Published online Aug 27, 2025. doi: 10.4254/wjh.v17.i8.107738
Revised: May 21, 2025
Accepted: July 23, 2025
Published online: August 27, 2025
Processing time: 152 Days and 10.2 Hours
Vitamin A is essential for vision, immunity, and cellular function, but excessive intake, known as hypervitaminosis A, leads to liver toxicity. Toxicity can be acute (from high single doses) or chronic (from prolonged overconsumption), causing symptoms like nausea, bone pain, and liver damage. The normal values of vitamin A in adults, measured as serum retinol, can range from 0.3 mg/L to 1.2 mg/L. The liver, which stores vitamin A in hepatic stellate cells, becomes overwhelmed, leading to retinoid accumulation, oxidative stress, and inflammation. Pathologically, vitamin A toxicity progresses from hepatic steatosis (fatty liver) to fibrosis and cirrhosis. Histological changes include hepatocellular ballooning, stellate cell activation, and perisinusoidal fibrosis. Molecular mech
Core Tip: Vitamin A toxicity (hypervitaminosis A) causes serious liver damage, progressing from steatosis to fibrosis and cirrhosis. The liver’s storage capacity is overwhelmed, leading to retinoid accumulation, oxidative stress, and inflammation. Key mechanisms include reactive oxygen species generation, apoptosis, and dysregulated pathways (tumor growth factor-beta, nuclear factor-kappa B), which drive stellate cell activation and fibrosis. Clinically, chronic toxicity manifests as hepatomegaly, portal hypertension, and potential liver failure. Management involves limiting vitamin A intake and exploring antioxidants (e.g., N-acetylcysteine) or anti-fibrotic therapies. Future research should focus on biomarkers, personalized risk assessment, and safer dietary guidelines. Public awareness and therapeutic advancements are crucial to prevent liver disease.
- Citation: Pestalardo ML, Bevilacqua CS, Amante MF. Vitamin A toxicity and hepatic pathology: A comprehensive review. World J Hepatol 2025; 17(8): 107738
- URL: https://www.wjgnet.com/1948-5182/full/v17/i8/107738.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i8.107738
Vitamin A, a fat-soluble vitamin, is essential for vision, immune function, cellular differentiation, and reproduction[1]. It exists in forms such as retinol, retinoic acid, and carotenoids, each with unique biological roles[2]. While crucial for health, its narrow therapeutic index means that both deficiency and excess can cause significant problems[3].
Vitamin A toxicity, known as hypervitaminosis A, is classified into acute and chronic forms[4]. Acute toxicity arises from a single, excessively high dose, often due to accidental ingestion or over-supplementation, and manifests rapidly with symptoms such as nausea, dizziness, and intracranial hypertension[5]. Chronic toxicity develops from sustained high intake, exceeding recommended daily allowances, and presents with gradual symptoms like fatigue, bone pain, and liver dysfunction[6]. Both forms highlight the narrow therapeutic index of vitamin A[7].
The liver, the primary site of vitamin A metabolism and storage, is particularly susceptible to damage from retinoid overload[8]. The pathological anatomy of liver damage in vitamin A toxicity includes hepatic steatosis, fibrosis, and cirrhosis. The early stages are characterized by lipid accumulation in hepatocytes, progressing to scar tissue formation and structural distortion of the liver[9].
Understanding the molecular mechanisms of vitamin A-induced liver toxicity is essential for developing targeted interventions[10]. Excessive vitamin A metabolism causes oxidative stress and the generation of reactive oxygen species (ROS), causing lipid peroxidation, DNA damage, and protein modifications[11]. Mitochondrial dysfunction and inflammatory cytokines induce apoptosis and necrosis, further contributing to hepatocyte loss[12]. Dysregulation of signaling pathways like tumor growth factor-beta (TGF-β) and nuclear factor-kappa B (NF-κB) exacerbates fibrogenesis and inflammation[13].
Clinicians and researchers must prioritize studying vitamin A toxicity due to its public health implications[14]. At-risk populations, such as those consuming high-dose supplements or diets rich in animal liver, require tailored guidelines to prevent toxicity[15]. Advancing our understanding of these mechanisms will inform therapeutic strategies to mitigate liver damage[16]. This review focuses on the pathological anatomy of liver damage induced by vitamin A toxicity, emphasizing its clinical significance and future research directions[17].
Vitamin A toxicity is associated with hepatic steatosis, fibrosis, and cirrhosis, arising from the liver's central role in vitamin A metabolism and storage. Excessive intake overwhelms the liver’s capacity to store retinol in stellate cells, leading to hepatocellular damage and systemic toxicity[18].
Hepatic steatosis is an early manifestation and results from disrupted lipid homeostasis and triglyceride accumulation due to excessive retinoid metabolism[19]. Over time, oxidative stress, inflammation, and stellate cell activation contribute to fibrosis, marked by extracellular matrix deposition and architectural distortion. Advanced cases progress to cirrhosis, characterized by bridging fibrosis and irreversible liver damage[20].
Chronic toxicity also leads to portal hypertension, hepatomegaly, and impaired liver function, manifesting as abdominal pain, jaundice, and ascites. Severe cases may result in liver failure and death[21].
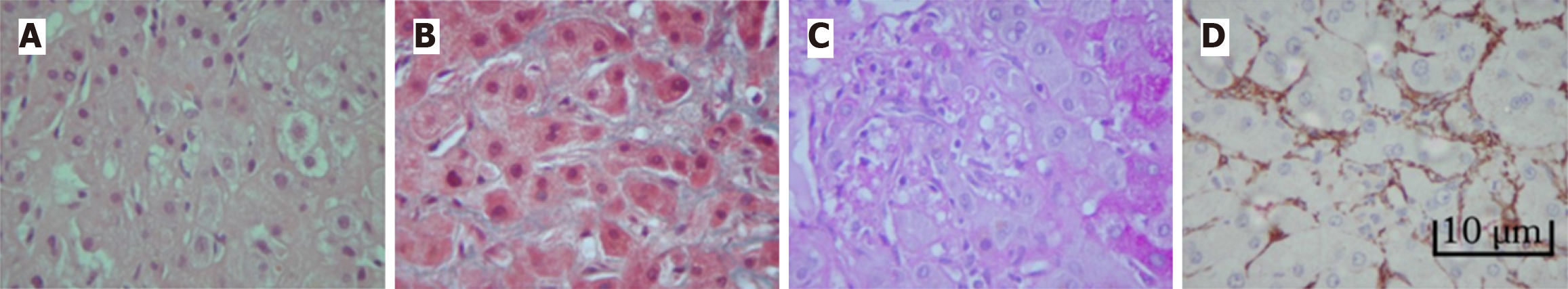
Histopathological examination reveals vacuolar degeneration, hepatocellular ballooning, and Mallory-Denk bodies in early stages, indicating cellular stress[22]. A key feature is the activation of hepatic stellate cells, which expand, occupy the space of Disse, and develop cytoplasmic microvacuoles. These cells transform into myofibroblasts, driving collagen production and fibrosis[23]. Perisinusoidal fibrosis, particularly in the space of Disse, often progresses to bridging fibrosis and cirrhosis[24]. Additional findings include inflammatory infiltrates, bile duct proliferation, and cholestasis. Advanced cases show regenerative nodules and extensive fibrosis, disrupting normal lobular architecture (Figure 1)[25].
Excessive retinoid intake disrupts cellular metabolism, generating ROS and causing oxidative stress[26,27]. ROS damage organelles, impair hepatocyte function, and trigger apoptosis and necrosis[28]. Retinoic acid influences gene expression via nuclear receptors (i.e. retinoic acid receptors, and retinoid X receptors), altering cellular proliferation, apoptosis, and fibrogenesis[29].
Although retinoic acid tends to inhibit fibrogenesis in most studied contexts, in diseases such as liver fibrosis retinoic acid reduces the activation of stellate cells by regulating the expression of factors like TGF-β (a key mediator of fibrosis) and modulating the activity of matrix metalloproteinases. However, experimental models show that high doses of retinoic acid can indirectly promote profibrotic effects by inducing inflammation[30].
Toxic accumulation of retinyl esters in stellate cells activates them and impairs vitamin A storage, perpetuating fibrosis[31]. Retinoid-induced changes in lipid metabolism also contribute to steatosis and liver damage[32].
Oxidative stress: Oxidative stress is central to vitamin A-induced liver damage. Excessive retinol and retinoic acid generate ROS through mitochondrial dysfunction and peroxisomal oxidation, causing lipid peroxidation, protein oxidation, and DNA damage[33]. This can lead to increased levels of malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), products of lipid peroxidation and indicators of oxidative damage to cell membranes. Additionally, thiol groups in proteins (e.g., cysteine) are sensitive to oxidation. Vitamin A toxicity reduces the levels of free thiol groups, reflecting oxidative damage. Hypervitaminosis A can induce DNA damage due to increased ROS, elevating levels of 8-hydroxydeoxyguanosine.
ROS also activate Kupffer cells, releasing pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6, amplifying inflammation and fibrosis[34].
Apoptosis and necrosis: Vitamin A-induced hepatocyte death involves mitochondrial dysfunction, cytochrome c release, and caspase activation in apoptosis, while oxidative stress depletes adenosine triphosphate, leading to necrosis. These processes result in significant hepatocellular loss[35].
Alterations in signaling pathways: Retinoic acid dysregulates TGF-β and NF-κB pathways, promoting stellate cell activation and fibrosis. Excess retinoids also disrupt Wnt/β-catenin signaling, impairing hepatic regeneration[36].
Retinoid metabolism and storage: Chronic hypervitaminosis A saturates the liver’s storage capacity, causing retinoid leakage and systemic toxicity. Retinol ester accumulation in hepatocytes and stellate cells leads to lipotoxicity, steatosis, and organelle dysfunction. Impaired retinoid metabolism exacerbates oxidative stress and inflammation[19,34]. Inflammatory response and fibrogenesis: Hypervitaminosis A triggers inflammation by activating Kupffer cells and macrophages to release TNF-α and IL-1β, subsequently promoting stellate cell activation and fibrosis. Retinoic acid also influences matrix metalloproteinases and tissue inhibitors of metalloproteinases, disrupting extracellular matrix turnover[37].
Targeted therapies, such as antioxidants (e.g., N-acetylcysteine) and TGF-β inhibitors, show promise in mitigating oxidative stress and fibrosis[38]. Limiting vitamin A intake is crucial for at-risk populations[2,14].
Vitamin A toxicity poses a significant challenge in hepatology, with pathological effects ranging from steatosis to cirrhosis. The molecular mechanisms involve oxidative stress, apoptosis, necrosis, and dysregulated signaling pathways[39]. While progress has been made, translating these insights into clinical applications remains a priority[40].
Excessive vitamin A intake overwhelms the liver’s storage capacity, leading to oxidative stress, inflammation, and fibrosis[2,35]. Apoptosis and necrosis contribute to hepatocellular loss, while dysregulated signaling pathways exacerbate fibrogenesis[35,37].
Biomarker development: Identifying reliable biomarkers for early detection is critical. Oxidative stress markers (e.g., MDA, 4-HNE,) and pro-inflammatory cytokines (e.g., IL-6, TNF-α) are potential candidates[11].
Therapeutic interventions: Antioxidants and TGF-β inhibitors show promise in mitigating liver damage[41]. Individualized risk assessment: Genetic polymorphisms in retinoid metabolism enzymes and hepatic storage capacity should be studied to tailor dietary recommendations[42].
Dietary guidelines: Establishing safe upper limits for vitamin A intake is essential, particularly for vulnerable po
Experimental models: Developing physiologically relevant models, such as organ-on-a-chip systems, will enhance translational research[43].
Addressing vitamin A toxicity requires interdisciplinary collaborations, public awareness, and policy development. Clinical trials evaluating antioxidants and anti-fibrotic agents are necessary to advance therapeutic strategies[44].
Vitamin A toxicity remains a significant contributor to liver disease. Although toxicity is not as prevalent as deficiency, severe cases may require hospitalization and treatment, generating costs for healthcare systems. Additionally, congenital defects related to toxicity during pregnancy have a significant impact on both maternal and child health. In contexts of mass supplementation, the lack of data on the short-term kinetics of high doses and their effects on lactating women and children limits the ability to prevent toxicity. This underscores the need for further research on subclinical toxicity and its long-term impact. Advances in understanding its mechanisms provide a foundation for future research, but gaps in biomarker development, therapeutic strategies, and dietary guidelines must be addressed. Balancing the benefits and risks of vitamin A is essential for optimal liver health and public well-being.
| 1. | Lazarus R, Gore CJ, Booth M, Owen N. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr. 1998;68:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 343] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Sanchez M, El-Khoury AE, Castillo L, Chapman TE, Basile Filho A, Beaumier L, Young VR. Twenty-four-hour intravenous and oral tracer studies with L-[1-13C]phenylalanine and L-[3,3-2H2]tyrosine at a tyrosine-free, generous phenylalanine intake in adults. Am J Clin Nutr. 1996;63:532-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR. Modern nutrition in health and disease: Eleventh edition. Amsterdam: Wolters Kluwer Health Adis (ESP), 2012: 415. |
| 5. | Myhre AM, Carlsen MH, Bøhn SK, Wold HL, Laake P, Blomhoff R. Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am J Clin Nutr. 2003;78:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Shannon M. Alternative medicines toxicology: a review of selected agents. J Toxicol Clin Toxicol. 1999;37:709-713. [PubMed] |
| 7. | Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 874] [Cited by in RCA: 802] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 8. | Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr. 2012;96:1166S-1172S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C. Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases. Gastroenterology. 1991;100:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 115] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Blaner WS, O'Byrne SM, Wongsiriroj N, Kluwe J, D'Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2270] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 12. | Teoh NC. Hepatic ischemia reperfusion injury: Contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection-the good, bad and deadly. J Gastroenterol Hepatol. 2011;26 Suppl 1:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260-7276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 289] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (4)] |
| 14. | Grogan S, Preuss CV. Pharmacokinetics. 2023 Jul 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 15. | Slomiany A, Piotrowski E, Grabska M, Piotrowski J, Slomiany BL. Chronic ethanol-initiated apoptosis in hepatocytes is induced by changes in membrane biogenesis and intracellular transport. Alcohol Clin Exp Res. 1999;23:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Rivera-Caravaca JM, Roldán V, Esteve-Pastor MA, Valdés M, Vicente V, Lip GYH, Marín F. Cessation of oral anticoagulation is an important risk factor for stroke and mortality in atrial fibrillation patients. Thromb Haemost. 2017;117:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1295] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 18. | Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 2010;34:1247-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Trasino SE, Benoit YD, Gudas LJ. Vitamin A deficiency causes hyperglycemia and loss of pancreatic β-cell mass. J Biol Chem. 2015;290:1456-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Matsumura Y, Sakaida I, Uchida K, Kimura T, Ishihara T, Okita K. Prolyl 4-hydroxylase inhibitor (HOE 077) inhibits pig serum-induced rat liver fibrosis by preventing stellate cell activation. J Hepatol. 1997;27:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Russell RM, Boyer JL, Bagheri SA, Hruban Z. Hepatic injury from chronic hypervitaminosis a resulting in portal hypertension and ascites. N Engl J Med. 1974;291:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 131] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Zimmerman HJ. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Amsterdam: Wolters Kluwer Health Adis (ESP), 1999: 491-498. |
| 23. | Senoo H. Structure and function of hepatic stellate cells. Med Electron Microsc. 2004;37:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2165] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 25. | Rubbia-Brandt L, Giostra E, Mentha G, Quadri R, Negro F. Expression of liver steatosis in hepatitis C virus infection and pattern of response to alpha-interferon. J Hepatol. 2001;35:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47:344-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 627] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 27. | Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 28. | Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 436] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 29. | Tarugi P, Ballarini G, Bembi B, Battisti C, Palmeri S, Panzani F, Di Leo E, Martini C, Federico A, Calandra S. Niemann-Pick type C disease: mutations of NPC1 gene and evidence of abnormal expression of some mutant alleles in fibroblasts. J Lipid Res. 2002;43:1908-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2201] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 31. | Upadhya AG, Harvey RP, Howard TK, Lowell JA, Shenoy S, Strasberg SM. Evidence of a role for matrix metalloproteinases in cold preservation injury of the liver in humans and in the rat. Hepatology. 1997;26:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Cassim Bawa FN, Xu Y, Gopoju R, Plonski NM, Shiyab A, Hu S, Chen S, Zhu Y, Jadhav K, Kasumov T, Zhang Y. Hepatic retinoic acid receptor alpha mediates all-trans retinoic acid's effect on diet-induced hepatosteatosis. Hepatol Commun. 2022;6:2665-2675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Klaunig JE, Wang Z. Oxidative stress in carcinogenesis. Curr Opin Toxicol. 2018;7:116-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Allameh A, Niayesh-Mehr R, Aliarab A, Sebastiani G, Pantopoulos K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants (Basel). 2023;12:1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 138] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 35. | Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 36. | Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 394] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 37. | Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 38. | Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr. 2000;71:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Friedman SL. Hepatic Fibrosis: Emerging Therapies. Dig Dis. 2015;33:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 2000] [Article Influence: 250.0] [Reference Citation Analysis (0)] |
| 41. | Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 518] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 42. | Tull TM, Bramlage LR. Racing prognosis after cumulative stress-induced injury of the distal portion of the third metacarpal and third metatarsal bones in Thoroughbred racehorses: 55 cases (2000-2009). J Am Vet Med Assoc. 2011;238:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Ewart L, Fabre K, Chakilam A, Dragan Y, Duignan DB, Eswaraka J, Gan J, Guzzie-Peck P, Otieno M, Jeong CG, Keller DA, de Morais SM, Phillips JA, Proctor W, Sura R, Van Vleet T, Watson D, Will Y, Tagle D, Berridge B. Navigating tissue chips from development to dissemination: A pharmaceutical industry perspective. Exp Biol Med (Maywood). 2017;242:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 534] [Article Influence: 76.3] [Reference Citation Analysis (0)] |