Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.107803
Revised: May 6, 2025
Accepted: July 7, 2025
Published online: August 27, 2025
Processing time: 151 Days and 18.9 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD) and Wilson's disease (WD) are common clinical conditions characterized by hepatic steatosis. Copper has been associated with the progression of hepatic steatosis, but its precise mechanism remains unclear. Emerging research on hepatic copper home
Core Tip: Hepatic steatosis diseases are strongly associated with copper, and it has been shown that copper levels promote hepatic fat synthesis or lipolysis by triggering different mechanisms, which is extremely important for unravelling the molecular mechanisms of hepatic steatosis in diseases such as metabolic dysfunction-associated steatotic liver disease and Wilson's disease. This article reviews the potential mechanisms of copper-induced hepatic lipid dysregulation and explores new therapeutic targets for hepatic steatosis diseases.
- Citation: Gao DJ, Zeng T, Chong YT, Li XH. Copper and hepatic lipid dysregulation: Mechanisms and implications. World J Hepatol 2025; 17(8): 107803
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/107803.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.107803
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common liver disease worldwide and a leading cause of liver-related disease and death, with pathogenic factors including obesity, type 2 diabetes mellitus (T2DM), hyperlipidemia, and metabolic syndrome[1]. As an essential trace element in the human body, copper is a key regulator of various physiological processes, and its dysregulated homeostasis can significantly impair normal liver function. Recent studies have shown that intracellular copper homeostasis in hepatocytes is not only involved in the regulation of liver aging but also influences the disease process by mediating cuproptosis, a novel form of regulated cuproptosis triggered by copper ion and copper ionophore[2,3]. Moreover, clinical evidence suggests that copper levels are associated with several metabolic disorders, including obesity, diabetes, and MASLD[4-6]. It has been suggested that hepatic copper is a potential etiological factor in hepatic steatosis and that cuproptosis may contribute to the development of MASLD[7,8]. Additionally, Wilson's disease (WD) is a typical disease of hepatic copper overload, in which the inci
However, current evidence regarding the effects of copper on hepatic lipid metabolism remains heterogeneous, suggesting involvement of multiple underlying mechanisms. Elevated hepatic copper regulates hepatic lipid metabolism bidirectionally by either inducing hepatic steatosis or promoting liver lipolysis[10,11]. In contrast, hepatic copper defi
Copper is an essential trace element required as a cofactor for the synthesis of copper-dependent enzymes, including copper/zinc superoxide dismutase (SOD1), ceruloplasmin (CP), cytochrome C oxidase (CcO), dopamine-β-hydroxylase, copper-containing amine oxidases, and tyrosinase. These enzymes play crucial physiological roles, including antioxidant defense, iron homeostasis, oxidative phosphorylation, and myelin sheath formation[13,14].
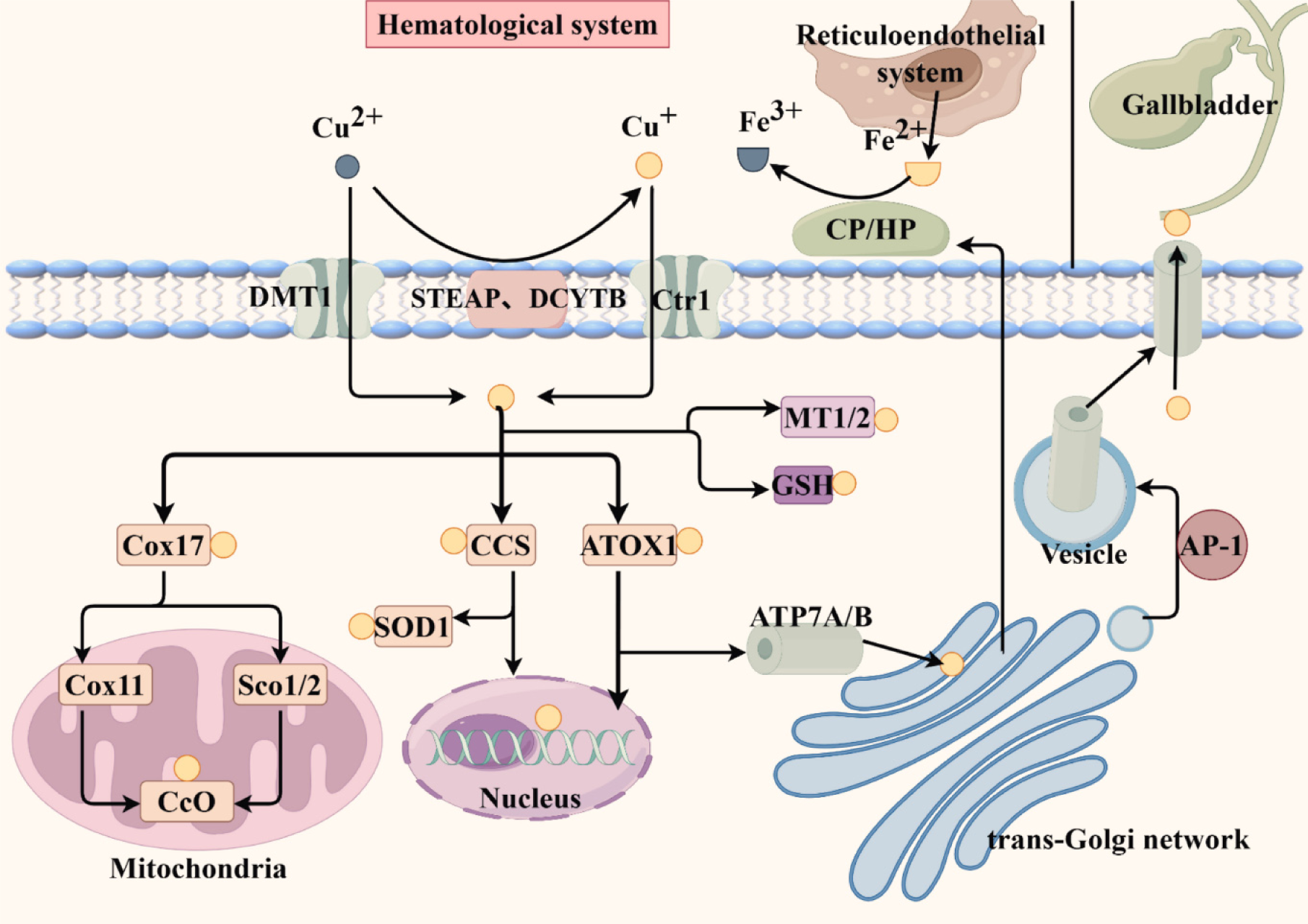
Copper absorption occurs mainly in the small intestine. The extracellular copper ions exist in the Cu(II) form. Although divalent metal transporter 1 can incorporate Cu(II), these cupric ions are not directly useable by cells. Most Cu(II) is reduced into Cu(I) by copper reductases such as DCYTB and STEAP. It is transported to the intestinal epithelial cells by the copper transport protein (Ctr1) and then excreted into the portal system by basolateral copper-transporting ATP7A, where it is transported in combination with albumin, macroglobulin, and histidine. Upon entering the liver, copper ions are transported by the copper-transporting ATP7B in hepatocytes to the trans-Golgi network (TGN) to synthesize holo-CP, which is secreted into blood circulation for distribution to tissues and organs. Excess copper in hepatocytes is transported via the ATP7B protein and excreted in bile[15-17].
Copper ions enter the cell via Ctr1 and have three main destinations: (1) Formation of copper-dependent enzymes mediated by copper chaperone proteins (CCS); (2) Secretion into the blood or bile; and (3) Intracellular storage. The SOD CCS supplies copper ions to SOD1 in the cytoplasm and mitochondrial membrane space (IMS); it also transports copper ions to the nucleus, activates the transcription factor HIF1, and increases the transcription of metallothionein (MT) genes in the presence of excess copper. The Cox17 Located in the IMS transfers cytoplasmic copper to the IMS, delivering it to CcO synthase (Sco1/2) and Cox11, respectively, both of which insert copper ions into CcO[18,19]. ATOX1 delivers copper ions to ATP7A and ATP7B proteins located in the TGN membrane, facilitating the synthesis of copper-dependent enzymes. For example, ATP7A-delivered copper is involved in lysyl oxidase and tyrosinase synthesis, while ATP7B delivers copper to CP[16,20,21]. CP and its homologue hephaestin are involved in iron oxidation in the reticuloendothelial system and intestinal epithelial cells, respectively[22]. ATOX1 also transports copper to the nucleus and functions as a copper-dependent transcription factor[23]. When intracellular copper levels increase, ATP7A/B proteins are localized in vesicles that fuse with the plasma membrane to excrete excess copper into the bile ducts or blood circulation. Upon restoration of physiological copper levels, ATP7A/B proteins are recycled back to the TGN[24]. Furthermore, AP-1 can recognize and package specific membrane proteins from the Golgi membrane into vesicles within the cell[25]. Additionally, it can bind to MT1/2 and glutathione (GSH) to prevent cytotoxicity when intracellular copper levels exceed its processing capacity[16]. The figure below shows the process of copper homeostasis regulation in the cells (Figure 1).
In mammalian cells, two highly homologous copper-transporting ATPases, ATP7A and ATP7B, precisely regulate intracellular copper concentrations within the physiological range by hydrolyzing ATP. While both ATPases are expr
In 2019, Tsvetkov’s team identified the molecular mechanism of cuproptosis, revealing that excess Cu²+ triggered proteotoxic stress by binding to lipid-acylated proteins in the tricarboxylic acid (TCA) cycle, leading to protein agg
Copper is closely linked to the development of lipid metabolic diseases, and understanding its regulatory mechanisms is an important research focus. Elevated serum copper levels are associated with multiple cardiometabolic risk factors, including dyslipidemia, T2DM, obesity, and MASLD, which are potential disease predictors[32]. Cohort studies of United States adults demonstrated that high serum copper was associated with elevated serum concentrations of total cholesterol (TC) and high-density lipoprotein (HDL) cholesterol and was associated with higher risks of high TC and high low-density lipoprotein cholesterol (LDL-C)[33]. These findings are consistent with those of meta-analyses, which reported significantly higher serum copper levels in obese adults and children compared to healthy-weight controls, with higher copper levels associated with increased prevalence of obesity in the United States[34,35]. A Mendelian randomization analysis found that obesity mediated the association between serum copper and inflammation, and high serum copper could be a cause of obesity[36].
Dietary copper intake also exerted dose-dependent effects on lipid metabolism. A nationwide Chinese cohort study revealed a U-shaped relationship between dietary copper intake and obesity risk, indicating that both excessive and insufficient intake increased general and abdominal obesity risks[37]. For instance, higher copper intake elevated the prevalence of hypertriglyceridemia, particularly among United States adolescents with a body mass index ≥ 23 kg/m²[38]. Conversely, another study reported inverse associations between dietary copper and fasting glucose, TC, and LDL-C[39]. In rats, moderate copper supplementation reduced triglyceride (TG), TC, and LDL-C levels, although it did not significantly affect body weight, food intake, or HDL[40]. While copper deficiency was associated with adverse lipid profiles, supplementation was not universally recommended due to its potential pro-inflammatory and pro-oxidative effects[39].
However, inconsistencies in findings may be attributed to geographical, demographic (e.g., age, sex), and lifestyle (e.g., exercise, smoking, alcohol) variations that influenced copper-lipid metabolism interactions[41]. For instance, a European study found no correlation between copper levels and body fat percentage in children, whereas obese Indian children exhibited reduced serum copper and zinc levels[42,43]. Methodological differences in copper measurement and control group selection may have introduced additional bias[6]. These discrepancies suggest that copper played a dual role in lipid metabolism, with its biological effects dependent on homeostatic balance and individual metabolic regulation.
The effects of copper on hepatic lipid metabolism include modulating lipoprotein synthesis, regulating de novo lipogenesis (DNL) and fatty acid β-oxidation (FAO), and disrupting glucose homeostasis. The pathogenic mechanisms will be elaborated below.
Excessive intracellular copper levels increase the generation of reactive oxygen species (ROS) and trigger OxS through multiple mechanisms. These included: (1) Copper-catalyzed ROS production via Fenton-like reactions; (2) GSH depletion under high copper exposure; and (3) Mitochondrial dysfunction due to copper toxicity and ROS disrupt membrane potential, impair electron transport chain activity, and exacerbate ROS. Notably, copper deficiency increases ROS and OxS by reducing the activity of antioxidant enzymes such as SOD1 and CcO[44,45].
Copper-induced ROS and OxS play a key role in hepatic lipogenesis through several mechanisms: (1) Generation of cytotoxic metabolites (e.g., malondialdehyde, lipid peroxides, 8-isoprostane, and 4-hydroxy-2-nonenal) via OxS-mediated lipid peroxidation, leading to cellular membrane damage[46]; (2) Activation of JNK and SREBP pathways by ROS, stimulating lipogenesis and lipid droplet (LD) formation. LDs may serve as a cellular defense mechanism against OxS[47,48]; (3) At the molecular level, Nrf2 acts as a central regulator. Copper overload-induced ROS activates Nrf2, promoting its nuclear translocation and binding to the PPARγ promoter, thereby upregulating PPARγ-mediated lipogenesis. Additionally, copper upregulates Nrf2 expression by enhancing MT transcription factor 1 binding to the Nrf2 promoter while inhibiting the SP1/Fyn pathway, further amplifying Nrf2-driven lipogenic gene transcription and TG deposition[49-51]; (4) ROS impairs mitochondrial integrity, disrupting oxidative phosphorylation and FAO, a key mechanism in copper-induced steatosis[52]. Concurrently, copper toxicity and suppressed mitophagy exacerbate mitochondrial dys
In summary, copper disrupts lipid metabolism by inducing OxS and mitochondrial dysfunction, altering hepatocyte structure, activating the JNK/SREBP pathway, increasing Nrf2 nuclear accumulation, and impairing FAO, ultimately promoting hepatic steatosis.
The endoplasmic reticulum (ER) is essential for hepatocytes to maintain lipid metabolism, with its regulatory roles including: (1) ER membrane-localized sterol regulatory element-binding proteins (SREBP-1c, SREBP-2), which regulate DNL-SREBP-1c primarily mediates fatty acid synthesis, while SREBP-2 controls cholesterol synthesis; (2) ER-resident acyltransferases that catalyze hepatocellular TG synthesis; and (3) ER-mediated VLDL formation. Consequently, ER dysfunction is closely associated with hepatic steatosis[56].
Impaired protein-folding capacity due to adverse conditions results in misfolded protein accumulation, triggering ER stress and activating the unfolded protein response (UPR). The UPR involves three transmembrane ER-resident stress sensors: Inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6). Under normal conditions, these sensors remain inactive through binding to 78-kDa glucose-regulated protein (GRP78). However, during protein misfolding stress, GRP78 dissociates from ATF6, IRE1α, and PERK, activating the UPR to restore ER homeostasis[57].
ER stress induced by hepatic copper overload contributed to steatosis through multiple mechanisms: (1) OxS-mediated UPR activation: Intracellular OxS and ROS accumulation activate the UPR and ER stress[56]; (2) Epigenetic dysregulation of ER chaperones: Hypermethylation of Grp78 induced by ROS-mediated copper overload suppresses its mRNA expression and exacerbates ER stress[55]; (3) CREB-dependent lipogenic programming: Copper-activated CREB binding sites in the promoters of Grp78, Perk, Ire1α, and Atf6α, upregulating lipogenic genes[58] such as SREBP-1c, ACC, SCD1, fatty acid synthase (FAS); and (4) Insig-1 depletion and SREBP-1c activation: ER stress/UPR-induced depletion of Insig-1 promoted copper-dependent activation of hepatic SREBP-1c[59].
Collectively, copper overload activates SREBP-1c and upregulates lipogenic gene expression by inducing ER stress and Insig-1 depletion. Additionally, ER stress reduces ApoB secretion, impairing VLDL assembly and contributing to hepatic IR, thereby exacerbating hepatic steatosis[56,60]. Moreover, studies have demonstrated that, in addition to copper-induced OxS triggering ER stress, copper ions themselves can directly induce ER stress.
Clinical studies indicated that abnormal copper levels significantly contribute to the development of diabetes and its complications[4]. A prospective cohort study demonstrated that higher dietary copper intake was associated with an increased risk of T2DM[61]. Elevated plasma copper concentrations also correlated positively with impaired glucose metabolism and T2DM, an association potentially modulated by SOD1 gene polymorphisms[62].
The mechanisms underlying copper’s impact on glucose metabolism may involve the following pathways: (1) ROS-mediated IR: Copper-induced IR occurs through ROS generation, as evidenced by reductions in ROS levels and improvements in IR following copper chelator therapy[63]; (2) Pancreatic β-cell dysfunction: Elevated copper levels impair β-cell function, consistent with negative correlations between copper and homeostasis model assessment of β-cell function[64]. This effect may involve copper’s interaction with human islet amyloid polypeptide (hIAPP). While copper inhibits hIAPP fibrillization, it stabilizes toxic oligomeric aggregates, exacerbating β-cell degeneration in T2DM[65]; and (3) Hepatic farnesoid X receptor modulation: Farnesoid X receptor (FXR) activation suppresses glycolysis, enhances hepatic glycogen synthesis, and inhibits gluconeogenesis via intestinal FGF19 release[66]. However, copper exerts tissue-specific effects on FXR: Intestinal copper activates the FXR-FGF19 axis, whereas hepatic copper accumulation downregulates FXR expression[67,68].
In summary, copper dysregulation influenced glucose metabolism through IR, β-cell dysfunction, hIAPP aggregation, and FXR modulation. These findings highlight the extensive impact of copper on metabolic regulation. However, current research has focused on copper’s role in diabetic complications. Due to the limited research on copper-induced glucose metabolism dysregulation, it remains unclear whether such metabolic disturbances subsequently disrupt hepatic lipid metabolism. Further investigation is needed to elucidate this potential link.
AMPK is a key regulator of cellular energy homeostasis, modulating metabolic pathways in response to intracellular AMP/ATP ratios. In lipid metabolism, AMPK inhibits fatty acid and cholesterol synthesis by phosphorylating and inactivating ACC and HMG-CoA reductase while enhancing catabolic processes such as glycolysis and FAO. Given these roles, AMPK activation has emerged as a potential therapeutic strategy for MASLD[69,70].
Current evidence indicates that both copper overload and deficiency can activate the AMPK signaling pathway, primarily through ATP depletion or ROS generation. Notably, copper-induced AMPK activation appears to be mediated through upregulation of the p-AMPK/AMPK ratio rather than through changes in AMPK or p-AMPK transcriptional levels[71-75]. In addition to the two prevalent mechanisms discussed above, a study also suggested that increased copper levels activate AMPK by promoting the assembly of the SCO1-LKB1-AMPK complex. SCO1, a copper chaperone of CcO, also functions as a scaffold protein that tethers the upstream kinase LKB1 to AMPK, facilitating AMPK phosphorylation. LKB1 is a master upstream kinase that directly phosphorylates and activates AMPK[11,76]. Importantly, copper-mediated AMPK activation stimulates catabolic processes, including FAO, mitochondrial biogenesis, and oxidative phos
However, Liu et al[79] reported contrasting findings in their study of hepatic lipid metabolism in Monopterus albus during chronic copper exposure. They observed significant downregulation of AMPK subunit transcripts (ampkβ1 and ampkγ1) and fatty acid catabolism-related genes (hsl, atgl), while lipid synthesis genes remained unchanged, ultimately leading to hepatic lipid accumulation.
This discrepant outcome may suggest two key implications. First, the mechanism by which copper overload leads to hepatic lipid accumulation (or reduction) involves multiple pathways and may be highly influenced by exposure duration and concentration. Supporting this interpretation, a previous study found that hepatic lipids in the javelin goby Synechogobius hasta exhibited concentration-dependent accumulation at 30 days of copper exposure, but demonstrated concentration-dependent reduction at 60 days of copper exposure[80]. Thus, despite AMPK activation, hepatic lipid accumulation may still occur. Second, copper-induced AMPK activation may exhibit concentration dependence. Studies on copper cytotoxicity have shown that relatively low copper concentrations induce AMPK activation, whereas higher concentrations suppress it[81].
Therefore, establishing a quantitative relationship between copper concentration, AMPK activation, and lipid meta
The nuclear receptor family in the liver includes PPARs, liver X receptor (LXR), and FXR, which can form heterodimers with RXR to participate in hepatic lipid metabolism, storage, transport, and elimination[82]. In adult and pediatric WD patients as well as Atp7b-/- mice, elevated copper impairs hepatic nuclear receptor function, leading to reduced binding of FXR, RXR, HNF4α, and LRH-1 to promoter response elements and decreased mRNA expression of their target genes[67]. The downregulation of cholesterol biosynthesis in Atp7b-/- mice was found to result from the inhibition of signaling by nuclear receptors, as evidenced by reduced expression of LXR/RXR targets such as FAS and HMG-CoA reductase. This reduced LXR/RXR activity may stem from decreased levels of endogenous activating ligands or lower expression of nuclear receptors[83,84].
The PPAR family comprises three isoforms: PPARα, PPARβ/δ, and PPARγ. PPARα is highly expressed in metabolically active tissues such as the liver, heart, and skeletal muscle, where it plays a key role in regulating genes involved in FAO. In contrast, PPARγ is predominantly found in adipose tissue, where it governs adipocyte differentiation, and it is also expressed at lower levels in the liver[85]. PPARγ activation is related to fat synthesis and hepatic steatosis[86]. Within specific concentration and time ranges, copper overload promotes hepatic lipid accumulation and modulates PPAR expression levels. For instance, in a study on copper exposure and hepatic lipid metabolism in Synechogobius hasta, 30-day copper treatment increased hepatic lipid content in a dose-dependent manner, accompanied by decreased PPARα mRNA expression and elevated PPARγ mRNA levels. Similar patterns were observed in Takifugu fasciatus and Monopterus albus exposed to copper[79,80,87]. However, excessive or prolonged copper exposure may reduce hepatic lipid content. For instance, in Synechogobius hasta, prolonged copper exposure (60 days) led to a dose-dependent decrease in liver lipids despite upregulation of both PPARα and PPARγ mRNA levels. Furthermore, dietary copper supplementation in rabbits reduced hepatic lipid deposition while increasing PPARα expression[78,80,88].
In contrast, in copper exposure experiments conducted on yellow catfish Pelteobagrus fulvidraco, variations in copper concentration did not significantly alter hepatic PPARα and PPARγ mRNA expression levels despite inducing noticeable changes in hepatic lipid metabolism[59,89]. The difference in hepatic lipid accumulation may be attributed to levels of PPAR expression in the liver between animals. For instance, PPARγ expression was most abundantly expressed in the liver of javelin goby Synechogobius hasta but significantly lower in Pelteobagrus fulvidraco[80].
Although PPAR transcript levels are strongly associated with copper exposure, the regulatory mechanisms of PPAR under copper overload remain unclear. When studying the effects of copper overload on hepatic steatosis, it is essential to consider the animal model, copper concentration, and exposure duration, as these factors also influence the expression levels of hepatic PPARα and PPARγ. A study has found that excessive Cu²+ may disrupt PPAR/RXR heterodimer formation, thereby inhibiting the PPARα pathway. Additionally, the zinc finger domains of PPAR could be a key target for copper-mediated interference[79,90].
Adipose tissue serves as the primary site of fat storage. Impaired or excessive adipocyte lipogenesis disrupts lipid metabolic homeostasis, potentially contributing to hepatic steatosis[91]. Elevated serum levels of the copper-dependent proteins semicarbazide-sensitive amine oxidase (SSAO) and CP in obese individuals were linked to increased copper levels, driven by upregulated Ctr1 and ATP7A expression in adipose tissue[92]. One study identified CP as a new lipocalin and was secreted by adipose tissue during obesity[93], suggesting a key role of adipose tissue copper in lipid metabolism.
Copper plays a critical role in regulating adipose tissue differentiation, lipogenesis, and lipolysis. During adipocyte differentiation, the Wnt/β-catenin pathway-essential for mesenchymal stem cell adipogenesis-was inhibited upon activation. Elevated copper levels in preadipocytes stabilized β-catenin, suppressing adipogenesis[94,95]. Adipocyte-specific ATP7A knockout in mice increased adipose copper content, resulting in age-dependent white adipose atrophy, IR, and hepatic steatosis[96].
Copper also modulates lipid metabolism via SSAO, a copper-dependent enzyme highly expressed in adipose tissue. Normal SSAO activity supports adipocyte differentiation and energy metabolism, while copper deficiency impairs SSAO function, promoting adipocyte hypertrophy and fat accumulation[97]. Regarding lipolysis, a classic study demonstrated that copper enhances fat lipolysis by binding to a critical conserved cysteine residue in phosphodiesterase 3B (PDE3B), which is responsible for cAMP degradation. This interaction inhibits PDE3B activity, leading to increased cAMP levels and subsequent activation of downstream target genes[98].
Overall, copper regulates multiple aspects of adipose tissue metabolism. Generally, elevated copper levels in adipose tissue reduce lipid accumulation, whereas copper deficiency exacerbates it. Given that obesity is a major contributor to hepatic steatosis, modulating copper homeostasis may represent a potential therapeutic strategy for improving lipid met
microRNAs: microRNAs (miRNAs) are a highly conserved class of endogenous single-stranded non-coding RNAs that inhibit gene expression. There was a review emphasized that miRNAs are involved in various processes of hepatic steatosis, including enhancing hepatic lipid uptake and promoting DNL, impairing lipid oxidation, reducing hepatic lipid export, as well as influencing hepatic glucose metabolism, autophagy, and ER stress-related cellular processes[99]. In Pelteobagrus fulvidraco, copper exposure downregulated miR-205, which appeared critical in copper-induced lipid metabolic dysfunction. Copper overload reduced miR-205 expression, elevated TG levels, and FAS activity. These effects were attenuated by a LXR antagonist. LXRα, as a potential target of miR-205, may be a key target of miR-205 and mediate copper-induced lipid metabolic disruption[100].
This research provides another perspective into copper-induced hepatic lipid metabolic disorders, specifically through epigenetic mechanisms. The miRNAs are involved in multiple processes of hepatic steatosis, which establishes a theoretical foundation for future studies on miRNAs-mediated mechanisms underlying copper-induced liver steatosis.
Hepatic iron overload: Hepatic iron overload disrupts systemic lipid homeostasis via multiple pathways, including impaired insulin signaling, OxS, mitochondrial dysfunction, and activation of the HIF1α-PPARγ pathway[101-103]. Clinical studies have observed hepatic iron overload in patients with hepatic steatosis, which correlates with diminished ferroxidase activity of copper-containing proteins due to reduced hepatic and serum copper levels[104]. Additionally, iron metabolism abnormalities have been documented in some patients with WD[105]. Although both hepatic copper and iron dysregulation influence lipid metabolism, their mechanisms operate independently[106]. Hepatic iron overload may provide valuable insights into the effects of copper on hepatic lipid metabolism.
Wnt/β-catenin signaling pathway: Classical Wnt signaling in hepatocytes plays a crucial role in the development of diet-induced hepatic steatosis and obesity[107]. A study demonstrated that hepatic β-catenin deficiency impaired mito
Although the Wnt/β-catenin pathway regulates various hepatic pathophysiological processes, its involvement in copper-induced hepatic steatosis remains unclear. Thus, investigating whether copper influences β-catenin expression could serve as a feasible starting point for further research.
Activation of autophagy: Autophagy is a conserved process involving the sequestration and degradation of cytoplasmic components via autophagosomes to maintain intracellular homeostasis[112]. Dysregulated autophagy disrupts cellular homeostasis and contributes to hepatic steatosis, and autophagy activation appears to be a critical therapeutic target for alleviating hepatic steatosis[113,114]. Copper overload induces OxS, which modulates autophagy through multiple pathways, including inhibition of the PI3K/AKT/mTOR pathway, activation of the AMPK-mTOR axis, or direct suppression of mTOR signaling[72,115,116]. Additionally, the p38 MAPK pathway participates in copper-mediated autophagy regulation[117]. In lipid metabolism, autophagy plays a protective role against copper-induced lipid depo
Collectively, the four proposed mechanisms-miRNAs alterations, excess hepatic iron, aberrant Wnt/β-catenin signaling pathway, and activation of autophagy-all play important roles in lipid metabolism. However, current evidence sup
MASLD is a chronic liver condition defined as abnormal lipid accumulation in hepatocytes and is diagnosed as steatotic liver disease when hepatic fat content reaches ≥ 5%, as measured by proton density fat fraction via magnetic resonance imaging. The global prevalence of hepatic steatosis has increased significantly owing to rising rates of obesity, diabetes, and metabolic syndrome. As an early indicator of hepatic dysfunction, steatosis could progress to steatohepatitis, fibrosis, cirrhosis, and even hepatocellular carcinoma, posing a serious health risk[119,120]. Emerging evidence indicates that copper plays a significant role in MASLD pathogenesis, although the precise mechanisms remain complex and debated.
Many studies have consistently reported lower hepatic copper levels in MASLD patients, which are negatively correlated with the severity of hepatic steatosis[121]. Hepatic copper deficiency has been suggested as a potential factor in hepatic steatosis, supported by the findings of several studies. In obese patients, hepatic copper levels were significantly higher in those with no or mild steatosis and significantly lower in those with severe steatosis than in controls[92]. Additionally, research showed significantly lower concentrations of copper in the dry, defatted liver tissues of ob/ob obese mice. This study provides compelling evidence of true hepatic copper deficiency, independent of volume effect, as hepatic copper levels were already significantly reduced by the age at which ob/ob mice exhibited signs of metabolic liver disease[122]. Research involving dietary copper restriction in animals has shown that a low-copper diet results in decreased copper levels in the liver and causes hepatic steatosis[12]. Consistent with these findings, hepatic copper overload was found to reduce fat accumulation in MASLD by activating AMPK.
Hepatic lipid metabolism may play a role in regulating hepatic copper levels. Future rigorous prospective studies are necessary to establish the causal relationship between hepatic copper and hepatic steatosis. Studies have found that mice fed a high-fat diet (HFD) exhibit reduced hepatic copper levels, which may be associated with increased synthesis of the major copper exporting proteins (ATP7A and ATP7B) and CP[11,123], in addition to inhibition of intestinal copper absorption by the high fructose component of the HFD[124]. In vitro studies revealed that fatty acid uptake in hepatocytes induced early mitochondrial dysfunction, elevated cytoplasmic copper, and activated export mechanisms, reducing intracellular copper[125]. Conversely, some studies reported elevated serum and hepatic copper levels in HFD-fed mice, correlating with increased CTR1 and ATP7B mRNA expression[126].
Although MASLD is associated with hepatic copper deficiency, the exact molecular mechanisms remain unclear. Early animal studies suggested that copper deficiency promotes the rate of hepatic fatty acid synthesis and assembly into triacylglycerol and phospholipids[127]. Copper deficiency also enhanced cholesterol synthesis via elevated HMG-CoA reductase activity, raising plasma cholesterol and TG levels[128]. Conversely, hepatocellular cholesterol levels decreased due to reduced cholesterol 7-hydroxylase expression and increased Apo (B and A1)-mediated lipoprotein secretion[129-131]. Additionally, copper-deficient rats exhibited higher nuclear SREBP-1 Levels, attributed to reduced hepatic cho
Several key issues require attention in contemporary research on MASLD and hepatic copper levels. First, it is essential to clarify whether decreased hepatic copper levels are a causative factor in MASLD or a secondary consequence of hepatic steatosis, as this distinction is crucial for understanding the mechanisms of the disease. Second, although patients with MASLD typically have lower copper levels in the liver, serum copper levels are not necessarily reduced[132-134]. This discrepancy underscores the need for further investigation into the role of hepatic copper in MASLD and for the development of more reliable serum biomarkers to assess copper status. Third, greater emphasis should be placed on the importance of maintaining hepatic copper homeostasis in preserving liver health and treating liver diseases. A previous study suggested that the reduction of hepatic copper in MASLD was associated with increased CP synthesis. Experimental CP ablation demonstrated restored copper levels and improved hepatic steatosis in MASLD mice, indicating that CP may be an important therapeutic target for MASLD[11]. Moreover, a recent study developed bio-friendly copper ionophores (HQFs) and showed that HQF-mediated copper delivery can safely and effectively mitigate the progression of fatty liver in mice[135]. Additionally, hepatic copper dysregulation plays a significant role in hepatocyte aging, and it is proposed that reducing overall copper intake or increasing antioxidant consumption may be an important strategy to promote liver longevity and counteract the effects of aging. Dietary copper intake levels are strongly associated with lipid metabolism. Thus, maintaining the delicate hepatic copper balance should be a key focus in future studies.
The chief pathological feature of WD was significant hepatic steatosis, with hepatic copper levels positively correlating with the degree of steatosis[136]. Hepatocyte-specific inactivation of the ATP7B gene confirmed that steatosis resulted directly from copper overload[10]. Factors such as patient age, ATP7B mutation type, and variants in the PNPLA3 gene, which encodes a triacylglycerol lipase, also influence the severity of steatosis. The PNPLA3 G allele and the age of the child were independent correlates of moderate and severe steatosis. Patients with a pure mutation in the ATP7B gene exhibited significantly lower hepatic fat content compared to those with other mutations. Conversely, patients with a mutation in exon 14 demonstrate a notably higher proportion of hepatic LDs than patients with mutations in other exons[9].
Studies on hepatic copper show that copper overload can lead to OxS, mitochondrial dysfunction, ER stress, abnor
Beyond hepatic steatosis, WD also alters serum lipid profiles. While most WD patients had normal serum cholesterol and TG, those with hepatic manifestations exhibited reduced cholesterol levels[149]. Similarly, WD mice showed decreased serum TG, likely due to the upregulation of lipoprotein lipase[150]. In WD mice, hepatic copper accumulation suppressed SREBP-2 and HMG-CoA reductase expression, downregulating cholesterol biosynthesis and potentially explaining the hypocholesterolemia[151]. Notably, this cholesterol reduction was likely a metabolic adaptation rather than a direct driver of steatosis[152].
Despite significant progress, several important questions regarding WD remain unresolved. First, in clinical practice, attention should be paid to the differential diagnosis between WD and MASLD to avoid delaying the treatment of WD. One study found that some WD patients were misdiagnosed with MASLD; subtle histologic features such as distribution and type of steatosis may help differentiate WD from MASLD. Consequently, it is necessary to test serum CP and 24-hour urinary copper to exclude WD in cases of unexplained hepatic steatosis[153,154]. Second, currently, the main therapeutic agents are copper chelators, such as D-penicillamine, zinc salts, and trientine[155]. However, a previous study dem
In WD patients, copper overload causes ISC damage, and significantly lower levels of lipoylase and ISC proteins were observed in WD mice compared to wild-type mice, suggesting a potential role of cuproptosis in WD pathogenesis[157]. ISC proteins are critical for enzymes in the TCA cycle and mitochondrial respiratory chain complexes I-III[158]; their impairment could disrupt metabolic homeostasis. Although no direct evidence links cuproptosis to hepatic steatosis, copper overload may influence lipid metabolism through OxS, mitochondrial dysfunction, IR, and dysregulated lipid-signaling pathways, implying a possible association[8].
Bioinformatics analyses have revealed that cuproptosis-related genes (CRGs) were involved in multiple pathological processes associated with MASLD, including glucose metabolism, lipid metabolism, OxS, and TCA cycle regulation. Among these, 13 CRGs were upregulated, and six were downregulated[159]. Wu et al[160] reported increased expression of DLD (affecting redox homeostasis) and PDHB (promoting pyruvate entry into the TCA cycle) in both MASLD patients and mouse models, suggesting their potential role in hepatic steatosis progression via cuproptosis. Similarly, Ouyang et al[161] identified three key CRGs-NFE2 L2 (attenuating hepatic steatosis and OxS), POLD1 (linked to lipid deposition), and DLD-and developed a diagnostic model for MASLD based on these genes. Additionally, Liu et al[162] identified three CRGs (ENO3, SLC16A1, and LEPR) involved in glycolysis, lactate transport, TG metabolism, and energy homeostasis, which could serve as predictive markers for MASLD risk.
These studies suggest that cuproptosis may play a significant role in hepatic steatosis. Interestingly, despite the reduced hepatic copper levels observed in MASLD patients, the expression of certain CRGs was found to be elevated. This finding highlights the complexity of copper metabolism in hepatic steatosis and indicates the need to reconsider its mechanistic role.
Copper is a key trace element with a tightly regulated metabolism. Clinical studies have demonstrated that copper meta
However, key questions remained unresolved: (1) Most mechanistic insights were derived from aquatic animal studies, necessitating validation in clinical settings; (2) Copper exhibits dual regulatory effects on hepatic lipid metabolism, promoting both lipolysis and lipogenesis. However, uncertainties persist regarding how copper regulates hepatic steatosis; (3) The contributions of cuproptosis and other pathological mechanisms to hepatic steatosis in WD and MASLD require further direct evidence; and (4) Current research still lacks comprehensive strategies for effectively regulating copper homeostasis for disease treatment. In addition to the significant potential of cuproptosis in cancer therapy, restoring hepatic copper homeostasis may represent a critical therapeutic target for liver diseases, particularly in preventing hepatocyte senescence and steatosis. Future studies should prioritize elucidating copper’s specific role in hepatic steatosis in WD and MASLD, investigating trace element effects, and identifying therapeutic targets or diagnostic markers.
| 1. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1186] [Article Influence: 395.3] [Reference Citation Analysis (1)] |
| 2. | Zhao Z, Lucero MY, Su S, Chaney EJ, Xu JJ, Myszka M, Chan J. Activity-based sensing reveals elevated labile copper promotes liver aging via hepatic ALDH1A1 depletion. Nat Commun. 2025;16:1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 675] [Article Influence: 225.0] [Reference Citation Analysis (0)] |
| 4. | Jia D, Liu L, Liu W, Li J, Jiang X, Xin Y. Copper metabolism and its role in diabetic complications: A review. Pharmacol Res. 2024;206:107264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, Stickel F, Datz C. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105:1978-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Gu K, Li X, Xiang W, Jiang X. The Relationship Between Serum Copper and Overweight/Obesity: a Meta-analysis. Biol Trace Elem Res. 2020;194:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Morrell A, Tallino S, Yu L, Burkhead JL. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life. 2017;69:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Li Y, Qi P, Song SY, Wang Y, Wang H, Cao P, Liu Y, Wang Y. Elucidating cuproptosis in metabolic dysfunction-associated steatotic liver disease. Biomed Pharmacother. 2024;174:116585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Stättermayer AF, Traussnigg S, Dienes HP, Aigner E, Stauber R, Lackner K, Hofer H, Stift J, Wrba F, Stadlmayr A, Datz C, Strasser M, Maieron A, Trauner M, Ferenci P. Hepatic steatosis in Wilson disease--Role of copper and PNPLA3 mutations. J Hepatol. 2015;63:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Muchenditsi A, Yang H, Hamilton JP, Koganti L, Housseau F, Aronov L, Fan H, Pierson H, Bhattacharjee A, Murphy R, Sears C, Potter J, Wooton-Kee CR, Lutsenko S. Targeted inactivation of copper transporter Atp7b in hepatocytes causes liver steatosis and obesity in mice. Am J Physiol Gastrointest Liver Physiol. 2017;313:G39-G49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Xie L, Yuan Y, Xu S, Lu S, Gu J, Wang Y, Wang Y, Zhang X, Chen S, Li J, Lu J, Sun H, Hu R, Piao H, Wang W, Wang C, Wang J, Li N, White MF, Han L, Jia W, Miao J, Liu J. Downregulation of hepatic ceruloplasmin ameliorates NAFLD via SCO1-AMPK-LKB1 complex. Cell Rep. 2022;41:111498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Jiang X, Hu R, Huang Y, Xu Y, Zheng Z, Shi Y, Miao J, Liu Y. Fructose aggravates copper-deficiency-induced non-alcoholic fatty liver disease. J Nutr Biochem. 2023;119:109402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Locatelli M, Farina C. Role of copper in central nervous system physiology and pathology. Neural Regen Res. 2025;20:1058-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem. 2009;284:717-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 15. | Campbell CH, Brown R, Linder MC. Circulating ceruloplasmin is an important source of copper for normal and malignant animal cells. Biochim Biophys Acta. 1981;678:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Chen J, Jiang Y, Shi H, Peng Y, Fan X, Li C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch. 2020;472:1415-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 17. | Liu N, Lo LS, Askary SH, Jones L, Kidane TZ, Trang T, Nguyen M, Goforth J, Chu YH, Vivas E, Tsai M, Westbrook T, Linder MC. Transcuprein is a macroglobulin regulated by copper and iron availability. J Nutr Biochem. 2007;18:597-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim Biophys Acta. 2006;1763:759-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Feng W, Ye F, Xue W, Zhou Z, Kang YJ. Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol. 2009;75:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Petris MJ, Strausak D, Mercer JF. The Menkes copper transporter is required for the activation of tyrosinase. Hum Mol Genet. 2000;9:2845-2851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Tchaparian EH, Uriu-Adams JY, Keen CL, Mitchell AE, Rucker RB. Lysyl oxidase and P-ATPase-7A expression during embryonic development in the rat. Arch Biochem Biophys. 2000;379:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 23. | Beaino W, Guo Y, Chang AJ, Anderson CJ. Roles of Atox1 and p53 in the trafficking of copper-64 to tumor cell nuclei: implications for cancer therapy. J Biol Inorg Chem. 2014;19:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys. 2007;463:149-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 339] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Rackova M, Mattera R, Svaton M, Fencl F, Kanderova V, Spicakova K, Park SY, Fabian O, Koblizek M, Fronkova E, Bonifacino JS, Skvarova Kramarzova K. Revising pathogenesis of AP1S1-related MEDNIK syndrome: a missense variant in the AP1S1 gene as a causal genetic lesion. J Mol Med (Berl). 2024;102:1343-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Menkes protein contributes to the function of peptidylglycine alpha-amidating monooxygenase. Endocrinology. 2003;144:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Schmidt K, Ralle M, Schaffer T, Jayakanthan S, Bari B, Muchenditsi A, Lutsenko S. ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-β-hydroxylase. J Biol Chem. 2018;293:20085-20098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 629] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 29. | Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, Eaton JK, Frenkel E, Kocak M, Corsello SM, Lutsenko S, Kanarek N, Santagata S, Golub TR. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 2491] [Article Influence: 830.3] [Reference Citation Analysis (1)] |
| 30. | Yang L, Yang P, Lip GYH, Ren J. Copper homeostasis and cuproptosis in cardiovascular disease therapeutics. Trends Pharmacol Sci. 2023;44:573-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 31. | Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 386] [Reference Citation Analysis (0)] |
| 32. | Vazquez-Moreno M, Sandoval-Castillo M, Rios-Lugo MJ, Klünder-Klünder M, Cruz M, Martínez-Navarro I, Romero-Guzmán ET, Victoria-Campos CI, Vilchis-Gil J, Hernández-Mendoza H. Overweight and Obesity Are Positively Associated with Serum Copper Levels in Mexican Schoolchildren. Biol Trace Elem Res. 2023;201:2744-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | Song X, Wang W, Li Z, Zhang D. Association between Serum Copper and Serum Lipids in Adults. Ann Nutr Metab. 2018;73:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Ge W, Liu W, Liu G. The relationships between serum copper levels and overweight/total obesity and central obesity in children and adolescents aged 6-18 years. J Trace Elem Med Biol. 2020;61:126557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Liu M, Fang C, Mei K, Ling J, Fu W, Qi X, Yu P, Yan Z, Xu L, Zhao Y, Li X, Liu X. Serum copper and obesity among healthy adults in the National Health and Nutrition Examination Survey. PLoS One. 2024;19:e0300795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Zhao J, Cao X, Li Q, Xie J, Wu H. Obesity Mediates the Association Between Serum Copper and Inflammation: A Cross-sectional and Mendelian Randomization Study. Biol Trace Elem Res. 2025;203:3009-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Wang W, Liu L, Shan R, Wang C. Associations between dietary copper intake, general obesity and abdominal obesity risk: A nationwide cohort study in China. Front Nutr. 2022;9:1009721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Shi Y, Hu H, Wu Z, Wu J, Chen Z, Cheng X, Li P. Associations between dietary copper intake and hypertriglyceridemia among children and adolescents in the US. Nutr Metab Cardiovasc Dis. 2023;33:809-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Bo S, Durazzo M, Gambino R, Berutti C, Milanesio N, Caropreso A, Gentile L, Cassader M, Cavallo-Perin P, Pagano G. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J Nutr. 2008;138:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Galhardi CM, Diniz YS, Rodrigues HG, Faine LA, Burneiko RC, Ribas BO, Novelli EL. Beneficial effects of dietary copper supplementation on serum lipids and antioxidant defenses in rats. Ann Nutr Metab. 2005;49:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Sánchez C, López-Jurado M, Aranda P, Llopis J. Plasma levels of copper, manganese and selenium in an adult population in southern Spain: influence of age, obesity and lifestyle factors. Sci Total Environ. 2010;408:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Vivek SM, Dayal D, Khaiwal R, Bharti B, Bhalla A, Singh S, Kaur H, Attri SV. Low serum copper and zinc concentrations in North Indian children with overweight and obesity. Pediatr Endocrinol Diabetes Metab. 2020;26:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Jaksic M, Martinovic M, Gligorovic-Barhanovic N, Vujacic A, Djurovic D, Nedovic-Vukovic M. Association between inflammation, oxidative stress, vitamin D, copper and zinc with pre-obesity and obesity in school children from the city of Podgorica, Montenegro. J Pediatr Endocrinol Metab. 2019;32:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26:268-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 573] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 45. | Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1849] [Cited by in RCA: 2284] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 46. | Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants (Basel). 2021;10:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 47. | Petan T, Jarc E, Jusović M. Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules. 2018;23:1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 265] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 48. | Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 636] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 49. | Zhong CC, Zhao T, Hogstrand C, Song CC, Zito E, Tan XY, Xu YC, Song YF, Wei XL, Luo Z. Copper induces liver lipotoxicity disease by up-regulating Nrf2 expression via the activation of MTF-1 and inhibition of SP1/Fyn pathway. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 50. | Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291-13295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2104] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 51. | Zhong CC, Zhao T, Hogstrand C, Chen F, Song CC, Luo Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem. 2022;100:108883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 52. | García-Ruiz C, Baulies A, Mari M, García-Rovés PM, Fernandez-Checa JC. Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radic Res. 2013;47:854-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Yu W, Liao J, Yang F, Zhang H, Chang X, Yang Y, Bilal RM, Wei G, Liang W, Guo J, Tang Z. Chronic tribasic copper chloride exposure induces rat liver damage by disrupting the mitophagy and apoptosis pathways. Ecotoxicol Environ Saf. 2021;212:111968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes. 2009;58:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 55. | Xu YH, Xu YC, Hogstrand C, Zhao T, Wu LX, Zhuo MQ, Luo Z. Waterborne copper exposure up-regulated lipid deposition through the methylation of GRP78 and PGC1α of grass carp Ctenopharyngodon idella. Ecotoxicol Environ Saf. 2020;205:111089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69:927-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 658] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 57. | Byun JH, Lebeau PF, Trink J, Uppal N, Lanktree MB, Krepinsky JC, Austin RC. Endoplasmic reticulum stress as a driver and therapeutic target for kidney disease. Nat Rev Nephrol. 2025;21:299-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 58. | Song YF, Xu YH, Zhuo MQ, Wu K, Luo Z. CREB element is essential for unfolded protein response (UPR) mediating the Cu-induced changes of hepatic lipogenic metabolism in Chinese yellow catfish (Pelteobagrus fulvidraco). Aquat Toxicol. 2018;203:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Song YF, Luo Z, Zhang LH, Hogstrand C, Pan YX. Endoplasmic reticulum stress and disturbed calcium homeostasis are involved in copper-induced alteration in hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Chemosphere. 2016;144:2443-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 61. | Eshak ES, Iso H, Maruyama K, Muraki I, Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population-based prospective cohort study. Clin Nutr. 2018;37:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 62. | Yin J, Wang X, Li S, Zhu Y, Chen S, Li P, Luo C, Huang Y, Li X, Hu X, Yang W, Bao W, Shan Z, Liu L. Interactions between plasma copper concentrations and SOD1 gene polymorphism for impaired glucose regulation and type 2 diabetes. Redox Biol. 2019;24:101172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 63. | Tanaka A, Kaneto H, Miyatsuka T, Yamamoto K, Yoshiuchi K, Yamasaki Y, Shimomura I, Matsuoka TA, Matsuhisa M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr J. 2009;56:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 64. | Li K, Yang Y, Zhao J, Zhou Q, Li Y, Yang M, Hu Y, Xu J, Zhao M, Xu Q. Associations of metals and metal mixtures with glucose homeostasis: A combined bibliometric and epidemiological study. J Hazard Mater. 2024;470:134224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Roy D, Maity NC, Kumar S, Maity A, Ratha BN, Biswas R, Maiti NC, Mandal AK, Bhunia A. Modulatory role of copper on hIAPP aggregation and toxicity in presence of insulin. Int J Biol Macromol. 2023;241:124470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 66. | Panzitt K, Wagner M. FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 67. | Wooton-Kee CR, Jain AK, Wagner M, Grusak MA, Finegold MJ, Lutsenko S, Moore DD. Elevated copper impairs hepatic nuclear receptor function in Wilson's disease. J Clin Invest. 2015;125:3449-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 68. | Liu Y, Zhang S, Deng H, Chen A, Chai L. Lead and copper influenced bile acid metabolism by changing intestinal microbiota and activating farnesoid X receptor in Bufo gargarizans. Sci Total Environ. 2023;863:160849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 69. | Huang R, Guo F, Li Y, Liang Y, Li G, Fu P, Ma L. Activation of AMPK by triptolide alleviates nonalcoholic fatty liver disease by improving hepatic lipid metabolism, inflammation and fibrosis. Phytomedicine. 2021;92:153739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 70. | Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 2736] [Article Influence: 342.0] [Reference Citation Analysis (0)] |
| 71. | Gybina AA, Prohaska JR. Copper deficiency results in AMP-activated protein kinase activation and acetylCoA carboxylase phosphorylation in rat cerebellum. Brain Res. 2008;1204:69-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Liao J, Yang F, Yu W, Qiao N, Zhang H, Han Q, Hu L, Li Y, Guo J, Pan J, Tang Z. Copper induces energy metabolic dysfunction and AMPK-mTOR pathway-mediated autophagy in kidney of broiler chickens. Ecotoxicol Environ Saf. 2020;206:111366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 73. | Chen J, Liao J, Yu W, Cao H, Hu G, Tang Z, Al-Mutairi KA, Yang F. Copper toxicity in the liver of broiler chicken: insights from metabolomics and AMPK-mTOR mediated autophagy perspective. Poult Sci. 2024;103:104011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 74. | Chen H, Wang Y, Luo J, Kang M, Hou J, Tang R, Zhao L, Shi F, Ye G, He X, Cui H, Guo H, Li Y, Tang H. Autophagy and apoptosis mediated nano-copper-induced testicular damage. Ecotoxicol Environ Saf. 2022;229:113039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Ramchandani D, Berisa M, Tavarez DA, Li Z, Miele M, Bai Y, Lee SB, Ban Y, Dephoure N, Hendrickson RC, Cloonan SM, Gao D, Cross JR, Vahdat LT, Mittal V. Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis. Nat Commun. 2021;12:7311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 76. | Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1524] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 77. | Chen GH, Luo Z, Hogstrand C, Wu K, Ling SC. SREBP1, PPARG and AMPK pathways mediated the Cu-induced change in intestinal lipogenesis and lipid transport of yellow catfish Pelteobagrus fulvidraco. Food Chem. 2018;269:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Lei L, Xiaoyi S, Fuchang L. Effect of dietary copper addition on lipid metabolism in rabbits. Food Nutr Res. 2017;61:1348866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Liu L, Fu J, Tang Q, Wang H, Lin C, Wei L. Combined transcriptomics and metabolomics analysis reveals lipid metabolic disruption in swamp eel (Monopterus albus) under chronic waterborne copper exposure. Aquat Toxicol. 2023;259:106520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 80. | Huang C, Chen QL, Luo Z, Shi X, Pan YX, Song YF, Zhuo MQ, Wu K. Time-dependent effects of waterborne copper exposure influencing hepatic lipid deposition and metabolism in javelin goby Synechogobius hasta and their mechanism. Aquat Toxicol. 2014;155:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Lan AP, Xiong XJ, Chen J, Wang X, Chai ZF, Hu Y. AMPK Inhibition Enhances the Neurotoxicity of Cu(II) in SH-SY5Y Cells. Neurotox Res. 2016;30:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1502] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 83. | Dev S, Muchenditsi A, Gottlieb A, Deme P, Murphy S, Gabrielson KL, Dong Y, Hughes R, Haughey NJ, Hamilton JP, Lutsenko S. Oxysterol misbalance critically contributes to Wilson disease pathogenesis. Sci Adv. 2022;8:eadc9022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Hamilton JP, Koganti L, Muchenditsi A, Pendyala VS, Huso D, Hankin J, Murphy RC, Huster D, Merle U, Mangels C, Yang N, Potter JJ, Mezey E, Lutsenko S. Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease in Atp7B(-/-) (Wilson disease) mice. Hepatology. 2016;63:1828-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 85. | Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, Lefebvre P, Taskinen MR, Van Hul W, Mertens I, Hubens G, Van Marck E, Michielsen P, Van Gaal L, Staels B. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 86. | Morán-Salvador E, López-Parra M, García-Alonso V, Titos E, Martínez-Clemente M, González-Périz A, López-Vicario C, Barak Y, Arroyo V, Clària J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 87. | Wang T, Wei X, Chen T, Wang W, Xia X, Miao J, Yin S. Studies of the mechanism of fatty liver formation in Takifugu fasciatus following copper exposure. Ecotoxicol Environ Saf. 2019;181:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Li F, Wu X, Liu H, Zhang B, Liu L, Li F. Dietary copper supplementation enhances lipolysis in Rex rabbits. J Trace Elem Med Biol. 2021;68:126851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Chen QL, Luo Z, Pan YX, Zheng JL, Zhu QL, Sun LD, Zhuo MQ, Hu W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat Toxicol. 2013;136-137:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Abbehausen C. Zinc finger domains as therapeutic targets for metal-based compounds - an update. Metallomics. 2019;11:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 91. | Jeon YG, Kim YY, Lee G, Kim JB. Physiological and pathological roles of lipogenesis. Nat Metab. 2023;5:735-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 92. | Yang H, Liu CN, Wolf RM, Ralle M, Dev S, Pierson H, Askin F, Steele KE, Magnuson TH, Schweitzer MA, Wong GW, Lutsenko S. Obesity is associated with copper elevation in serum and tissues. Metallomics. 2019;11:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 93. | Arner E, Forrest AR, Ehrlund A, Mejhert N, Itoh M, Kawaji H, Lassmann T, Laurencikiene J, Rydén M, Arner P; FANTOM Consortium. Ceruloplasmin is a novel adipokine which is overexpressed in adipose tissue of obese subjects and in obesity-associated cancer cells. PLoS One. 2014;9:e80274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Yang H, Kabin E, Dong Y, Zhang X, Ralle M, Lutsenko S. ATP7A-dependent copper sequestration contributes to termination of β-CATENIN signaling during early adipogenesis. Mol Metab. 2024;80:101872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 95. | Matsushita K, Morello F, Zhang Z, Masuda T, Iwanaga S, Steffensen KR, Gustafsson JÅ, Pratt RE, Dzau VJ. Nuclear hormone receptor LXRα inhibits adipocyte differentiation of mesenchymal stem cells with Wnt/beta-catenin signaling. Lab Invest. 2016;96:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Tao C, Wang Y, Zhao Y, Pan J, Fan Y, Liang X, Cao C, Zhao J, Petris MJ, Li K, Wang Y. Adipocyte-specific disruption of ATPase copper transporting α in mice accelerates lipoatrophy. Diabetologia. 2019;62:2340-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Yang H, Ralle M, Wolfgang MJ, Dhawan N, Burkhead JL, Rodriguez S, Kaplan JH, Wong GW, Haughey N, Lutsenko S. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 2018;16:e2006519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 98. | Krishnamoorthy L, Cotruvo JA Jr, Chan J, Kaluarachchi H, Muchenditsi A, Pendyala VS, Jia S, Aron AT, Ackerman CM, Wal MN, Guan T, Smaga LP, Farhi SL, New EJ, Lutsenko S, Chang CJ. Copper regulates cyclic-AMP-dependent lipolysis. Nat Chem Biol. 2016;12:586-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 99. | Gjorgjieva M, Sobolewski C, Dolicka D, Correia de Sousa M, Foti M. miRNAs and NAFLD: from pathophysiology to therapy. Gut. 2019;68:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 100. | Cui HY, Chen QL, Tan XY, Zhang DG, Ling SC, Chen GH, Luo Z. MiR-205 Mediated Cu-Induced Lipid Accumulation in Yellow Catfish Pelteobagrus fulvidraco. Int J Mol Sci. 2018;19:2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Maniscalchi A, Benzi Juncos ON, Conde MA, Funk MI, Fermento ME, Facchinetti MM, Curino AC, Uranga RM, Alza NP, Salvador GA. New insights on neurodegeneration triggered by iron accumulation: Intersections with neutral lipid metabolism, ferroptosis, and motor impairment. Redox Biol. 2024;71:103074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 102. | Song CC, Pantopoulos K, Chen GH, Zhong CC, Zhao T, Zhang DG, Luo Z. Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1α-PPARγ pathway. Cell Mol Life Sci. 2022;79:394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 103. | Fernández-Real JM, McClain D, Manco M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care. 2015;38:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 104. | Aigner E, Theurl I, Haufe H, Seifert M, Hohla F, Scharinger L, Stickel F, Mourlane F, Weiss G, Datz C. Copper availability contributes to iron perturbations in human nonalcoholic fatty liver disease. Gastroenterology. 2008;135:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 105. | Gromadzka G, Wierzbicka D, Litwin T, Przybyłkowski A. Iron metabolism is disturbed and anti-copper treatment improves but does not normalize iron metabolism in Wilson's disease. Biometals. 2021;34:407-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Zhou J, Liu C, Francis M, Sun Y, Ryu MS, Grider A, Ye K. The Causal Effects of Blood Iron and Copper on Lipid Metabolism Diseases: Evidence from Phenome-Wide Mendelian Randomization Study. Nutrients. 2020;12:3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 107. | Behari J, Li H, Liu S, Stefanovic-Racic M, Alonso L, O'Donnell CP, Shiva S, Singamsetty S, Watanabe Y, Singh VP, Liu Q. β-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. Am J Pathol. 2014;184:3284-3298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, Wu T, Evans R, Monga SP. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 109. | Lehwald N, Tao GZ, Jang KY, Papandreou I, Liu B, Liu B, Pysz MA, Willmann JK, Knoefel WT, Denko NC, Sylvester KG. β-Catenin regulates hepatic mitochondrial function and energy balance in mice. Gastroenterology. 2012;143:754-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 110. | Xu YC, Xu YH, Zhao T, Wu LX, Yang SB, Luo Z. Waterborne Cu exposure increased lipid deposition and lipogenesis by affecting Wnt/β-catenin pathway and the β-catenin acetylation levels of grass carp Ctenopharyngodon idella. Environ Pollut. 2020;263:114420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 111. | Fan YT, Peng DQ, Shen JL, Cui JH, Yang XY, Zhang JG, Jin YC. Copper excess induces autophagy dysfunction and mitochondrial ROS-ferroptosis progression, inhibits cellular biosynthesis of milk protein and lipid in bovine mammary epithelial cells. Ecotoxicol Environ Saf. 2025;291:117783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 112. | Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 250] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 113. | Wang K. Molecular mechanism of hepatic steatosis: pathophysiological role of autophagy. Expert Rev Mol Med. 2016;18:e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 114. | Yoo J, Jun JE, Jeong IK, Ahn KJ, Chung HY, Lee MS, Hwang YC. DA-1241, a GPR119 Agonist, Ameliorates Fatty Liver Through the Upregulation of TFEB-Mediated Autophagy. Diabetes. 2025;74:1107-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 115. | Liu H, Deng H, Cui H, Jian Z, Guo H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Copper induces hepatocyte autophagy via the mammalian targets of the rapamycin signaling pathway in mice. Ecotoxicol Environ Saf. 2021;208:111656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Wang Y, Zhao H, Shao Y, Liu J, Li J, Luo L, Xing M. Copper or/and arsenic induces autophagy by oxidative stress-related PI3K/AKT/mTOR pathways and cascaded mitochondrial fission in chicken skeletal muscle. J Inorg Biochem. 2018;188:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 117. | Deng H, Lou Y, He R, Deng J, Zhu Y, Wu X, Guo H. RETRACTED ARTICLE: Copper Exposure Destroys the Integrity of the Blood-Testis Barrier (BTB) Through p38 MAPK-Meditated Autophagy Pathways. Biol Trace Elem Res. 2025;203:3987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Li X, Gu D, You J, Qiao T, Yu X. Gamma-aminobutyric acid coupled with copper ion stress stimulates lipid production of green microalga Monoraphidium sp. QLY-1 through multiple mechanisms. Bioresour Technol. 2022;352:127091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48 Suppl 1:S104-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 416] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 120. | Yang AH, Tincopa MA, Tavaglione F, Ajmera VH, Richards LM, Amangurbanova M, Butcher C, Hernandez C, Madamba E, Singh S, Bettencourt R, Schnabl B, Sirlin CB, Loomba R. Prevalence of steatotic liver disease, advanced fibrosis and cirrhosis among community-dwelling overweight and obese individuals in the USA. Gut. 2024;73:2045-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 121. | Stättermayer AF, Traussnigg S, Aigner E, Kienbacher C, Huber-Schönauer U, Steindl-Munda P, Stadlmayr A, Wrba F, Trauner M, Datz C, Ferenci P. Low hepatic copper content and PNPLA3 polymorphism in non-alcoholic fatty liver disease in patients without metabolic syndrome. J Trace Elem Med Biol. 2017;39:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 122. | Church SJ, Begley P, Kureishy N, McHarg S, Bishop PN, Bechtold DA, Unwin RD, Cooper GJ. Deficient copper concentrations in dried-defatted hepatic tissue from ob/ob mice: A potential model for study of defective copper regulation in metabolic liver disease. Biochem Biophys Res Commun. 2015;460:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Heffern MC, Park HM, Au-Yeung HY, Van de Bittner GC, Ackerman CM, Stahl A, Chang CJ. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2016;113:14219-14224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 124. | Song M, Schuschke DA, Zhou Z, Chen T, Pierce WM Jr, Wang R, Johnson WT, McClain CJ. High fructose feeding induces copper deficiency in Sprague-Dawley rats: a novel mechanism for obesity related fatty liver. J Hepatol. 2012;56:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 125. | Harder NHO, Lee HP, Flood VJ, San Juan JA, Gillette SK, Heffern MC. Fatty Acid Uptake in Liver Hepatocytes Induces Relocalization and Sequestration of Intracellular Copper. Front Mol Biosci. 2022;9:863296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Santini SJ, Tarantino G, Iezzi A, Alisi A, Balsano C. Copper-catalyzed dicarbonyl stress in NAFLD mice: protective effects of Oleuropein treatment on liver damage. Nutr Metab (Lond). 2022;19:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 127. | al-Othman AA, Rosenstein F, Lei KY. Copper deficiency increases in vivo hepatic synthesis of fatty acids, triacylglycerols, and phospholipids in rats. Proc Soc Exp Biol Med. 1993;204:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Yount NY, McNamara DJ, Al-Othman AA, Lei KY. The effect of copper deficiency on rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. J Nutr Biochem. 1990;1:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Reaves SK, Hoogeveen RC, Wang YR, Wu JY, Lei KY. Copper deficiency increases hepatic apolipoprotein B secretion and mRNA editing in rats. Am J Physiol. 1996;271:C595-C604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 130. | Tang Z, Gasperkova D, Xu J, Baillie R, Lee JH, Clarke SD. Copper deficiency induces hepatic fatty acid synthase gene transcription in rats by increasing the nuclear content of mature sterol regulatory element binding protein 1. J Nutr. 2000;130:2915-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Hoogeveen RC, Reaves SK, Lei KY. Copper deficiency increases hepatic apolipoprotein A-I synthesis and secretion but does not alter hepatic total cellular apolipoprotein A-I mRNA abundance in rats. J Nutr. 1995;125:2935-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 132. | Chen Y, Wu C, Li G, Wang W, Tang S. Comparison of copper concentration between non-alcoholic fatty liver disease patients and normal individuals: A meta-analysis. Front Public Health. 2023;11:1095916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 133. | Lan Y, Wu S, Wang Y, Chen S, Liao W, Zhang X, Pan L, Jiang X, Zhang Y, Wang L. Association between blood copper and nonalcoholic fatty liver disease according to sex. Clin Nutr. 2021;40:2045-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 134. | Chen C, Zhou Q, Yang R, Wu Z, Yuan H, Zhang N, Zhi M, Zhang Y, Ni X, Wang Z, Gao D, Zhu X, Cai J, Yang Z, Sun L. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011-2014). Ecotoxicol Environ Saf. 2021;218:112295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 135. | Jiang J, Chen G, Zhang W, Qin S, Li M, Zhong S, Yang Y, Yang L, Shao M, Wang K, Li Q, Jiang C, Yang J, Wang F, Qiu S, Li X. Pseudonatural Flavonols as Novel Copper Ionophores for NAFLD Intervention via Synergistic Copper Delivery and Flavonoid Activity. J Med Chem. 2025;68:6450-6461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 136. | Liggi M, Murgia D, Civolani A, Demelia E, Sorbello O, Demelia L. The relationship between copper and steatosis in Wilson's disease. Clin Res Hepatol Gastroenterol. 2013;37:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Day PL, Fyffe-Freil R, Vanderboom P, Spears G, Wangensteen K, Bornhorst J, Hassan S, Jannetto PJ. Iron and Copper Liver Concentrations in Wilson Disease. J Gastrointestin Liver Dis. 2024;33:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 138. | Polishchuk EV, Merolla A, Lichtmannegger J, Romano A, Indrieri A, Ilyechova EY, Concilli M, De Cegli R, Crispino R, Mariniello M, Petruzzelli R, Ranucci G, Iorio R, Pietrocola F, Einer C, Borchard S, Zibert A, Schmidt HH, Di Schiavi E, Puchkova LV, Franco B, Kroemer G, Zischka H, Polishchuk RS. Activation of Autophagy, Observed in Liver Tissues From Patients With Wilson Disease and From ATP7B-Deficient Animals, Protects Hepatocytes From Copper-Induced Apoptosis. Gastroenterology. 2019;156:1173-1189.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 139. | Cheng C, Wang Q, Huang Y, Xue Q, Wang Y, Wu P, Liao F, Miao C. Gandouling inhibits hepatic fibrosis in Wilson's disease through Wnt-1/β-catenin signaling pathway. J Ethnopharmacol. 2023;311:116445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 140. | Wooton-Kee CR, Robertson M, Zhou Y, Dong B, Sun Z, Kim KH, Liu H, Xu Y, Putluri N, Saha P, Coarfa C, Moore DD, Nuotio-Antar AM. Metabolic dysregulation in the Atp7b(-/-) Wilson's disease mouse model. Proc Natl Acad Sci U S A. 2020;117:2076-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 141. | Roberts EA, Robinson BH, Yang S. Mitochondrial structure and function in the untreated Jackson toxic milk (tx-j) mouse, a model for Wilson disease. Mol Genet Metab. 2008;93:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 142. | Oe S, Miyagawa K, Honma Y, Harada M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp Cell Res. 2016;347:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 143. | Li J, Jiang Y, Xu T, Zhang Y, Xue J, Gao X, Yang X, Wang X, Jia X, Cheng W, Jin S. Wilson Disease With Novel Compound Heterozygote Mutations in the ATP7B Gene Presenting With Severe Diabetes. Diabetes Care. 2020;43:1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 144. | Wooton-Kee CR. Therapeutic implications of impaired nuclear receptor function and dysregulated metabolism in Wilson's disease. Pharmacol Ther. 2023;251:108529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Nagasaka H, Miida T, Inui A, Inoue I, Tsukahara H, Komatsu H, Hiejima E, Fujisawa T, Yorifuji T, Hiranao K, Okajima H, Inomata Y. Fatty liver and anti-oxidant enzyme activities along with peroxisome proliferator-activated receptors γ and α expressions in the liver of Wilson's disease. Mol Genet Metab. 2012;107:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Mordaunt CE, Kieffer DA, Shibata NM, Członkowska A, Litwin T, Weiss KH, Zhu Y, Bowlus CL, Sarkar S, Cooper S, Wan YY, Ali MR, LaSalle JM, Medici V. Epigenomic signatures in liver and blood of Wilson disease patients include hypermethylation of liver-specific enhancers. Epigenetics Chromatin. 2019;12:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 147. | Gholizadeh M, Szelag-Pieniek S, Post M, Kurzawski M, Prieto J, Argemi J, Drozdzik M, Kaderali L. Identifying Differentially Expressed MicroRNAs, Target Genes, and Key Pathways Deregulated in Patients with Liver Diseases. Int J Mol Sci. 2020;21:7368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 148. | Mi X, Li Z, Yan J, Li Y, Zheng J, Zhuang Z, Yang W, Gong L, Shi J. Activation of HIF-1 signaling ameliorates liver steatosis in zebrafish atp7b deficiency (Wilson's disease) models. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Seessle J, Gohdes A, Gotthardt DN, Pfeiffenberger J, Eckert N, Stremmel W, Reuner U, Weiss KH. Alterations of lipid metabolism in Wilson disease. Lipids Health Dis. 2011;10:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 150. | Huster D, Lutsenko S. Wilson disease: not just a copper disorder. Analysis of a Wilson disease model demonstrates the link between copper and lipid metabolism. Mol Biosyst. 2007;3:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 151. | Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, Olson NE, Teupser D, Lutsenko S. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem. 2007;282:8343-8355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 152. | Gottlieb A, Dev S, DeVine L, Gabrielson KL, Cole RN, Hamilton JP, Lutsenko S. Hepatic Steatosis in the Mouse Model of Wilson Disease Coincides with a Muted Inflammatory Response. Am J Pathol. 2022;192:146-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 153. | Schroeder SM, Matsukuma KE, Medici V. Wilson disease and the differential diagnosis of its hepatic manifestations: a narrative review of clinical, laboratory, and liver histological features. Ann Transl Med. 2021;9:1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 154. | Hassan MK, Alswat K, El-Kassas M. Shedding lights on diagnostic challenges in Wilson's disease: The positive impact of introducing steatotic liver disease new nomenclature umbrella. Liver Int. 2024;44:1267-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 155. | Mohr I, Schmitt T, Weber C, Schall N, Leidner VY, Langel A, Langel J, Poujois A, Weiss KH, Merle U. Clinical experience on switching trientine tetrahydrochloride to trientine dihydrochloride in Wilson disease patients. JIMD Rep. 2024;65:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 156. | Einer C, Leitzinger C, Lichtmannegger J, Eberhagen C, Rieder T, Borchard S, Wimmer R, Denk G, Popper B, Neff F, Polishchuk EV, Polishchuk RS, Hauck SM, von Toerne C, Müller JC, Karst U, Baral BS, DiSpirito AA, Kremer AE, Semrau J, Weiss KH, Hohenester S, Zischka H. A High-Calorie Diet Aggravates Mitochondrial Dysfunction and Triggers Severe Liver Damage in Wilson Disease Rats. Cell Mol Gastroenterol Hepatol. 2019;7:571-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 157. | Sarode GV, Kim K, Kieffer DA, Shibata NM, Litwin T, Czlonkowska A, Medici V. Metabolomics profiles of patients with Wilson disease reveal a distinct metabolic signature. Metabolomics. 2019;15:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 158. | Stiban J, So M, Kaguni LS. Iron-Sulfur Clusters in Mitochondrial Metabolism: Multifaceted Roles of a Simple Cofactor. Biochemistry (Mosc). 2016;81:1066-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 159. | Tan W, Zhang J, Chen L, Wang Y, Chen R, Zhang H, Liang F. Copper homeostasis and cuproptosis-related genes: Therapeutic perspectives in non-alcoholic fatty liver disease. Diabetes Obes Metab. 2024;26:4830-4845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 160. | Wu C, Liu X, Zhong L, Zhou Y, Long L, Yi T, Chen S, Li Y, Chen Y, Shen L, Zeng Q, Tang S. Identification of Cuproptosis-Related Genes in Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2023;2023:9245667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 161. | Ouyang G, Wu Z, Liu Z, Pan G, Wang Y, Liu J, Guo J, Liu T, Huang G, Zeng Y, Wei Z, He S, Yuan G. Identification and validation of potential diagnostic signature and immune cell infiltration for NAFLD based on cuproptosis-related genes by bioinformatics analysis and machine learning. Front Immunol. 2023;14:1251750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 162. | Liu C, Fang Z, Yang K, Ji Y, Yu X, Guo Z, Dong Z, Zhu T, Liu C. Identification and validation of cuproptosis-related molecular clusters in non-alcoholic fatty liver disease. J Cell Mol Med. 2024;28:e18091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |