation. Moreover, nanoparticles allow the co-encapsulation of multiple drugs within a single nanoparticle and therefore enable a more homogeneous multi-drug DEB-TACE[171].
This review provides a comprehensive examination of current and emerging HCC treatment strategies and future directions in this intricate and multidisciplinary field. Emphasis is placed on the role of intraprocedural cross-sectional imaging-guided locoregional therapies, the effectiveness of targeted therapies, and the identification of novel biomarkers for both prognostic assessment and prediction of treatment response.
| 1. | Tümen D, Heumann P, Gülow K, Demirci CN, Cosma LS, Müller M, Kandulski A. Pathogenesis and Current Treatment Strategies of Hepatocellular Carcinoma. Biomedicines. 2022;10:3202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 2. | Chakraborty E, Sarkar D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers (Basel). 2022;14:2798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 204] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 3. | Singal AG, Kudo M, Bruix J. Breakthroughs in Hepatocellular Carcinoma Therapies. Clin Gastroenterol Hepatol. 2023;21:2135-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 4. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109:djx030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1111] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 5. | Daher S, Massarwa M, Benson AA, Khoury T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J Clin Transl Hepatol. 2018;6:69-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, Pawlik TM. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023;158:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 363] [Reference Citation Analysis (1)] |
| 7. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4267] [Article Influence: 237.1] [Reference Citation Analysis (2)] |
| 8. | Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 533] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 9. | Rich NE, Yopp AC, Singal AG, Murphy CC. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin Gastroenterol Hepatol. 2020;18:242-248.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1228] [Article Influence: 409.3] [Reference Citation Analysis (41)] |
| 11. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2612] [Article Influence: 870.7] [Reference Citation Analysis (59)] |
| 12. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 750] [Article Influence: 375.0] [Reference Citation Analysis (23)] |
| 13. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 449] [Article Influence: 449.0] [Reference Citation Analysis (1)] |
| 14. | Hasegawa K, Takemura N, Yamashita T, Watadani T, Kaibori M, Kubo S, Shimada M, Nagano H, Hatano E, Aikata H, Iijima H, Ueshima K, Ohkawa K, Genda T, Tsuchiya K, Torimura T, Ikeda M, Furuse J, Akahane M, Kobayashi S, Sakurai H, Takeda A, Murakami T, Motosugi U, Matsuyama Y, Kudo M, Tateishi R; committee for Revision of the Clinical Practice Guidelines for Hepatocellular Carcinoma, Tokyo, Japan. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol Res. 2023;53:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 15. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 16. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 17. | Primavesi F, Maglione M, Cipriani F, Denecke T, Oberkofler CE, Starlinger P, Dasari BVM, Heil J, Sgarbura O, Søreide K, Diaz-Nieto R, Fondevila C, Frampton AE, Geisel D, Henninger B, Hessheimer AJ, Lesurtel M, Mole D, Öllinger R, Olthof P, Reiberger T, Schnitzbauer AA, Schwarz C, Sparrelid E, Stockmann M, Truant S, Aldrighetti L, Braunwarth E, D'Hondt M, DeOliveira ML, Erdmann J, Fuks D, Gruenberger T, Kaczirek K, Malik H, Öfner D, Rahbari NN, Göbel G, Siriwardena AK, Stättner S. E-AHPBA-ESSO-ESSR Innsbruck consensus guidelines for preoperative liver function assessment before hepatectomy. Br J Surg. 2023;110:1331-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Mizutani Y, Hirai T, Nagamachi S, Nanashima A, Yano K, Kondo K, Hiyoshi M, Imamura N, Terada T. Prediction of Posthepatectomy Liver Failure Proposed by the International Study Group of Liver Surgery: Residual Liver Function Estimation With 99mTc-Galactosyl Human Serum Albumin Scintigraphy. Clin Nucl Med. 2018;43:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Smet H, Martin D, Uldry E, Duran R, Girardet R, Schaefer N, Prior JO, Denys A, Halkic N, Demartines N, Melloul E. Tc-99m mebrofenin hepatobiliary scintigraphy to assess future liver remnant function before major liver surgery. J Surg Oncol. 2023;128:1312-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Di Benedetto F, Magistri P, Di Sandro S, Sposito C, Oberkofler C, Brandon E, Samstein B, Guidetti C, Papageorgiou A, Frassoni S, Bagnardi V, Clavien PA, Citterio D, Kato T, Petrowsky H, Halazun KJ, Mazzaferro V; Robotic HPB Study Group. Safety and Efficacy of Robotic vs Open Liver Resection for Hepatocellular Carcinoma. JAMA Surg. 2023;158:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 21. | Hobeika C, Nault JC, Barbier L, Schwarz L, Lim C, Laurent A, Gay S, Salamé E, Scatton O, Soubrane O, Cauchy F. Influence of surgical approach and quality of resection on the probability of cure for early-stage HCC occurring in cirrhosis. JHEP Rep. 2020;2:100153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Azoulay D, Ramos E, Casellas-Robert M, Salloum C, Lladó L, Nadler R, Busquets J, Caula-Freixa C, Mils K, Lopez-Ben S, Figueras J, Lim C. Liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. JHEP Rep. 2021;3:100190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Liu R, Abu Hilal M, Wakabayashi G, Han HS, Palanivelu C, Boggi U, Hackert T, Kim HJ, Wang XY, Hu MG, Choi GH, Panaro F, He J, Efanov M, Yin XY, Croner RS, Fong YM, Zhu JY, Wu Z, Sun CD, Lee JH, Marino MV, Ganpati IS, Zhu P, Wang ZZ, Yang KH, Fan J, Chen XP, Lau WY. International experts consensus guidelines on robotic liver resection in 2023. World J Gastroenterol. 2023;29:4815-4830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 24. | Kam JH, Goh BK, Chan CY, Wong JS, Lee SY, Cheow PC, Chung AY, Ooi LL. Robotic hepatectomy: initial experience of a single institution in Singapore. Singapore Med J. 2016;57:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Choi GH, Chong JU, Han DH, Choi JS, Lee WJ. Robotic hepatectomy: the Korean experience and perspective. Hepatobiliary Surg Nutr. 2017;6:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Goja S, Singh MK, Vohra V, Soin AS. Robotic Left Hepatectomy: a Case Report (First Reported Case of Robotic Hepatectomy in India). Indian J Surg. 2015;77:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5312] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 28. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1573] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 29. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 432] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, Kneteman NM. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. 2015;62:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 31. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 32. | Shimamura T, Akamatsu N, Fujiyoshi M, Kawaguchi A, Morita S, Kawasaki S, Uemoto S, Kokudo N, Hasegawa K, Ohdan H, Egawa H, Furukawa H, Todo S; Japanese Liver Transplantation Society. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: the 5-5-500 rule - a retrospective study. Transpl Int. 2019;32:356-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 34. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 35. | Seehofer D, Petrowsky H, Schneeberger S, Vibert E, Ricke J, Sapisochin G, Nault JC, Berg T. Patient Selection for Downstaging of Hepatocellular Carcinoma Prior to Liver Transplantation-Adjusting the Odds? Transpl Int. 2022;35:10333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 36. | Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Booker SE, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021;21 Suppl 2:208-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 37. | Shah SA, Levy GA, Greig PD, Smith R, McGilvray ID, Lilly LB, Girgrah N, Cattral MS, Grant DR. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant. 2007;7:998-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Berg CL, Gillespie BW, Merion RM, Brown RS Jr, Abecassis MM, Trotter JF, Fisher RA, Freise CE, Ghobrial RM, Shaked A, Fair JH, Everhart JE; A2ALL Study Group. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Abecassis MM, Fisher RA, Olthoff KM, Freise CE, Rodrigo DR, Samstein B, Kam I, Merion RM; A2ALL Study Group. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant. 2012;12:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Cheah YL, Simpson MA, Pomposelli JJ, Pomfret EA. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl. 2013;19:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 41. | Jackson WE, Malamon JS, Kaplan B, Saben JL, Schold JD, Pomposelli JJ, Pomfret EA. Survival Benefit of Living-Donor Liver Transplant. JAMA Surg. 2022;157:926-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 42. | Koh JH, Tan DJH, Ong Y, Lim WH, Ng CH, Tay PWL, Yong JN, Muthiah MD, Tan EX, Pang NQ, Kim BK, Syn N, Kow A, Goh BKP, Huang DQ. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr. 2022;11:78-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Koniaris LG, Levi DM, Pedroso FE, Franceschi D, Tzakis AG, Santamaria-Barria JA, Tang J, Anderson M, Misra S, Solomon NL, Jin X, DiPasco PJ, Byrne MM, Zimmers TA. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254:527-37; discussion 537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6060] [Article Influence: 865.7] [Reference Citation Analysis (3)] |
| 45. | Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021;74:2342-2352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 326] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 46. | Lucatelli P, Guiu B. 2022 Update of BCLC Treatment Algorithm of HCC: What's New for Interventional Radiologists? Cardiovasc Intervent Radiol. 2022;45:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Bapst B, Lagadec M, Breguet R, Vilgrain V, Ronot M. Cone Beam Computed Tomography (CBCT) in the Field of Interventional Oncology of the Liver. Cardiovasc Intervent Radiol. 2016;39:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Pung L, Ahmad M, Mueller K, Rosenberg J, Stave C, Hwang GL, Shah R, Kothary N. The Role of Cone-Beam CT in Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. J Vasc Interv Radiol. 2017;28:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012;81:3985-3992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Lucatelli P, Corona M, Argirò R, Anzidei M, Vallati G, Fanelli F, Bezzi M, Catalano C. Impact of 3D Rotational Angiography on Liver Embolization Procedures: Review of Technique and Applications. Cardiovasc Intervent Radiol. 2015;38:523-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. 2025;82:315-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 52. | Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M; ESMO Guidelines Committee. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 300] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 53. | Keane G, Lam M, Braat A, Bruijnen R, Kaufmann N, de Jong H, Smits M. Transarterial Radioembolization (TARE) Global Practice Patterns: An International Survey by the Cardiovascular and Interventional Radiology Society of Europe (CIRSE). Cardiovasc Intervent Radiol. 2024;47:1224-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Ronot M, Loffroy R, Arnold D, Greget M, Sengel C, Pinaquy JB, Pellerin O, Maleux G, Peynircioglu B, Pelage JP, Schaefer N, Sangro B, de Jong N, Zeka B, Urdaniz M, Helmberger T, Vilgrain V. Transarterial Radioembolisation with Y90 Resin Microspheres and the Effect of Reimbursement Criteria in France: Final Results of the CIRT-FR Prospective Observational Study. Cardiovasc Intervent Radiol. 2025;48:205-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Zane KE, Nagib PB, Jalil S, Mumtaz K, Makary MS. Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma. World J Hepatol. 2022;14:885-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 56. | Inchingolo R, Cortese F, Pisani AR, Acquafredda F, Calbi R, Memeo R, Anagnostopoulos F, Spiliopoulos S. Selective internal radiation therapy segmentectomy: A new minimally invasive curative option for primary liver malignancies? World J Gastroenterol. 2024;30:2379-2386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Stella M, Braat AJAT, van Rooij R, de Jong HWAM, Lam MGEH. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardiovasc Intervent Radiol. 2022;45:1634-1645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 58. | Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, Baker T, Kulik L, Gupta R, Abecassis M, Benson AB 3rd, Omary R, Millender L, Kennedy A, Salem R. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 59. | Prachanronarong K, Kim E. Radiation Segmentectomy. Semin Intervent Radiol. 2021;38:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 60. | Gabr A, Riaz A, Johnson GE, Kim E, Padia S, Lewandowski RJ, Salem R. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 61. | Montazeri SA, De la Garza-Ramos C, Lewis AR, Lewis JT, LeGout JD, Sella DM, Paz-Fumagalli R, Devcic Z, Ritchie CA, Frey GT, Vidal L, Croome KP, McKinney JM, Harnois D, Krishnan S, Patel T, Toskich BB. Hepatocellular carcinoma radiation segmentectomy treatment intensification prior to liver transplantation increases rates of complete pathologic necrosis: an explant analysis of 75 tumors. Eur J Nucl Med Mol Imaging. 2022;49:3892-3897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Serhal M, Dadrass F, Kim E, Lewandowski RJ. Radiation Segmentectomy for Hepatocellular Carcinoma. Curr Oncol. 2024;31:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 63. | Core JM, Frey GT, Sharma A, Bussone ST, Legout JD, McKinney JM, Lewis AR, Ritchie C, Devcic Z, Paz-Fumagalli R, Toskich BB. Increasing Yttrium-90 Dose Conformality Using Proximal Radioembolization Enabled by Distal Angiosomal Truncation for the Treatment of Hepatic Malignancy. J Vasc Interv Radiol. 2020;31:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, Gnesin S, Bodei L, Kracmerova T, Luster M, Garin E, Herrmann K. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2022;49:1682-1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 65. | Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, Kulik L, Ganger D, Desai K, Thornburg B, Mouli S, Hickey R, Caicedo JC, Abecassis M, Riaz A, Salem R. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 66. | Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, Patel RS, Nowakowski FS, Fischman AM, Kim E. Radiation Segmentectomy versus TACE Combined with Microwave Ablation for Unresectable Solitary Hepatocellular Carcinoma Up to 3 cm: A Propensity Score Matching Study. Radiology. 2017;283:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Arndt L, Villalobos A, Wagstaff W, Cheng B, Xing M, Ermentrout RM, Bercu Z, Cristescu M, Shah A, Wedd J, Majdalany BS, Magliocca JF, Sellers MT, Kokabi N. Evaluation of Medium-Term Efficacy of Y90 Radiation Segmentectomy vs Percutaneous Microwave Ablation in Patients with Solitary Surgically Unresectable < 4 cm Hepatocellular Carcinoma: A Propensity Score Matched Study. Cardiovasc Intervent Radiol. 2021;44:401-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | De la Garza-Ramos C, Montazeri SA, Croome KP, LeGout JD, Sella DM, Cleary S, Burns J, Mathur AK, Overfield CJ, Frey GT, Lewis AR, Paz-Fumagalli R, Ritchie CA, McKinney JM, Mody K, Patel T, Devcic Z, Toskich BB. Radiation Segmentectomy for the Treatment of Solitary Hepatocellular Carcinoma: Outcomes Compared with Those of Surgical Resection. J Vasc Interv Radiol. 2022;33:775-785.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 69. | Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, Assenat E, Tacher V, Robert C, Terroir-Cassou-Mounat M, Mariano-Goulart D, Amaddeo G, Palard X, Hollebecque A, Kafrouni M, Regnault H, Boudjema K, Grimaldi S, Fourcade M, Kobeiter H, Vibert E, Le Sourd S, Piron L, Sommacale D, Laffont S, Campillo-Gimenez B, Rolland Y; DOSISPHERE-01 Study Group. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 70. | Kim E, Sher A, Abboud G, Schwartz M, Facciuto M, Tabrizian P, Knešaurek K, Fischman A, Patel R, Nowakowski S, Llovet J, Taouli B, Lookstein R. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. Lancet Gastroenterol Hepatol. 2022;7:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 71. | Mahvash A, Chartier S, Turco M, Habib P, Griffith S, Brown S, Kappadath SC. A prospective, multicenter, open-label, single-arm clinical trial design to evaluate the safety and efficacy of (90)Y resin microspheres for the treatment of unresectable HCC: the DOORwaY90 (Duration Of Objective Response with arterial Ytrrium-90) study. BMC Gastroenterol. 2022;22:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (30)] |
| 73. | Dhondt E, Lambert B, Hermie L, Huyck L, Vanlangenhove P, Geerts A, Verhelst X, Aerts M, Vanlander A, Berrevoet F, Troisi RI, Van Vlierberghe H, Defreyne L. (90)Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology. 2022;303:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 74. | Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, Vaidya S, Monsky WL, Park JO, Bhattacharya R, Hippe DS, Harris WP. Segmental Yttrium-90 Radioembolization versus Segmental Chemoembolization for Localized Hepatocellular Carcinoma: Results of a Single-Center, Retrospective, Propensity Score-Matched Study. J Vasc Interv Radiol. 2017;28:777-785.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (37)] |
| 75. | Biederman DM, Titano JJ, Korff RA, Fischman AM, Patel RS, Nowakowski FS, Lookstein RA, Kim E. Radiation Segmentectomy versus Selective Chemoembolization in the Treatment of Early-Stage Hepatocellular Carcinoma. J Vasc Interv Radiol. 2018;29:30-37.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 76. | Villalobos A, Arndt L, Cheng B, Dabbous H, Loya M, Majdalany B, Bercu Z, Kokabi N. Yttrium-90 Radiation Segmentectomy of Hepatocellular Carcinoma: A Comparative Study of the Effectiveness, Safety, and Dosimetry of Glass-Based versus Resin-Based Microspheres. J Vasc Interv Radiol. 2023;34:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 77. | Sarwar A, Malik MS, Vo NH, Tsai LL, Tahir MM, Curry MP, Catana AM, Bullock AJ, Parker JA, Eckhoff DE, Nasser IA, Weinstein JL, Ahmed M. Efficacy and Safety of Radiation Segmentectomy with (90)Y Resin Microspheres for Hepatocellular Carcinoma. Radiology. 2024;311:e231386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 78. | Piron L, Le Roy J, Cassinotto C, Delicque J, Belgour A, Allimant C, Beregi JP, Greffier J, Molinari N, Guiu B. Radiation Exposure During Transarterial Chemoembolization: Angio-CT Versus Cone-Beam CT. Cardiovasc Intervent Radiol. 2019;42:1609-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Yao X, Yan D, Jiang X, Li X, Zeng H, Liu D, Li H. Dual-phase Cone-beam CT-based Navigation Imaging Significantly Enhances Tumor Detectability and Aids Superselective Transarterial Chemoembolization of Liver Cancer. Acad Radiol. 2018;25:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Takai Takamatsu R, Okano A, Yamakawa G, Mizukoshi K, Obayashi H, Ohana M. Impact of an ultrasound-guided radiofrequency ablation training program on the outcomes in patients with hepatocellular carcinoma. Diagn Interv Imaging. 2019;100:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Hermida M, Cassinotto C, Piron L, Assenat E, Pageaux GP, Escal L, Pierredon-Foulongne MA, Verzilli D, Jaber S, Guiu B. Percutaneous thermal ablation of hepatocellular carcinomas located in the hepatic dome using artificial carbon dioxide pneumothorax: retrospective evaluation of safety and efficacy. Int J Hyperthermia. 2018;35:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Kobe A, Tselikas L, Deschamps F, Roux C, Delpla A, Varin E, Hakime A, de Baère T. Thermal ablation of ultrasound and non-contrast computed tomography invisible primary and secondary liver tumors: targeting by selective intra-arterial lipiodol injection. Diagn Interv Radiol. 2023;29:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 83. | Schembri V, Piron L, Le Roy J, Hermida M, Lonjon J, Escal L, Pierredon MA, Belgour A, Cassinotto C, Guiu B. Percutaneous ablation of obscure hypovascular liver tumours in challenging locations using arterial CT-portography guidance. Diagn Interv Imaging. 2020;101:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4108] [Article Influence: 586.9] [Reference Citation Analysis (6)] |
| 85. | Renzulli M, Peta G, Vasuri F, Marasco G, Caretti D, Bartalena L, Spinelli D, Giampalma E, D'Errico A, Golfieri R. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann Hepatol. 2021;22:100278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 86. | Veloso Gomes F, de Baère T, Verset G, Coimbra É, Tovar-Felice G, Malagari K, Bruix J. Transarterial Chemoembolization with Anthracyclines-Loaded Polyethylene Glycol Drug Eluting Microspheres for the Treatment of Hepatocellular Carcinoma: A Pooled Multicentric Analysis of Survival in 580 Patients. Cardiovasc Intervent Radiol. 2023;46:436-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 87. | Peng CW, Teng W, Lui KW, Hung CF, Jeng WJ, Huang CH, Chen WT, Lin CC, Lin CY, Lin SM, Sheen IS. Complete response at first transarterial chemoembolization predicts favorable outcome in hepatocellular carcinoma. Am J Cancer Res. 2021;11:4956-4965. [PubMed] |
| 88. | Irie T, Kuramochi M, Takahashi N. Dense accumulation of lipiodol emulsion in hepatocellular carcinoma nodule during selective balloon-occluded transarterial chemoembolization: measurement of balloon-occluded arterial stump pressure. Cardiovasc Intervent Radiol. 2013;36:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 89. | Hatanaka T, Arai H, Kakizaki S. Balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2018;10:485-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 90. | Park C, Gwon DI, Chu HH, Kim JW, Kim JH, Ko GY. Correlation of tumor response on CT with pathologically proven necrosis in hepatocellular carcinoma treated by conventional transcatheter arterial chemoembolization: threshold value of intratumoral Lipiodol accumulation predicting tumor necrosis. Abdom Radiol (NY). 2021;46:3729-3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Lucatelli P, De Rubeis G, Rocco B, Basilico F, Cannavale A, Abbatecola A, Nardis PG, Corona M, Brozzetti S, Catalano C, Bezzi M. Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: a single center retrospective case control study. BMC Gastroenterol. 2021;21:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 92. | Golfieri R, Bezzi M, Verset G, Fucilli F, Mosconi C, Cappelli A, Paccapelo A, Lucatelli P, Magand N, Rode A, De Baere T. Balloon-Occluded Transarterial Chemoembolization: In Which Size Range Does It Perform Best? A Comparison of Its Efficacy versus Conventional Transarterial Chemoembolization, Using Propensity Score Matching. Liver Cancer. 2021;10:522-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Chu HH, Gwon DI, Kim GH, Kim JH, Ko GY, Shin JH, Ko HK, Yoon HK. Balloon-occluded transarterial chemoembolization versus conventional transarterial chemoembolization for the treatment of single hepatocellular carcinoma: a propensity score matching analysis. Eur Radiol. 2023;33:2655-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Lucatelli P, Rocco B, De Beare T, Verset G, Fucilli F, Damato E, Paccapelo A, Braccischi L, Taninokuchi Tomassoni M, Bucalau AM, Catalano C, Mosconi C. Long-Term Outcomes of Balloon TACE for HCC: An European Multicentre Single-Arm Retrospective Study. Cardiovasc Intervent Radiol. 2024;47:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 95. | Jiang C, Cheng G, Liao M, Huang J. Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: a time-to-event meta-analysis. World J Surg Oncol. 2021;19:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 96. | Xu Z, Xie H, Zhou L, Chen X, Zheng S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Vogl TJ, Stefan H, Gruber-Rouh T, Trojan J, Bechstein WO, Bielfeldt J, Adwan H. The combination of transarterial chemoembolization and microwave ablation is superior to microwave ablation alone for liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2024;150:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: State of the art. World J Gastroenterol. 2016;22:1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Li W, Ni CF. Current status of the combination therapy of transarterial chemoembolization and local ablation for hepatocellular carcinoma. Abdom Radiol (NY). 2019;44:2268-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Liu C, Li T, He JT, Shao H. TACE combined with microwave ablation therapy vs. TACE alone for treatment of early- and intermediate-stage hepatocellular carcinomas larger than 5 cm: a meta-analysis. Diagn Interv Radiol. 2020;26:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Yang Y, Yu H, Qi L, Liu C, Feng Y, Qi J, Li J, Zhu Q. Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: a systematic review and meta-analysis. Int J Hyperthermia. 2022;39:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 102. | Iezzi R, Posa A, Tanzilli A, Carchesio F, Pompili M, Manfredi R. Balloon-Occluded MWA (b-MWA) Followed by Balloon-Occluded TACE (b-TACE): Technical Note on a New Combined Single-Step Therapy for Single Large HCC. Cardiovasc Intervent Radiol. 2020;43:1702-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 103. | Lucatelli P, Argirò R, Crocetti L, Rocco B, Bozzi E, Gasparrini F, Tanzilli A, Catalano C, Iezzi R. Percutaneous Thermal Segmentectomy: Proof of Concept. Cardiovasc Intervent Radiol. 2022;45:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Lucatelli P, Rocco B, Argirò R, Semeraro V, Lai Q, Bozzi E, Crociati S, Barone M, Posa A, Catalano C, Crocetti L, Iezzi R. Percutaneous thermal segmentectomy for liver malignancies over 3 cm: mid-term oncological performance and predictors of sustained complete response from a multicentric Italian retrospective study. Radiol Med. 2024;129:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Cheng JF, Sun QL, Tang L, Xu XJ, Huang XZ. Meta-analysis of transarterial chemoembolization combined with cryoablation vs transarterial chemoembolization alone for ≥ 5 cm hepatocellular carcinoma. World J Gastrointest Oncol. 2024;16:2793-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 106. | Abdelmalak J, Lubel JS, Sinclair M, Majeed A, Kemp W, Roberts SK. Quality of care in hepatocellular carcinoma-A critical review. Hepatol Commun. 2025;9:e0595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 107. | Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, Li D, Mulla S, Verret W, Xu DZ, Hernandez S, Ding B, Liu J, Huang C, Lim HY, Cheng AL, Ducreux M. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 108. | Sangro B, Chan SL, Kelley RK, Lau G, Kudo M, Sukeepaisarnjaroen W, Yarchoan M, De Toni EN, Furuse J, Kang YK, Galle PR, Rimassa L, Heurgué A, Tam VC, Van Dao T, Thungappa SC, Breder V, Ostapenko Y, Reig M, Makowsky M, Paskow MJ, Gupta C, Kurland JF, Negro A, Abou-Alfa GK; HIMALAYA investigators. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2024;35:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 118] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 109. | Rimassa L, Chan S, Sangro B, Lau G, Kudo M, Breder V, Varela M, Crysler O, Bouattour M, Dao V, Faccio A, Furuse J, Jeng L, Kang Y, Kelley R, Paskow M, Makowsky M, Ran D, Negro A, Abou-alfa G. 947MO Five-year overall survival (OS) and OS by tumour response measures from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma (uHCC). Ann Oncol. 2024;35:S656. [DOI] [Full Text] |
| 110. | Galle PR, Decaens T, Kudo M, Qin S, Fonseca L, Sangro B, Karachiwala H, Park J, Gane E, Pinter M, Tai D, Santoro A, Pizarro G, Chiu C, Schenker M, He AR, Wang Q, Stromko C, Hreiki J, Yau T. Nivolumab (NIVO) plus ipilimumab (IPI) vs lenvatinib (LEN) or sorafenib (SOR) as first-line treatment for unresectable hepatocellular carcinoma (uHCC): First results from CheckMate 9DW. J Clin Oncol. 2024;42:LBA4008-LBA4008. [DOI] [Full Text] |
| 111. | Qin S, Kudo M, Meyer T, Bai Y, Guo Y, Meng Z, Satoh T, Marino D, Assenat E, Li S, Chen Y, Boisserie F, Abdrashitov R, Finn RS, Vogel A, Zhu AX. Tislelizumab vs Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2023;9:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 59.0] [Reference Citation Analysis (1)] |
| 112. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3827] [Article Influence: 546.7] [Reference Citation Analysis (1)] |
| 113. | Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 251] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 114. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 115. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4652] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 116. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2714] [Article Influence: 339.3] [Reference Citation Analysis (0)] |
| 117. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1769] [Article Influence: 252.7] [Reference Citation Analysis (0)] |
| 118. | Yau T, Kaseb A, Cheng AL, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Milwee S, Wang Z, Curran D, Kelley RK, Rimassa L. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): final results of a randomised phase 3 study. Lancet Gastroenterol Hepatol. 2024;9:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 119. | Chan SL, Ryoo BY, Mo F, Chan LL, Cheon J, Li L, Wong KH, Yim N, Kim H, Yoo C. Multicentre phase II trial of cabozantinib in patients with hepatocellular carcinoma after immune checkpoint inhibitor treatment. J Hepatol. 2024;81:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 120. | Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 653] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 121. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1249] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 122. | Wen N, Cai Y, Li F, Ye H, Tang W, Song P, Cheng N. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci Trends. 2022;16:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 123. | Koay EJ, Owen D, Das P. Radiation-Induced Liver Disease and Modern Radiotherapy. Semin Radiat Oncol. 2018;28:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 124. | Lewis S, Dawson L, Barry A, Stanescu T, Mohamad I, Hosni A. Stereotactic body radiation therapy for hepatocellular carcinoma: From infancy to ongoing maturity. JHEP Rep. 2022;4:100498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 125. | Inchingolo R, Acquafredda F, Tedeschi M, Laera L, Surico G, Surgo A, Fiorentino A, Spiliopoulos S, de'Angelis N, Memeo R. Worldwide management of hepatocellular carcinoma during the COVID-19 pandemic. World J Gastroenterol. 2021;27:3780-3789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Garibaldi C, Jereczek-Fossa BA, Marvaso G, Dicuonzo S, Rojas DP, Cattani F, Starzyńska A, Ciardo D, Surgo A, Leonardi MC, Ricotti R. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 127. | Bae SH, Chun SJ, Chung JH, Kim E, Kang JK, Jang WI, Moon JE, Roquette I, Mirabel X, Kimura T, Ueno M, Su TS, Tree AC, Guckenberger M, Lo SS, Scorsetti M, Slotman BJ, Kotecha R, Sahgal A, Louie AV, Kim MS. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Meta-Analysis and International Stereotactic Radiosurgery Society Practice Guidelines. Int J Radiat Oncol Biol Phys. 2024;118:337-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 128. | Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, Hallemeier CL, Koay EJ, Lasley F, Meyer J, Owen D, Pursley J, Schaub SK, Smith G, Venepalli NK, Zibari G, Cardenes H. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2022;12:28-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 129. | Yoon SM, Kim SY, Lim YS, Kim KM, Shim JH, Lee D, An J, Jung J, Kim JH, Lee HC. Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: Results of a single-arm, phase II clinical trial. Clin Mol Hepatol. 2020;26:506-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 130. | Mathew AS, Atenafu EG, Owen D, Maurino C, Brade A, Brierley J, Dinniwell R, Kim J, Cho C, Ringash J, Wong R, Cuneo K, Feng M, Lawrence TS, Dawson LA. Long term outcomes of stereotactic body radiation therapy for hepatocellular carcinoma without macrovascular invasion. Eur J Cancer. 2020;134:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 131. | Roquette I, Bogart E, Lacornerie T, Ningarhari M, Bibault JE, Le Deley MC, Lartigau EF, Pasquier D, Mirabel X. Stereotactic Body Radiation Therapy for the Management of Hepatocellular Carcinoma: Efficacy and Safety. Cancers (Basel). 2022;14:3892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 132. | Su TS, Liu QH, Zhu XF, Liang P, Liang SX, Lai L, Zhou Y, Huang Y, Cheng T, Li LQ. Optimal stereotactic body radiotherapy dosage for hepatocellular carcinoma: a multicenter study. Radiat Oncol. 2021;16:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 133. | Sapir E, Tao Y, Schipper MJ, Bazzi L, Novelli PM, Devlin P, Owen D, Cuneo KC, Lawrence TS, Parikh ND, Feng M. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2018;100:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 134. | Méndez Romero A, van der Holt B, Willemssen FEJA, de Man RA, Heijmen BJM, Habraken S, Westerveld H, van Delden OM, Klümpen HJ, Tjwa ETTL, Braam PM, Jenniskens SFM, Vanwolleghem T, Weytjens R, d'Archambeau O, de Vos-Geelen J, Buijsen J, van der Leij C, den Toom W, Sprengers D, IJzermans JNM, Moelker A. Transarterial Chemoembolization With Drug-Eluting Beads Versus Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Outcomes From a Multicenter, Randomized, Phase 2 Trial (the TRENDY Trial). Int J Radiat Oncol Biol Phys. 2023;117:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 135. | Dawson LA, Winter KA, Knox JJ, Zhu AX, Krishnan S, Guha C, Kachnic LA, Gillin MT, Hong TS, Craig TD, Williams TM, Hosni A, Chen E, Noonan AM, Koay EJ, Sinha R, Lock MI, Ohri N, Dorth JA, Delouya G, Swaminath A, Moughan J, Crane CH. Stereotactic Body Radiotherapy vs Sorafenib Alone in Hepatocellular Carcinoma: The NRG Oncology/RTOG 1112 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2025;11:136-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 136. | Nugent FW, Hunter K, Molgaard C, Qamar A, Gunturu K, Stuart KE, Gordon F, Flacke S. A randomized phase II feasibility study of individualized stereotactic body radiation therapy (SBRT) versus transarterial chemoembolization (TACE) with DEBDOX beads as a bridge to transplant in hepatocellular carcinoma (HCC). J Clin Oncol. 2020;38:4586-4586. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Chami P, Diab Y, Khalil DN, Azhari H, Jarnagin WR, Abou-Alfa GK, Harding JJ, Hajj J, Ma J, El Homsi M, Reyngold M, Crane C, Hajj C. Radiation and Immune Checkpoint Inhibitors: Combination Therapy for Treatment of Hepatocellular Carcinoma. Int J Mol Sci. 2023;24:16773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Tubin S, Vozenin MC, Prezado Y, Durante M, Prise KM, Lara PC, Greco C, Massaccesi M, Guha C, Wu X, Mohiuddin MM, Vestergaard A, Bassler N, Gupta S, Stock M, Timmerman R. Novel unconventional radiotherapy techniques: Current status and future perspectives - Report from the 2nd international radiation oncology online seminar. Clin Transl Radiat Oncol. 2023;40:100605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 139. | Moore C, Hsu CC, Chen WM, Chen BPC, Han C, Story M, Aguilera T, Pop LM, Hannan R, Fu YX, Saha D, Timmerman R. Personalized Ultrafractionated Stereotactic Adaptive Radiotherapy (PULSAR) in Preclinical Models Enhances Single-Agent Immune Checkpoint Blockade. Int J Radiat Oncol Biol Phys. 2021;110:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 140. | Verbus EA, Rossi AJ, Teke M, Nugent FW, Hernandez JM. Stereotactic Body Radiation Therapy (SBRT) Versus Transarterial Chemoembolization (TACE) as a Bridge to Transplant in Unresectable Hepatocellular Carcinoma. Ann Surg Oncol. 2022;29:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol. 2019;37:2141-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 142. | Chiang CL, Chiu KWH, Chan KSK, Lee FAS, Li JCB, Wan CWS, Dai WC, Lam TC, Chen W, Wong NSM, Cheung ALY, Lee VWY, Lau VWH, El Helali A, Man K, Kong FMS, Lo CM, Chan AC. Sequential transarterial chemoembolisation and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for patients with locally advanced, unresectable hepatocellular carcinoma (START-FIT): a single-arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 143. | Witt JS, Rosenberg SA, Bassetti MF. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21:e74-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 144. | Safavi AH, Louie AV, Boldt RG, Bruynzeel AM, Lagerwaard FJ, Senan S, Chen H. 108: Early Clinical Effectiveness, Toxicity, and Quality of Life Outcomes of Magnetic Resonance Imaging-Guided Stereotactic Ablative Radiotherapy: A Systematic Review. Radiother Oncol. 2022;174:S47. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 145. | Coffman AR, Sufficool DC, Kang JI, Hsueh CT, Swenson S, McGee PQ, Nagaraj G, Patyal B, Reeves ME, Slater JD, Yang GY. Proton stereotactic body radiation therapy for liver metastases-results of 5-year experience for 81 hepatic lesions. J Gastrointest Oncol. 2021;12:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 146. | Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 147. | Folkert MR, Meyer JJ, Aguilera TA, Yokoo T, Sanford NN, Rule WG, Mansour J, Yopp A, Polanco P, Hannan R, Nedzi LA, Timmerman RD. Long-Term Results of a Phase 1 Dose-Escalation Trial and Subsequent Institutional Experience of Single-Fraction Stereotactic Ablative Radiation Therapy for Liver Metastases. Int J Radiat Oncol Biol Phys. 2021;109:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 148. | Srinivas SM, Nasr EC, Kunam VK, Bullen JA, Purysko AS. Administered activity and outcomes of glass versus resin (90)Y microsphere radioembolization in patients with colorectal liver metastases. J Gastrointest Oncol. 2016;7:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | Meek J, Fletcher S, Gauss CH, Bezold S, Borja-Cacho D, Meek M. Temporary Balloon Occlusion for Hepatic Arterial Flow Redistribution during Yttrium-90 Radioembolization. J Vasc Interv Radiol. 2019;30:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 150. | Ramdhani K, Smits MLJ, Lam MGEH, Braat AJAT. Combining Selective Internal Radiation Therapy with Immunotherapy in Treating Hepatocellular Carcinoma and Hepatic Colorectal Metastases: A Systematic Review. Cancer Biother Radiopharm. 2023;38:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 151. | Brandi N, Renzulli M. The Synergistic Effect of Interventional Locoregional Treatments and Immunotherapy for the Treatment of Hepatocellular Carcinoma. Int J Mol Sci. 2023;24:8598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 152. | Liao C, Zhang G, Huang R, Zeng L, Chen B, Dai H, Tang K, Lin R, Huang Y. Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy. Pharmaceuticals (Basel). 2023;16:1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 153. | Lucas AP, Lewis AR, Kasi PM, Toskich BB, Paz-Fumagalli R. Abscopal downstaging of intermediate stage hepatocellular via combination cryoablation and immunotherapy with complete pathologic response. Radiol Case Rep. 2024;19:910-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 154. | Levine JM, Habib A, Silk M, Sacks GD, Winograd R, Hill CS, Javed AA, Wolfgang CL, Hewitt DB. Abscopal Effect with Liver-Directed Therapy: A Review of the Current Literature and Future Directions. Livers. 2024;4:601-614. [DOI] [Full Text] |
| 155. | Craciun L, de Wind R, Demetter P, Lucidi V, Bohlok A, Michiels S, Bouazza F, Vouche M, Tancredi I, Verset G, Garaud S, Naveaux C, Galdon MG, Gallo KW, Hendlisz A, Derijckere ID, Flamen P, Larsimont D, Donckier V. Retrospective analysis of the immunogenic effects of intra-arterial locoregional therapies in hepatocellular carcinoma: a rationale for combining selective internal radiation therapy (SIRT) and immunotherapy. BMC Cancer. 2020;20:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 156. | Yu S, Yu M, Keane B, Mauro DM, Helft PR, Harris WP, Sanoff HK, Johnson MS, O'Neil B, McRee AJ, Somasundaram A. A Pilot Study of Pembrolizumab in Combination With Y90 Radioembolization in Subjects With Poor Prognosis Hepatocellular Carcinoma. Oncologist. 2024;29:270-e413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 157. | Humeau J, Le Naour J, Galluzzi L, Kroemer G, Pol JG. Trial watch: intratumoral immunotherapy. Oncoimmunology. 2021;10:1984677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 158. | Nakamoto Y, Mizukoshi E, Tsuji H, Sakai Y, Kitahara M, Arai K, Yamashita T, Yokoyama K, Mukaida N, Matsushima K, Matsui O, Kaneko S. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin Exp Immunol. 2007;147:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 159. | Aroldi F, Sacco J, Harrington K, Olsson-brown A, Nanclares P, Menezes L, Bommareddy P, Thomas S, Kaufman H, Samakoglu S, Coffin R, Middleton M. 421 Initial results of a phase 1 trial of RP2, a first in class, enhanced potency, anti-CTLA-4 antibody expressing, oncolytic HSV as single agent and combined with nivolumab in patients with solid tumors. Regular and young investigator award abstracts. J Immunother Cancer. 2020;8 (Suppl 3):A1-A559. [DOI] [Full Text] |
| 160. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3882] [Article Influence: 970.5] [Reference Citation Analysis (3)] |
| 161. | Lee SC, Tan HT, Chung MC. Prognostic biomarkers for prediction of recurrence of hepatocellular carcinoma: current status and future prospects. World J Gastroenterol. 2014;20:3112-3124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (2)] |
| 162. | Lin X, Wei R, Xu Z, Zhuo S, Dou J, Sun H, Li R, Yang R, Lu Q, An C, Chen H. A deep learning model for personalized intra-arterial therapy planning in unresectable hepatocellular carcinoma: a multicenter retrospective study. EClinicalMedicine. 2024;75:102808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 163. | Albrecht MH, Vogl TJ, Wichmann JL, Martin SS, Scholtz JE, Fischer S, Hammerstingl RM, Harth M, Nour-Eldin NA, Thalhammer A, Zangos S, Bauer RW. Dynamic 4D-CT Angiography for Guiding Transarterial Chemoembolization: Impact on the Reduction of Contrast Material, Operator Radiation Exposure, Catheter Consumption, and Diagnostic Confidence. Rofo. 2018;190:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 164. | Moschovaki-Zeiger O, Arkoudis NA, Spiliopoulos S. Safety and feasibility study of a novel robotic system in an in vivo porcine vascular model. CVIR Endovasc. 2024;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 165. | Soulen MC, Rilling WS. Clinical Outcomes After Personalized Dosimetry for (90)Y Radioembolization of Advanced Hepatocellular Carcinoma: Defining the Role of a Device in a Pharma-Centric Landscape. J Nucl Med. 2024;65:270-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 166. | Boughdad S, Duran R, Prior JO, da Mota M, De Carvalho MM, Costes J, Firsova M, Gnesin S, Schaefer N. Measure of (90)Y-glass microspheres residue post-TARE using PET/CT and potential impact on tumor absorbed dose in comparison (99m)Tc-MAA SPECT/CT dosimetry. EJNMMI Rep. 2024;8:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 167. | Bucalau AM, Collette B, Tancredi I, Vouche M, Pezzullo M, Bouziotis J, Moreno-Reyes R, Trotta N, Levillain H, Van Laethem JL, Verset G. Clinical impact of (99m)Tc-MAA SPECT/CT-based personalized predictive dosimetry in selective internal radiotherapy: a real-life single-center experience in unresectable HCC patients. Eur J Hybrid Imaging. 2023;7:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 168. | Roosen J, van Wijk MWM, Westlund Gotby LEL, Arntz MJ, Janssen MJR, Lobeek D, van de Maat GH, Overduin CG, Nijsen JFW. Improving MRI-based dosimetry for holmium-166 transarterial radioembolization using a nonrigid image registration for voxelwise ΔR2* calculation. Med Phys. 2023;50:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 169. | Abraham RJ, Arepally A, Liu D, Lewandowski R, Kappadath SC, Verma A, Dobrowski D, Holden A. Imageable Radioembolization Microspheres for Treatment of Unresectable Hepatocellular Carcinoma: Interim Results from a First-in-Human Trial. J Vasc Interv Radiol. 2024;35:1464-1473.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 170. | Argyrou M, Valassi A, Andreou M, Lyra M. Rhenium-188 production in hospitals, by w-188/re-188 generator, for easy use in radionuclide therapy. Int J Mol Imaging. 2013;2013:290750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 171. | Escutia-Gutiérrez R, Sandoval-Rodríguez A, Zamudio-Ojeda A, Guevara-Martínez SJ, Armendáriz-Borunda J. Advances of Nanotechnology in the Diagnosis and Treatment of Hepatocellular Carcinoma. J Clin Med. 2023;12:6867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |