Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.108253
Revised: April 25, 2025
Accepted: June 23, 2025
Published online: July 27, 2025
Processing time: 107 Days and 21.6 Hours
Progressive familial intrahepatic cholestasis (PFIC) is a group of rare, inherited cholestatic liver disorders presenting in infants and children and are associated with impaired bile flow (i.e., cholestasis), pruritus and progressive liver disease. Historically there has been no effective or approved pharmacologic treatments for these disorders and standard medical treatment has only been supportive. The impaired bile flow within the liver, leads to accumulation in the liver and inflammation. Historically there has been no effective or approved pharmacologic treatments for these disorders and standard medical treatment has only been supportive. A potential for reducing pathologic bile accumulation in the liver is surgical biliary diversion, with an aim to interrupt the enterohepatic circulation. These procedures have demonstrated a positive effect in PFIC by normalizing serum bile acids, reducing pruritus and liver injury and improving the patient quality of life. Nonsurgical approach to interrupting the enterohepatic circulation is inhibition of the ileal bile acid transporter (IBAT). IBAT inhibition has demon
Core Tip: Progressive familial intrahepatic cholestasis is a heterogenous autosomal recessive progressive cholestatic liver disease with varying genotype and phenotype. Identifying the underlying genetic disorder allows better understanding of the clinical phenotype and associated complications. Management is aimed at nutritional support, trying to make bile more hydrophilic by administering ursodeoxycholic acid and control of pruritus. Surgical biliary diversion has proved to reduce bile acid levels and improve pruritis in some patients. New hope exists with ileal bile acid transporter receptor inhibitors, however more data is still required. The ultimate treatment for end stage liver disease and persistent pruritus is liver transplantation.
- Citation: Mkarem LE, Batika MAH, Bitar R. New hope in treating progressive familial intrahepatic cholestasis in children. World J Hepatol 2025; 17(7): 108253
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/108253.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.108253
Progressive familial intrahepatic cholestasis (PFIC) is a rare group of inherited liver diseases caused by impaired bile acid transport, resulting in cholestasis, ongoing liver damage, and, in severe instances, liver failure. PFIC usually manifests in infancy or early childhood, presenting with jaundice, itching (pruritus), liver enlargement (hepatomegaly), and poor growth. Patient with advanced PFIC require liver transplantation.
Initially classified into three main subtypes (PFIC1, PFIC2, and PFIC3), ongoing genetic research has expanded the spectrum to 13 distinct PFIC subtypes, each linked to mutations in genes critical for bile acid metabolism and hepatobiliary transport. These mutations impair bile secretion, causing toxic bile acid accumulation in hepatocytes and contributing to liver dysfunction.
Traditional PFIC management has relied on medications, surgical biliary diversion, and liver transplantation. Recent advancements in targeted therapy have introduced intestinal bile acid transport (IBAT) inhibitors, IBAT inhibitors reduce bile acid reabsorption in the intestine, thereby lowering hepatic bile acid load and alleviating cholestatic symptoms.
This review will explore the pathophysiology, genetic basis, and conventional management of PFIC, followed by an in-depth discussion of IBAT inhibitors as a promising therapeutic strategy for this complex pediatric liver disorder.
To understand the pathogenesis of PFIC we must go through the normal pathophysiology of bile acid synthesis and transport.
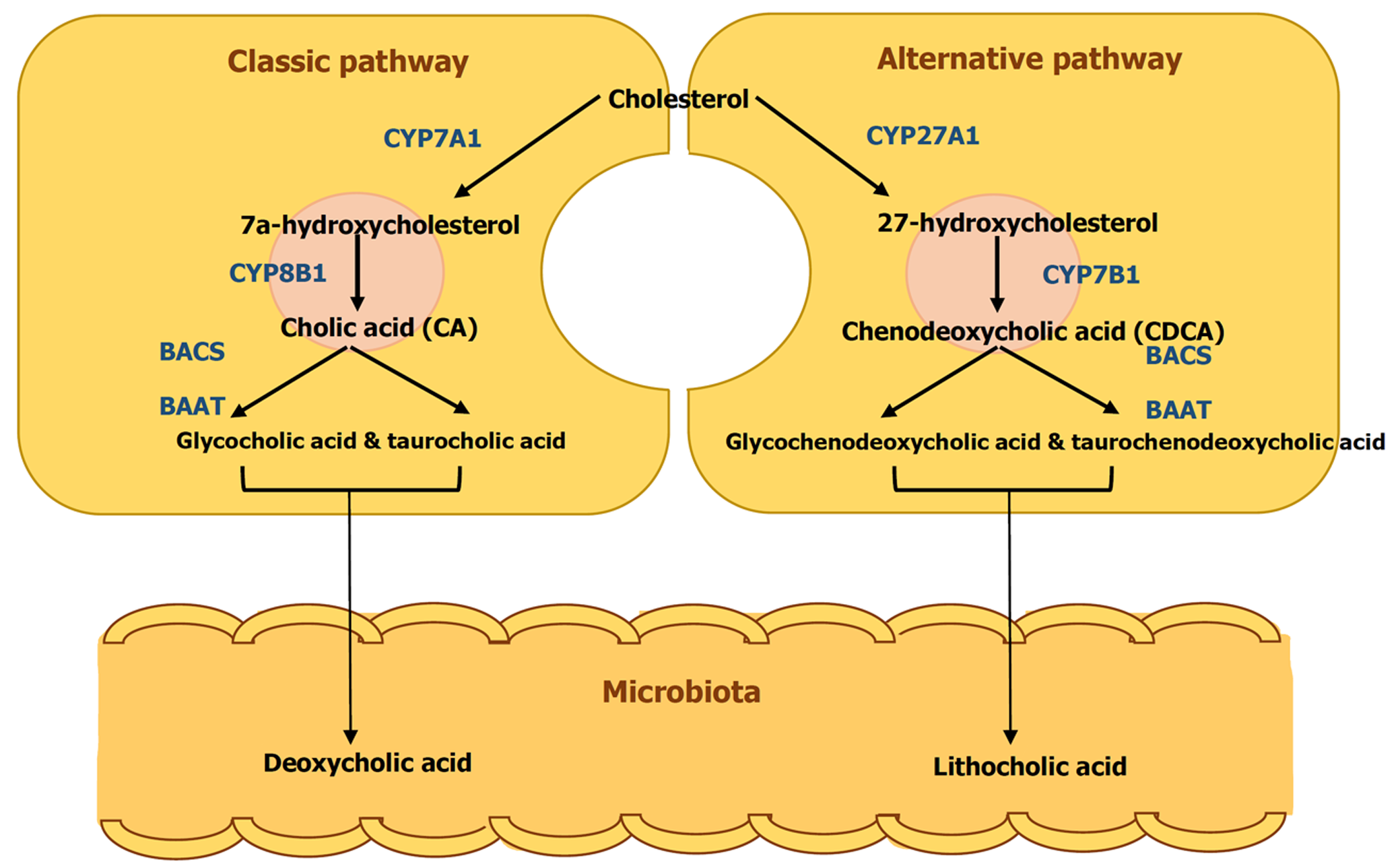
Bile is a naturally occurring aqueous fluid synthesized and secreted by the liver, composed of bile salts, phospholipids, cholesterol, conjugated bilirubin, electrolytes, and water[1]. Among these components, bile salts are particularly important for lipid digestion and cholesterol homeostasis. Hepatocytes break down cholesterol to produce two primary bile acids—cholic acid and chenodeoxycholic acid—through a multi-step process regulated by cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme. In the small intestine, bacterial enzymes convert these primary bile acids into secondary bile acids, deoxycholic acid and lithocholic acid (Figure 1). The liver then conjugates both primary and secondary bile acids with glycine or taurine, forming bile salts. These bile salts play a crucial role in lipid digestion by acting as amphipathic molecules that emulsify fats and facilitate micelle formation. They also regulate their own production by inhibiting cholesterol 7 alpha-hydroxylase. The majority of bile acids are recycled through the enterohepatic circulation, with only a small portion (about 5%) being synthesized by the liver to compensate for daily fecal losses, provided they remain below 20% of the total bile acid pool[1]. In the enterohepatic circulation, bile salts are reabsorbed by the apical sodium-dependent bile salt transporter in intestinal epithelial cells. They return to the liver via the superior mesenteric and portal veins, which carry the blood that feeds liver sinusoids. Uptake into hepatocytes is mediated by the sodium taurocholate co-transporting polypeptide and organic anion transporters. Bile salt transport by the bile salt export pump (BSEP) constitutes the rate-limiting step in bile formation and provides the major driving force for enterohepatic circulation.
PFIC is inherited in an autosomal recessive pattern. Historically, these conditions were diagnosed based on persistent cholestasis with normal gamma glutamyl transferase without an identifiable structural or molecular cause. However, recent advancements in genetic research have revealed up to 13 key proteins involved in bile transport, clarifying the underlying mechanisms. Additionally, the spectrum of PFIC-related diseases has since expanded, ranging from intermittent jaundice with a benign course to severe cholestasis progressing to liver failure[2].
PFIC is a genetically inherited condition, autosomal recessive, so it needs heterogeneous genes to be acquired. However, almost all types can be inherited even with homozygous mutation, and the phenotypic features might differ.
These mutations occur in the genes that encode the bile transportation process, which leads to cholestasis and progressive liver damage. Depending on the protein presence sites encoded by the mutated gene, it can involve other organs.
PFIC can be classified into five functional groups based on the underlying genetic mutation. Canalicular membrane stability defects are seen in PFIC1 and PFIC3, while bile acid transport defects are characteristic of PFIC2 and Progressive familial intrahepatic cholestasis type 6 (PFIC6). Disorders affecting intracellular trafficking and membrane transporter expression control include progressive familial intrahepatic cholestasis type 5 (PFIC5), progressive familial intrahepatic cholestasis type 8 (PFIC8), progressive familial intrahepatic cholestasis type 12 (PFIC12), PFIC10, and progressive familial intrahepatic cholestasis type 11 (PFIC11). Mutations disrupting tight junction integrity and hepatocyte structure are responsible for PFIC4 and PFIC7. Lastly, ciliopathies account for PFIC13 and progressive familial intrahepatic cholestasis type 9 (PFIC9).
Advances in next-generation sequencing and whole-exome sequencing have improved the identification of novel PFIC subtypes, allowing for better genotype-phenotype correlation and targeted therapeutic strategies (Table 1).
| PFIC type | Gene | Protein affected | Pathophysiology | Key features | GGT | Extrahepatic symptoms |
| PFIC1 | ATP8B1 | FIC1 (flippase) | Canalicular membrane instability; impaired bile salt resistance | Cholestasis, diarrhea, growth failure, deafness | Normal | Diarrhea, short stature, deafness, pancreatitis |
| PFIC2 | ABCB11 | BSEP | Impaired bile salt excretion | Severe cholestasis, early liver failure, Increased risk of Hepatocellular carcinoma risk | Normal | None typically |
| PFIC3 | ABCB4 | MDR3 | Deficient phosphatidylcholine secretion → toxic bile | Later onset, biliary fibrosis, cirrhosis | High | None typically |
| PFIC4 | TJP2 | ZO-2 | Tight junction disruption → bile leaks into liver tissue | Pruritus, portal hypertension, hearing loss | Low | Hearing loss, respiratory issues |
| PFIC5 | NR1H4 | FXR | Decreased bile acid regulation and BSEP expression | Neonatal cholestasis, bleeding, ↑ International Normalization Ratio, ↑ Alpha Feto Protein | Normal | Coagulopathy (vitamin K-resistant) |
| PFIC6 | SLC51A | OSTα | Impaired bile acid reabsorption from intestine | Diarrhea, jaundice, hepatosplenomegaly | High | Severe diarrhea, bleeding tendency |
| PFIC7 | USP53 | USP53 | Tight junction dysfunction | Episodic cholestasis, vitamin deficiency, deafness | Normal | Hearing loss, developmental delay, cardiomyopathy |
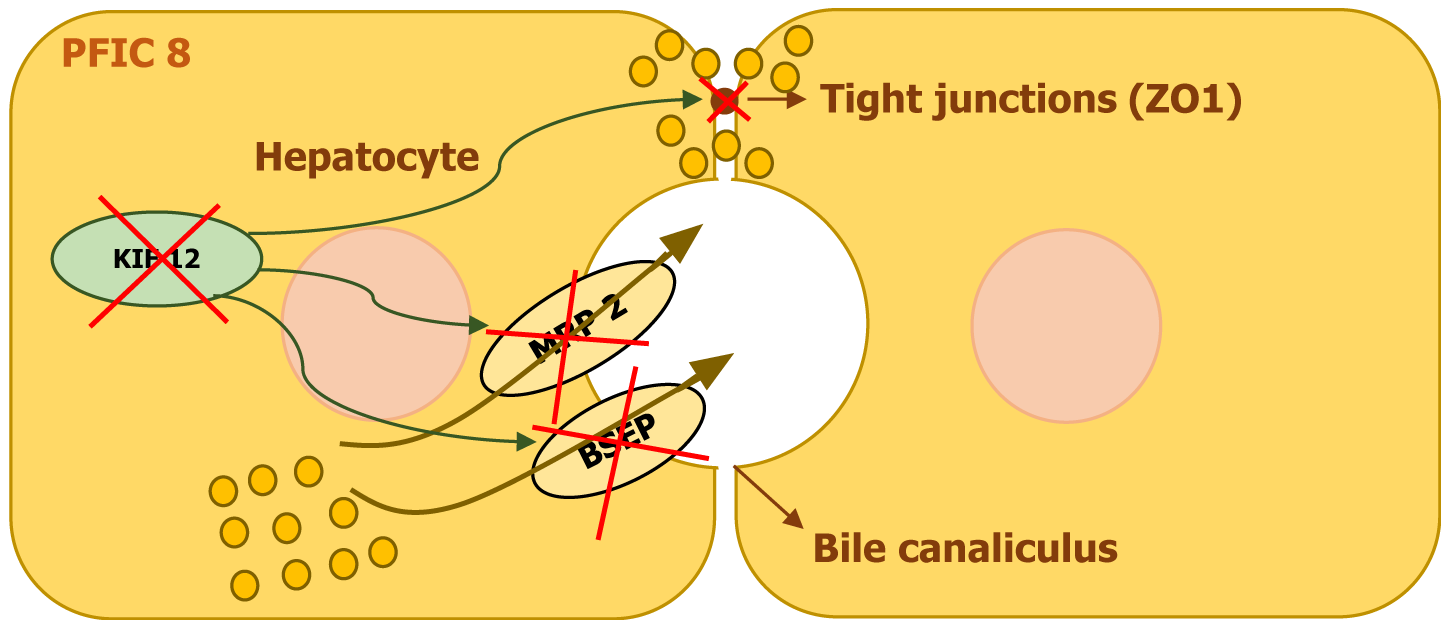
| PFIC8 | KIF12 | KIF12 | Disrupted intracellular transport, MRP2 mislocalization | Variable: Cholestasis to cirrhosis | High | None typically |
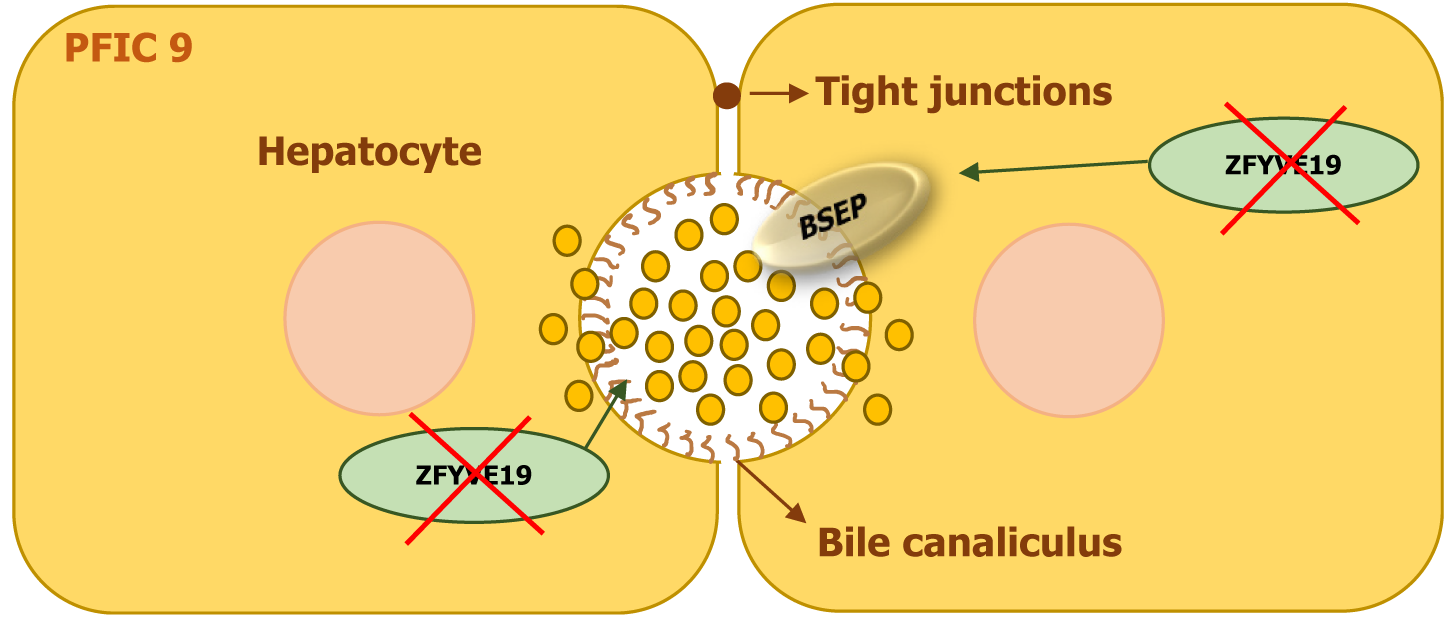
| PFIC9 | ZFYVE19 | ZFYVE19 | Ciliopathy affecting bile duct cilia and transporter localization | Cholestasis, hepatospleno-megaly, Gastrointestinal bleeding | High | Ciliary dysfunction; systemic involvement |
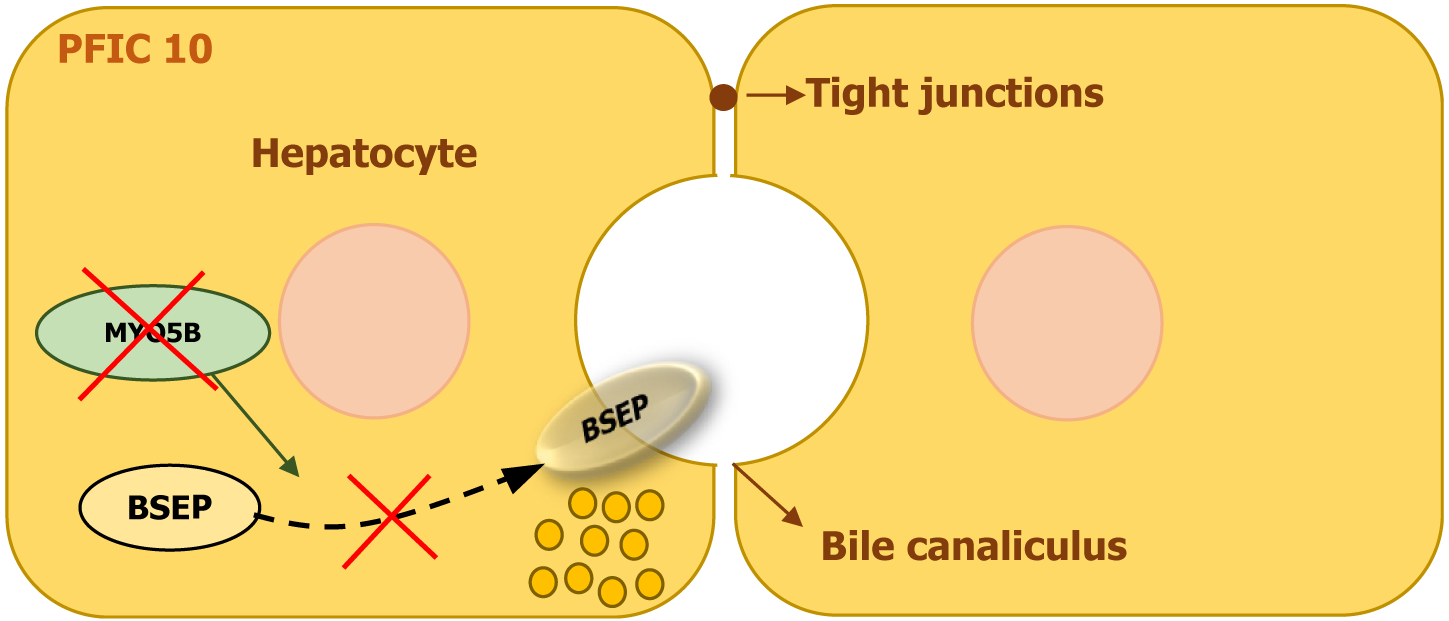
| PFIC10 | MYO5B | Myosin 5B | Defective transporter trafficking (e.g., BSEP) | Cholestasis ± microvillous inclusion disease | Normal/Low | Diarrhea, pruritus |
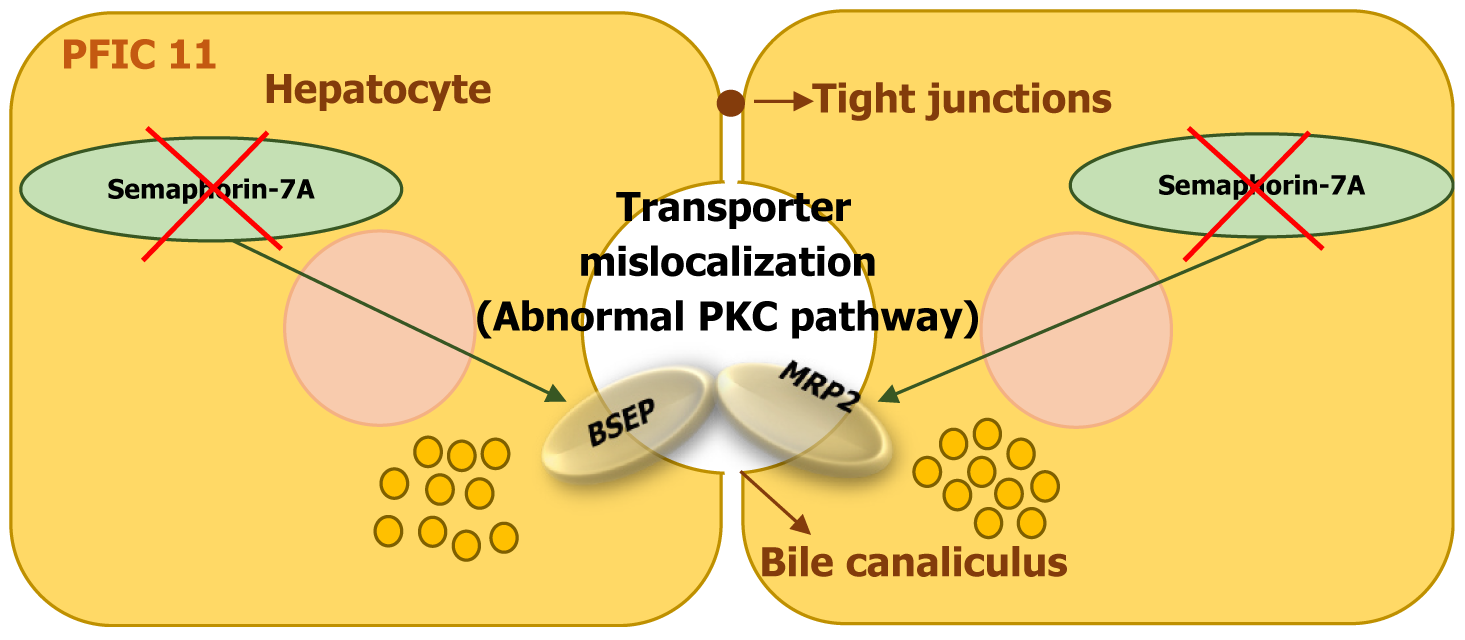
| PFIC11 | SEMA7A | Semaphorin-7A | Abnormal transporter placement (via PKC pathway) | Jaundice, ↑ ALT/AST, no pruritus | Normal | None reported |
| PFIC12 | VPS33B | VPS33B | Defective intracellular trafficking | Neonatal cholestasis, pruritus | Normal | Mild renal/skeletal issues |
| PFIC13 | PSKH1 | PSKH1 | Hepatorenal ciliopathy | Cholestasis + kidney disease | Variable | Renal disease, anemia, diaphragmatic hernia |
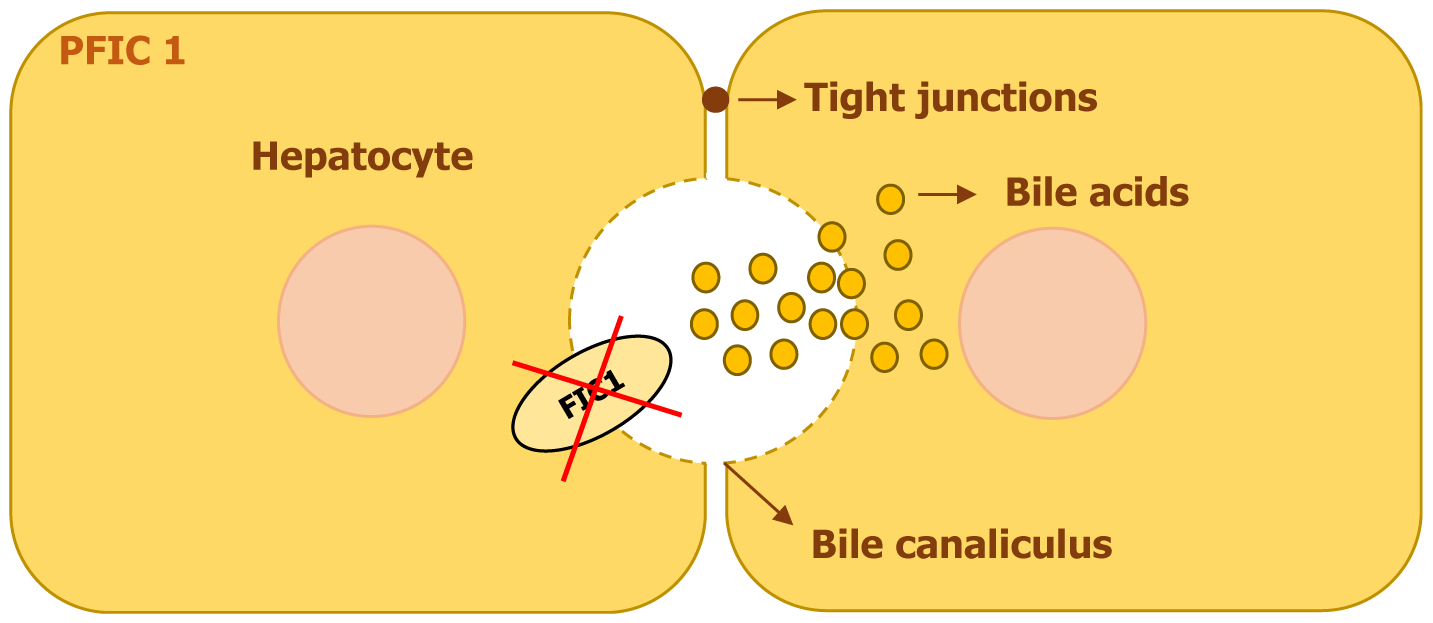
PFIC1 is an autosomal recessive disorder caused by mutations in the ATP8B1 gene, located on chromosome 18. This gene encodes ATPase, an enzyme crucial for maintaining the proper distribution of phospholipids in cell membranes[3]. Mutations in ATP8B1 Lead to a spectrum of liver diseases, ranging from the severe PFIC1 to the milder benign recurrent intrahepatic cholestasis type 1 and Greenland familial cholestasis[4]. The ATP8B1 gene is expressed in the apical membrane of epithelial cells of several organs, including the liver, small intestine, pancreas, kidney, and bladder, with particularly high levels in the intestinal epithelium[3]. Within the liver, its presence is concentrated in cholangiocytes and the canalicular membrane of hepatocytes, where it plays a vital role in bile transport and canalicular membrane stability.
The ATP8B1 protein is known as FIC1, functions as a flippase, responsible for maintaining the asymmetry of phospholipids within the plasma membrane. It specifically moves phosphatidylserine and phosphatidylethanolamine from the outer to the inner leaflet, which helps to protect the canalicular membrane from damage caused by the toxic accumulation of bile salts[3,5].
The pathophysiology of PFIC1 arises from increased vulnerability to bile acid toxicity. When the ATP8B1 function is lost, the canalicular membrane becomes unstable, making hepatocytes more susceptible to bile salt-induced damage (Figure 2). Additionally, ATP8B1 deficiency leads to reduced bile acid excretion into the intestines, disrupting enterohepatic circulation and contributing to chronic diarrhea, a common feature of PFIC1[4]. Studies have also shown that ATP8B1 mutations lead to downregulation of the farnesoid X receptor (FXR), a nuclear receptor that regulates bile acid metabolism. As a result, there is decreased expression of BSEP in hepatocytes, leading to intracellular bile acid accumulation and worsening cholestasis[4,6].
PFIC1 is not limited to liver dysfunction, as its effects extend to other organs. The disease has been linked to reduced expression of cystic fibrosis transmembrane conductance regulator in cholangiocytes, which may further impair bile secretion and contribute to extrahepatic symptoms such as chronic diarrhea, growth failure, pancreatitis, and short stature[6].
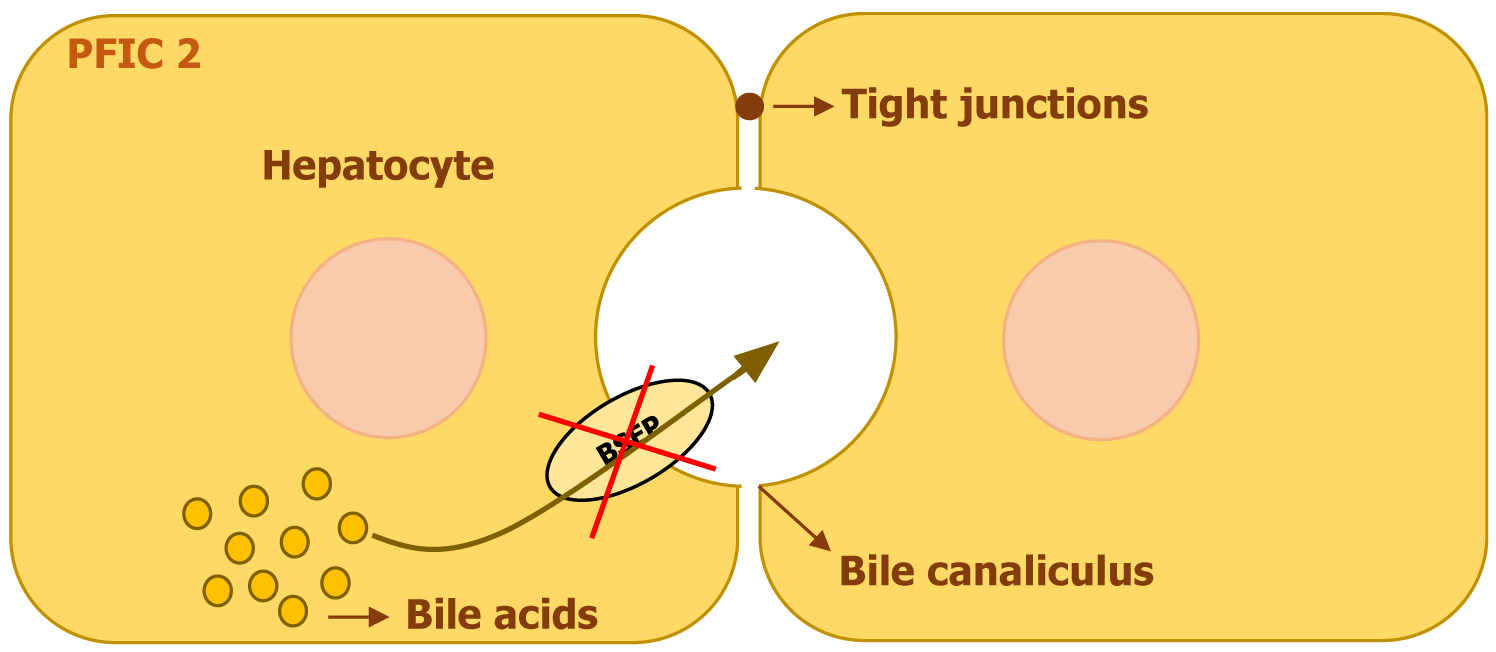
PFIC2, previously referred to as Byler syndrome, arises from mutations in the ABCB11 gene on chromosome 2. This gene encodes the BSEP, a transport protein that relies on ATP to facilitate the movement of bile acids into bile. BSEP is primarily localized on the canalicular membrane of hepatocytes, where it plays an essential role in maintaining bile flow by transporting bile acids against a steep concentration gradient[6]. Although ABCB11 mRNA has been detected in multiple tissues outside the liver, such as the thymus, trachea, lungs, kidney, colon, testes, and prostate, functional BSEP protein expression is restricted to hepatocytes[7]. Genetic alterations in ABCB11 disrupt bile acid secretion, leading to intrahepatic bile accumulation and progressive liver damage (Figure 3). The severity of the disease varies, with a spectrum ranging from the milder benign recurrent intrahepatic cholestasis type 2 (BRIC2) to the more severe PFIC2. While missense mutations are more frequently associated with BRIC2 and tend to occur outside the crucial nucleotide-binding regions, more severe cases of PFIC2 are often linked to truncating mutations, such as nonsense, splicing defects, insertions, or deletions, which significantly reduce or eliminate BSEP expression. Despite the range of clinical severity, no definitive genotype-phenotype correlation has been established. Notably, Heterozygous mutations in ABCB11 have also been linked to conditions like drug-induced cholestasis, transient neonatal cholestasis, and intrahepatic cholestasis of pregnancy[6].
Various missense mutations in ABCB11 impair BSEP function by disrupting protein folding, trafficking, or stability. Certain mutations, such as p.E297G and p.D482G, interfere with the proper transport of BSEP to the membrane, while others, including p.N490D, p.G562D, p.R832C, and p.A1110E, affect key structural regions necessary for its function. Depending on the mutation, BSEP may either be absent from the canalicular membrane or present in a non-functional form, both of which contribute to bile acid buildup and progressive liver disease[6].
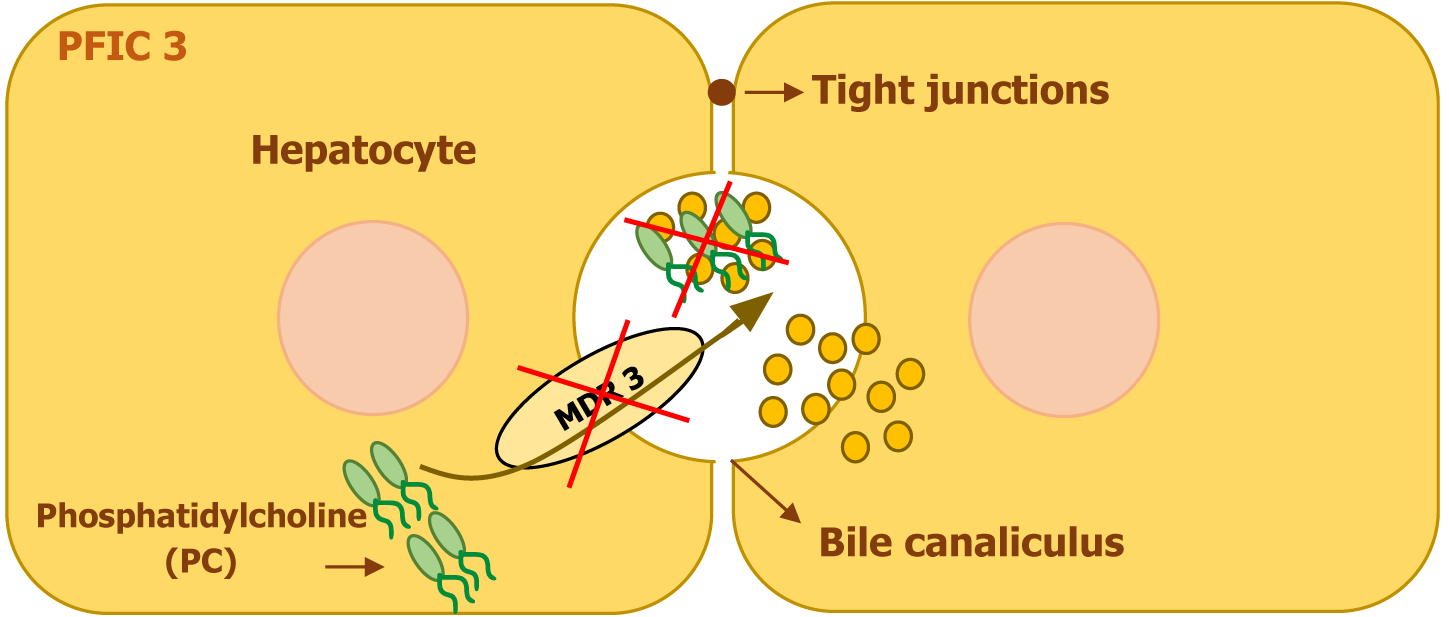
PFIC3 is caused by mutations in the ABCB4 gene, located on chromosome 7. This gene encodes the multidrug resistance protein 3 (MDR3) protein, a transmembrane phospholipid translocator that actively transports phosphatidylcholine from hepatocytes into bile. The presence of phosphatidylcholine in bile is essential for neutralizing the detergent effects of bile salts, thereby protecting the biliary epithelium and bile canaliculi from injury. In patients with PFIC3, MDR3 deficiency leads to impaired phospholipid secretion, exposing the bile ducts to the toxic effects of hydrophobic bile salts and resulting in progressive liver damage[4,6].
In addition, the absence of phospholipids in bile disrupts the normal three-phase system that maintains bile composition. Under normal conditions, bile salts, phospholipids, and cholesterol exist in a stable balance that facilitates cholesterol solubility and micelle formation. When phospholipid secretion is defective, bile becomes lithogenic, promoting cholesterol crystallization and increasing the risk of bile duct obstruction. Additionally, the continuous exposure of the bile canaliculi and biliary epithelium to detergent bile salts leads to progressive cholangiopathy, inflammation, and fibrosis, ultimately contributing to liver dysfunction (Figure 4)[6].
The clinical spectrum of PFIC3 varies widely, with manifestations ranging from neonatal cholestasis to progressive liver disease and cirrhosis in young adulthood. Genetic studies have identified a broad range of ABCB4 mutations, with most patients carrying mutations on both alleles. Approximately one-third of cases involve mutations that lead to premature protein truncation, resulting in either rapid degradation of the MDR3 protein or instability of ABCB4 mRNA, leading to a complete absence of MDR3 expression on liver immunostaining. The remaining two-thirds of patients have missense mutations, some of which affect critical ATP-binding domains required for protein function. These mutations can impair ATPase activity or disrupt intracellular trafficking, reducing MDR3 expression on the canalicular membrane and lowering phospholipid secretion into bile.
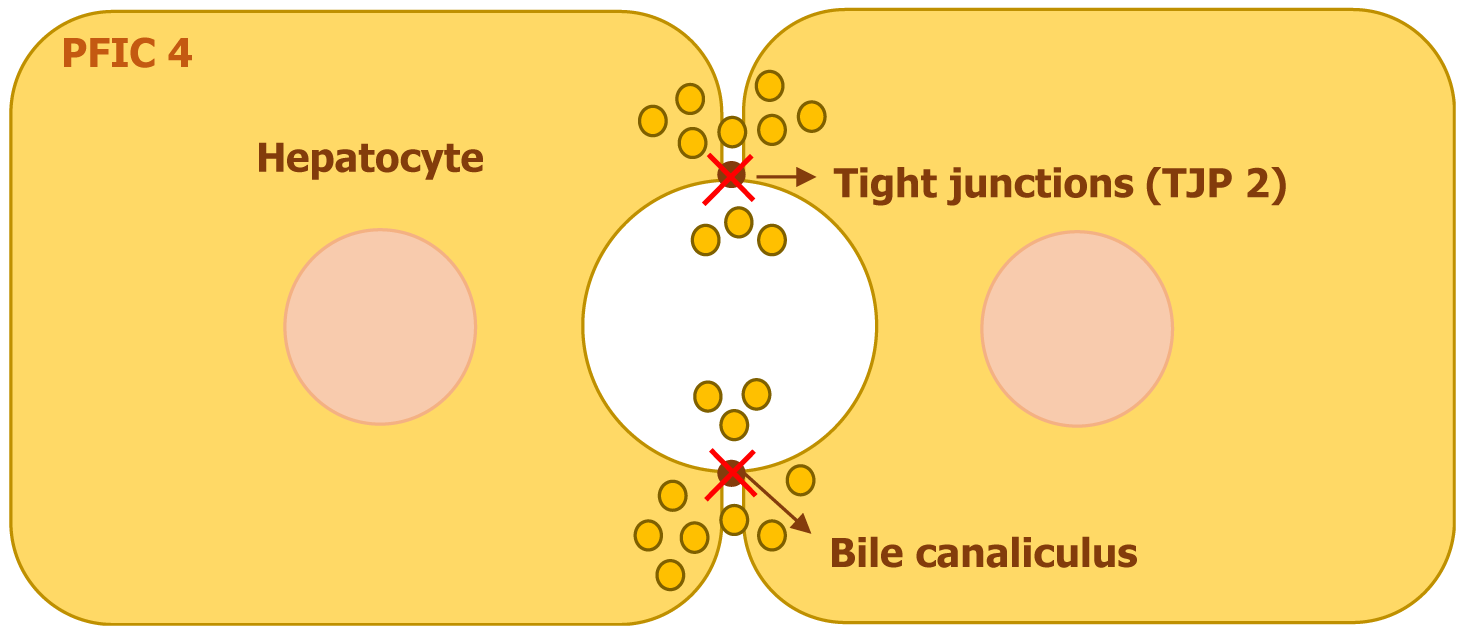
Mutations in the tight junction protein 2 (TJP2) gene, located on chromosome 9, are responsible for PFIC4. This gene encodes TJP2, also referred to as zona occludens 2. The protein plays a crucial role in maintaining tight junction stability by interacting with transmembrane proteins and anchoring them to the actin cytoskeleton[8].
TJP2 is essential for preserving the integrity of the tight junctions that separate bile from plasma within the liver. When TJP2 mutations lead to its dysfunction, bile escapes from canaliculi into surrounding hepatocytes, triggering an inflammatory cascade and progressive fibrosis (Figure 5). This disruption ultimately contributes to cholestasis and liver damage[8,9].
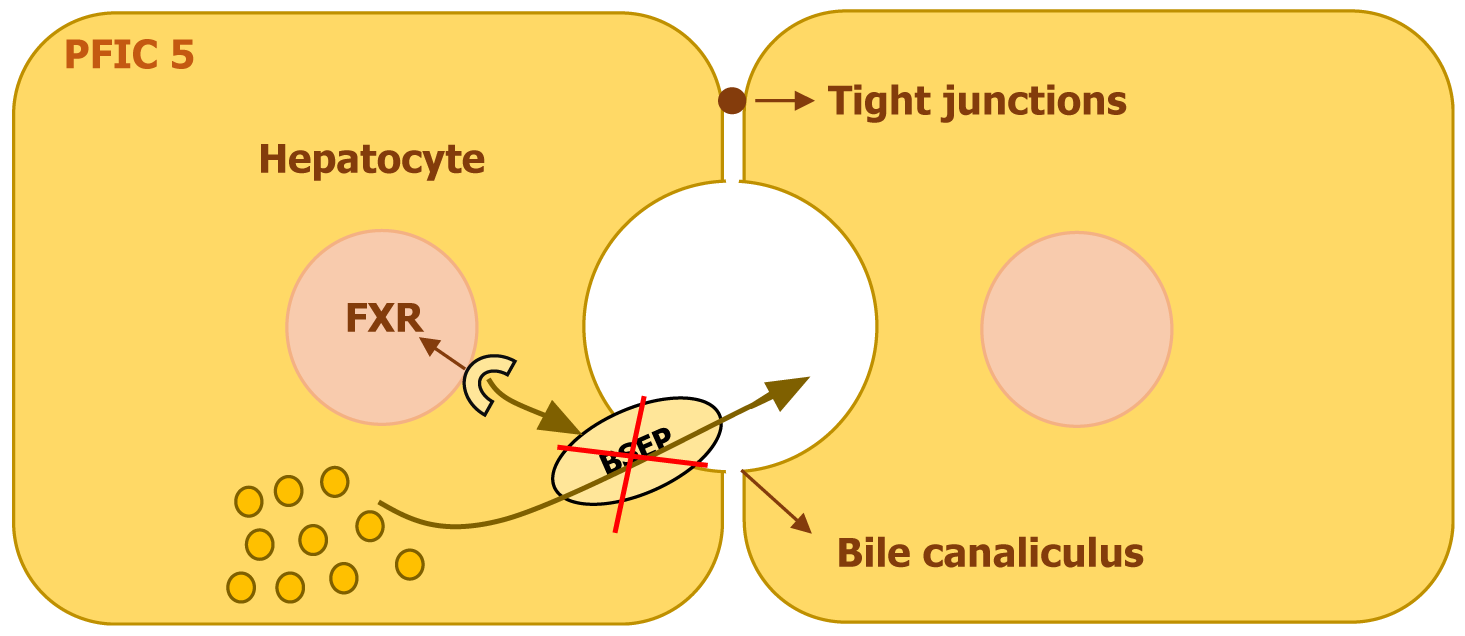
PFIC5 results from mutations in the NR1H4 gene, situated on chromosome 12. This gene encodes the FXR, a nuclear receptor essential for bile acid regulation. FXR controls the expression of key bile acid transporters, including the BSEP in liver cells and the ileal bile acid-binding protein in the intestines. It also plays a role in suppressing bile acid synthesis by inhibiting CYP7A1, a critical enzyme in this process, through the activation of fibroblast growth factor 19 (FGF19)[10].
A deficiency in FXR disrupts the balance of bile acid production and transport, leading to toxic bile acid buildup in liver cells. The reduction in FXR activity results in decreased BSEP expression, impairing bile acid excretion and contributing to liver cell damage (Figure 6). Additionally, FXR deficiency interferes with enterohepatic bile acid circulation due to lower FGF19 Levels, which leads to uninhibited bile acid synthesis in the liver. FXR also regulates the production of clotting factors, and its dysfunction can cause a form of coagulopathy that does not improve with vitamin K, even before liver failure develops[10,11].
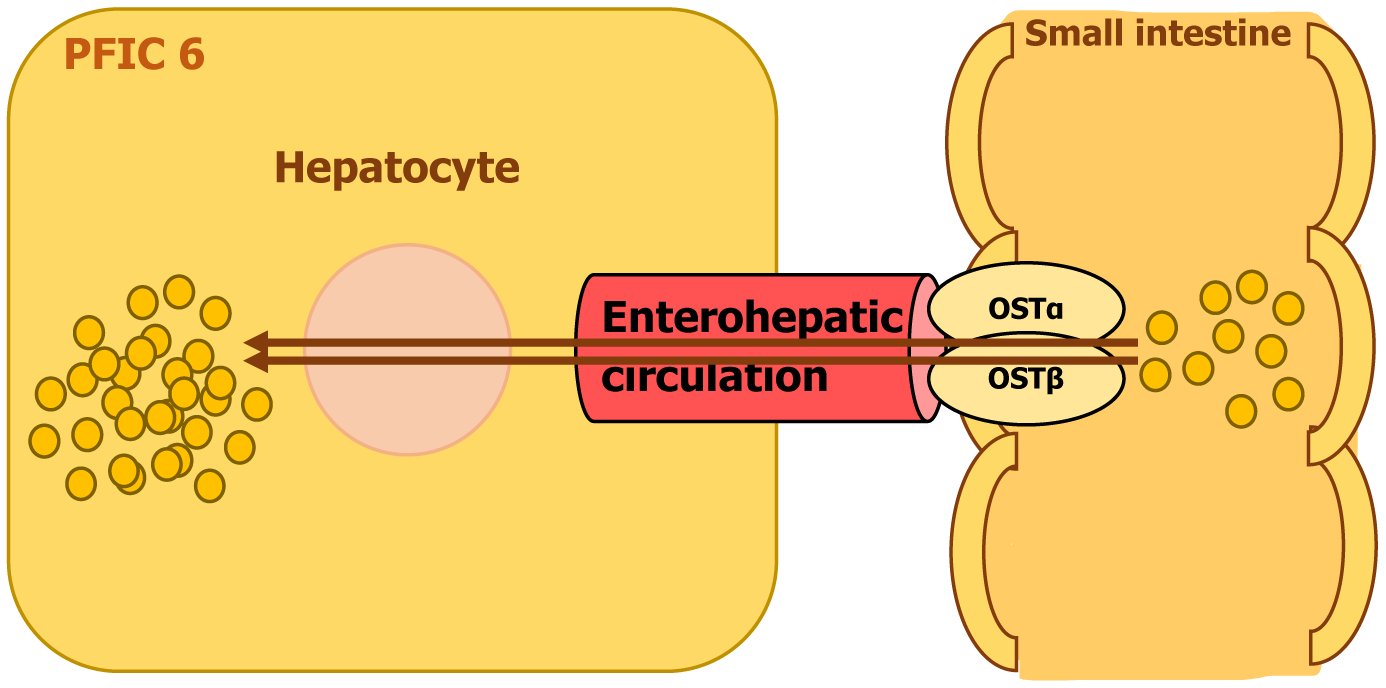
PFIC6 is a rare autosomal recessive disorder caused by homozygous mutations in the SLC51A gene on chromosome 3. This gene encodes organic solute transporter alpha (OSTα), a protein that functions as part of a bile acid transport system. OSTα forms a complex with OSTβ, working together to facilitate the reabsorption of bile acids from the intestine into liver cells for proper metabolism and secretion[12-14].
A defect in SLC51A disrupts bile acid transport, leading to reduced bile acid reabsorption and impaired bile flow. This deficiency affects fat digestion and stimulates bile acid synthesis in the liver which contributes to its accumulation in the liver, resulting in cholestatic liver disease. Over time, excessive bile acids in the liver can cause inflammation, fibrosis, and progressive liver damage (Figure 7). The impaired transport mechanism is also responsible for the severe diarrhea seen in affected individuals, as bile acids fail to be efficiently recycled through the enterohepatic circulation[12,13].
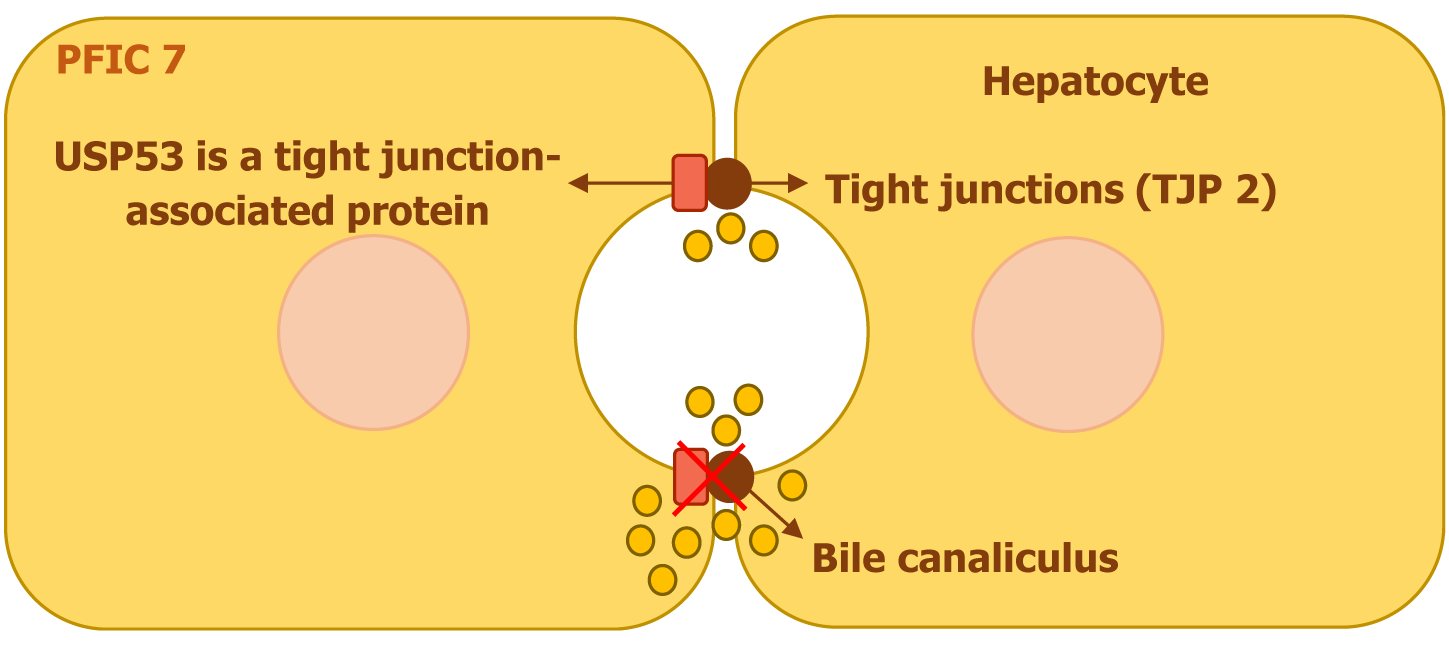
PFIC7 is linked to mutations in the ubiquitin-specific peptidases53 (USP53) gene, which is found on chromosome 4. This gene encodes a tight junction-associated protein that plays a crucial role in cellular barrier function. Unlike other ubiquitin-specific peptidases, USP53 Lacks the necessary histidine residue required for deubiquitination activity, rendering it catalytically inactive. Research in mice has demonstrated that USP53 interacts with TJP2 (ZO-2), contributing to the integrity of tight junctions (Figure 8)[10,15]. Additionally, it is essential for the maintenance of auditory hair cells, influencing both the stability and barrier properties of these junctions, which are critical for normal hearing[16].
Mutations in the KIF12 gene, located on chromosome 9, have been identified as a genetic cause of cholestatic liver disease. Studies have reported homozygous or compound heterozygous KIF12 variants in affected individuals, highlighting its role in PFIC8[17].
KIF12 encodes a kinesin-related protein that belongs to the kinesin superfamily. These proteins are essential for intracellular transport and cytoskeletal organization. KIF12 has also been linked to processes such as mitosis, Myosin II localization, and cytokinesis in certain cellular models. Given its role in cellular transport, disruptions in KIF12 are believed to contribute to cholestasis by impairing hepatocyte polarity and affecting the proper localization of key membrane transporters[18].
Research involving liver tissue analysis from affected individuals has revealed abnormal cytoplasmic expression of KIF12. Further examination of key transport proteins such as BSEP, MRP2, OATP1B1, and the tight junction protein ZO1 showed that while BSEP, OATP1B1, and ZO1 maintained normal positioning, MRP2 displayed an unusual cytoplasmic accumulation. This suggests that mutations in KIF12 may lead to defective MRP2 trafficking, which in turn results in impaired bile transport and hepatocellular dysfunction (Figure 9)[17-19].
Mutations in the ZFYVE19 gene, located on chromosome 15, have been identified as the underlying cause of PFIC9. The condition follows an autosomal recessive inheritance pattern, with affected individuals carrying either homozygous or compound heterozygous mutations[18].
The ZFYVE19 gene encodes a protein that plays a crucial role in cell division and the formation of primary cilia. It is primarily localized to centrosomes, which regulate cytokinesis and ciliogenesis. The protein is involved in coordinating interactions between the ESCRT-III complex and vacuolar protein sorting 4, processes that are essential for maintaining proper ciliary function. Disruptions in ZFYVE19 impair the function of primary cilia in bile duct cells, leading to cholestasis. The cilia serve as sensors for bile flow, and their dysfunction interferes with bile transport and secretion. Additionally, defects in the ESCRT-III machinery affect the proper localization of bile transport proteins, including BSEP, causing their accumulation within cells rather than at the apical membrane, further contributing to cholestasis (Figure 10)[20].
PFIC10 is caused by mutations in the myosin 5B (MYO5B) gene, which is located on chromosome 18q21.1. This gene encodes MYO5B, a motor protein essential for intracellular transport and recycling in polarized epithelial cells. MYO5B interacts with specific GTPases, including RAB11A and RAB8, facilitating the movement of key proteins such as the BSEP to the canalicular membrane of hepatocytes[9,10].
When MYO5B function is impaired, the proper trafficking of transporter proteins, including BSEP, is disrupted, leading to defective bile acid secretion and cholestasis (Figure 11). MYO5B also plays a vital role in maintaining cell polarity and plasma membrane recycling, particularly in enterocytes and respiratory epithelial cells. In some individuals, mutations in MYO5B are linked to microvillus inclusion disease, a severe intestinal disorder that causes early-onset diarrhea and malabsorption due to defective brush border enzyme localization[9,10].
PFIC11 is a rare autosomal recessive disorder caused by homozygous mutations in the SEMA7A gene, located on chromosome 15q24[13,14,21,22].
The SEMA7A gene encodes Semaphorin-7A, a protein that plays a role in cell signaling, axon growth, and cellular migration. It has also been implicated in maintaining hepatocyte polarity and bile acid transport[13,14,21].
Mutations in SEMA7A are believed to result in a gain-of-function effect, leading to disruptions in bile acid transporter expression. The mutation affects the canalicular bile acid transporters BSEP (ABCB11) and Mrp2 (ABCC2), reducing their presence on the hepatocyte membrane. This impairment contributes to bile acid accumulation in liver cells and cholestasis (Figure 12). Studies in mutant mouse models suggest that these disruptions are linked to increased protein kinase C activity, which interferes with transporter localization and function[13,14,21,22].
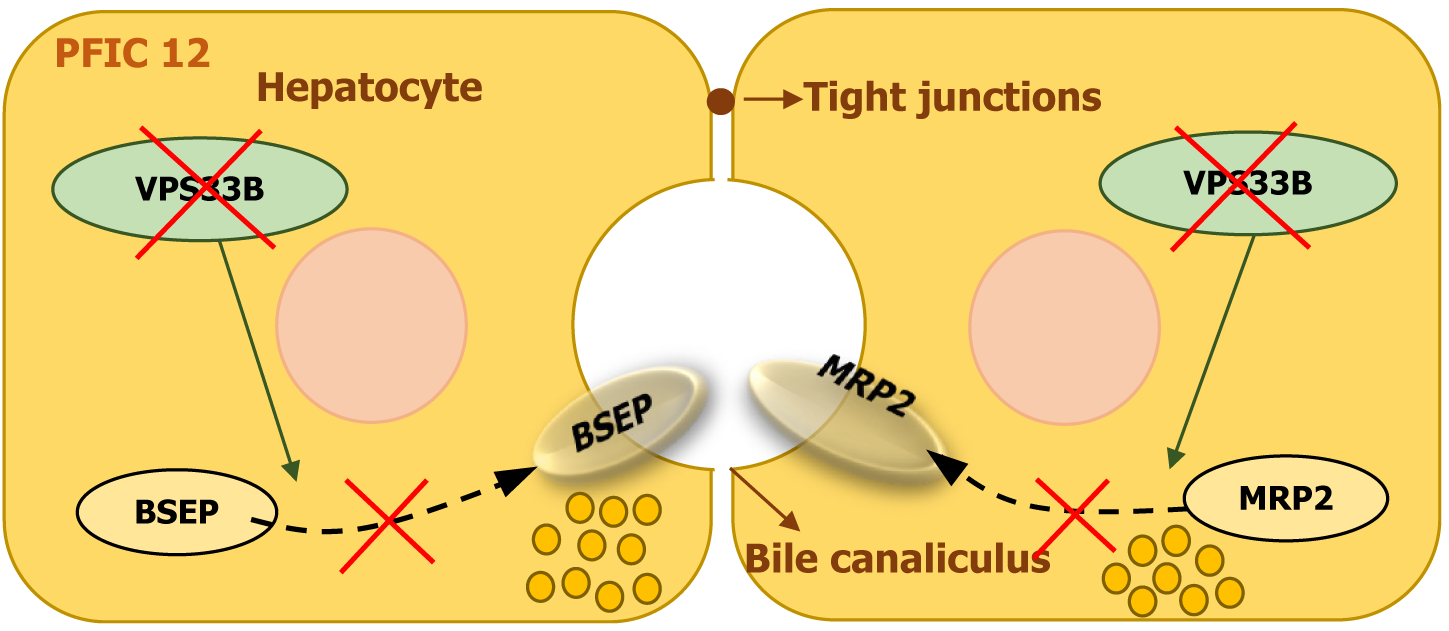
PFIC12 is a rare autosomal recessive disorder caused by homozygous or compound heterozygous mutations in the VPS33B gene, located on chromosome 15q26. The VPS33B gene encodes vacuolar sorting-associated protein 33B, which plays a key role in intracellular trafficking and lysosomal function. It is essential for maintaining hepatocyte polarity and bile flow[23].
A defect in VPS33B disrupts normal bile secretion, leading to cholestasis. The condition is characterized by neonatal jaundice, conjugated hyperbilirubinemia, and severe itching (Figure 13)[14,23]. While mutations in this gene are also linked to arthrogryposis-renal dysfunction-cholestasis syndrome, patients with PFIC12 generally present with hepatic dysfunction without significant renal or joint abnormalities, although mild proteinuria and skeletal issues have been observed in some cases[13,23].
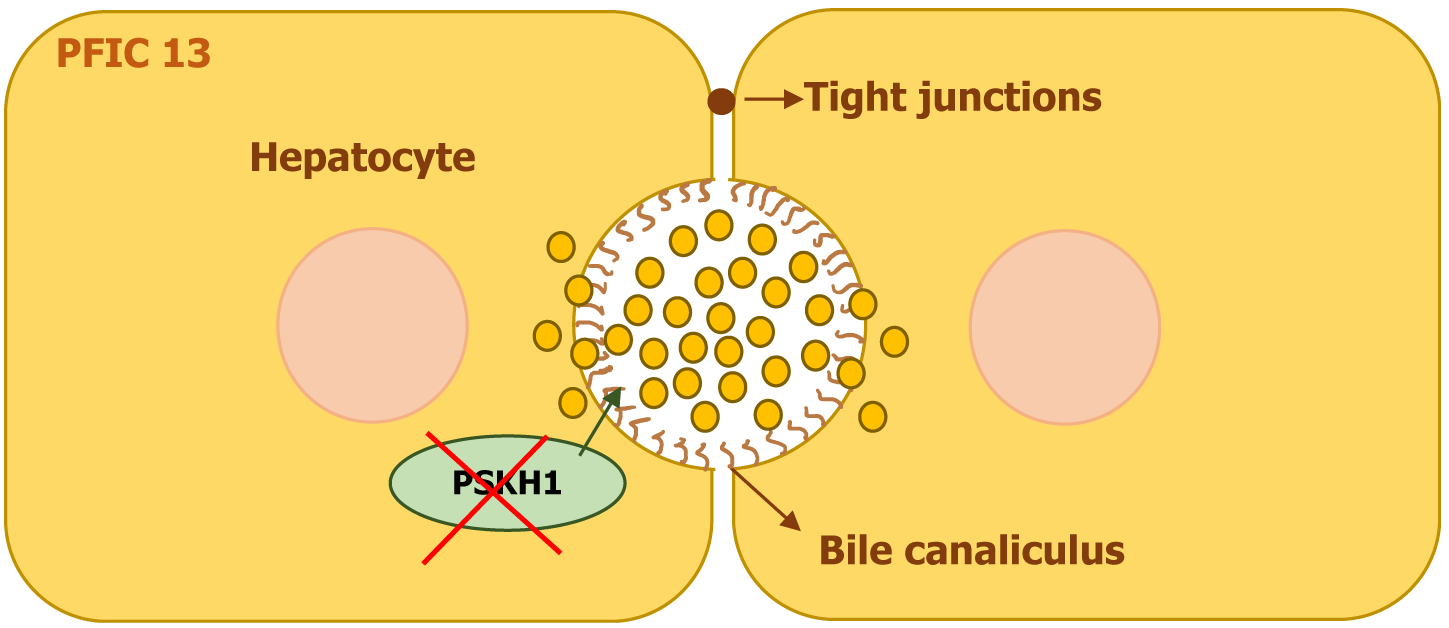
PFIC13 is linked to homozygous mutations in the protein serine kinase H1 (PSKH1) gene, which is located on chromosome 16q22. The PSKH1 gene encodes PSKH1, a protein essential for ciliary function and intracellular transport[24].
Mutations in PSKH1 contribute to hepatorenal ciliopathy, leading to cholestasis and progressive kidney disease. Dysfunction in bile duct cilia disrupts bile flow, resulting in intrahepatic and biliary cholestasis (Figure 14). Many affected individuals develop chronic kidney disease, often accompanied by unilateral renal agenesis and glomerulosclerosis. The defective bile excretion results in jaundice and pruritus, while renal abnormalities lead to kidney dysfunction[24].
The management of PFIC in pediatric patients involves a multifaceted approach aimed at controlling symptoms, slowing the progression of liver damage, and addressing related complications such as malnutrition, intense itching (pruritus), and liver injury. There is no definitive curative medical therapy. Treatment strategies encompass both non-invasive medical therapies and surgical interventions, depending on the severity of the condition, the genetic type and subtype.
Medical therapies focus on alleviating cholestasis, reducing the accumulation of bile acids and salts in the liver, and managing pruritus. In addition, patients often require nutritional support due to malabsorption of fat, and fat-soluble vitamins.
There are several medical treatments available, including ursodeoxycholic acid (UDCA) aimed to enhance bile flow, antipruritic like rifampicin, and more recently, bile acid transport inhibitors such as Odevixibat and Maralixibat.
Traditionally, when medical treatments are not effective, surgical options may be explored. For children with severe symptoms or liver dysfunction, procedures such as biliary diversion can help redirect bile and alleviate symptoms. In advanced stages of the disease, liver transplantation might be required to restore liver function and enhance the patient’s quality of life.
Bile plays a crucial role in the intestinal absorption of fats and fat-soluble vitamins. In cholestatic liver diseases, impaired absorption of fat and fat-soluble vitamins (A, D, E, and K) is common but often not clinically apparent.
Fat malabsorption can lead to insufficient calorie intake and failure to thrive, particularly in young children. Fat-soluble vitamins deficiency may result in various complications, including rickets, osteoporosis, coagulopathy, life threatening bleeding, impaired neurological, immune, and visual functions and insufficient antioxidant activity[25].
The total caloric intake is advised be about 125% of the recommended daily allowance. Dietary fat should primarily consist of medium-chain triglycerides oil, which doesn’t require bile salts for absorption and helps improve nutrition. Fat-soluble vitamins are supplemented in the following doses for children: Vitamin A (5000–25000 IU/day orally), vitamin D (400–800 IU/day orally), vitamin E (50–100 IU/day orally), and vitamin K (1–5 mg/day orally or 1–5 mg intravenously every 3–4 weeks). Adequate sunlight exposure and a calcium intake of 800–2000 mg/day are also essential. Additionally, clinical and biochemical monitoring (including serum vitamin levels) should be conducted to identify vitamin deficiencies, with supplement dosages adjusted accordingly[26].
UDCA is the most frequently prescribed off-label treatment for patients with PFIC[25]. As a hydrophilic bile acid, UDCA replaces hydrophobic bile acids in bile, reducing their toxic effects on the liver. Additionally, it is believed to enhance biliary secretion by upregulating the expression of BSEP and MDR3 transporters in bile canaliculi[26].
UDCA is administered at a dose of 20-30 mg/kg per day, divided into 2-3 doses[27]. It is considered a safe and well-tolerated medication with few adverse effects. Long-term use in PFIC patients has shown improvements in both clinical symptoms and biochemical liver markers. However, its efficacy varies by PFIC subtype, with the greatest benefit observed in PFIC type 3, where it improves symptoms and slows disease progression[26,27].
Rifampicin is commonly used as a second-line off-label therapy for managing pruritus associated with cholestasis. It alleviates itching by enhancing bile salt metabolism through the activation of pregene X Receptor, which upregulates the expression of cytochrome P450 enzymes, particularly CYP3A4, responsible for bile salt metabolism, thereby reducing their accumulation in the liver and plasma[26,28,29]. Additionally, rifampicin is thought to promote bilirubin excretion by increasing the expression of bilirubin transporters across the canalicular membrane, such as UGT1A1 and MRP2 proteins[29].
Rifampicin is frequently administered alongside UDCA at a dose of 10 mg/kg/day, given in one or two divided doses[30]. However, it has shown reduced efficacy in PFIC types 2 and 3. One of the most common adverse effects is orange discoloration of body fluids. Additionally, as an enzyme inducer of cytochrome P450, rifampicin can influence the metabolism of other drugs. While generally well-tolerated, its known hepatotoxic effects necessitate regular monitoring of liver function tests during treatment[26,28].
Cholestyramine and colesevelam are bile acid sequestrants commonly used off-label to manage pruritus associated with cholestasis. They primarily function by binding bile salts in the gastrointestinal tract, forming insoluble complexes that prevent reabsorption, thereby lowering plasma bile salt levels and alleviating itching[31].
While some patients experience relief with these treatments, there is limited evidence supporting their efficacy and long-term outcomes in the pediatric population. It is important to provide clear instructions for their use, as these agents may interfere with the absorption of other medications and fat-soluble vitamins[26,31].
Surgical intervention plays a role in the management of PFIC, particularly in patients who fail to respond to medical therapy but are not yet candidates for liver transplantation. The primary objective of surgical treatment is to interrupt the enterohepatic circulation of bile acids, thereby lowering systemic bile acid levels. This disruption is thought to alleviates cholestasis and pruritus, improves liver function, and helps delay disease progression. The main surgical strategies include biliary diversion procedures—such as partial biliary diversion and ileal bypass—and liver transplantation in advanced cases.
Biliary diversion procedures are designed to reduce bile acid reabsorption and are most effective when performed early in the disease course, particularly in non-cirrhotic patients. These procedures are often combined with UDCA to enhance therapeutic outcomes.
Partial external biliary diversion: Partial external biliary diversion (PEBD) is widely regarded as the first-line surgical option for patients with PFIC type 1 and 2, particularly when performed prior to the onset of cirrhosis. It involves the creation of a jejunal conduit between the gallbladder and abdominal wall, resulting in a permanent stoma that facilitates continuous external bile drainage. The procedure can be carried out via open or laparoscopic techniques, and modifications may use a gallbladder wall button or the appendix as a conduit.
Clinical studies have demonstrated that approximately 50% of patients undergoing PEBD achieve normalization of serum bile acid levels and remain free of cholestasis over a median follow-up of 9.8 years. A favorable biochemical response—particularly normalization of bile acids within one year—is considered a strong predictor of long-term clinical success[26,32].
Partial internal biliary drainage: Partial internal biliary drainage offers a similar bile diversion strategy without the need for an external stoma. It involves diverting bile from the gallbladder to the colon using a jejunal conduit or through a cholecysto-colostomy utilizing an anti-reflux colonic loop. Although this approach is less invasive cosmetically, its long-term efficacy remains under evaluation, and comparative data with PEBD are currently limited[26,32,33].
Originally developed for PFIC patients with a prior cholecystectomy, ileal bypass entails surgical exclusion of the distal 15% of the ileum—the primary site of bile acid reabsorption—via an ileocolic anastomosis. The main advantage of this procedure is the avoidance of an external stoma, which reduces the risk of fluid and electrolyte imbalances. However, clinical efficacy is limited, as symptom recurrence occurs in up to 50% of patients within one year, largely due to com
Liver transplantation (LT) remains the cornerstone and definitive treatment for PFIC, particularly in cases of refractory pruritus, end-stage liver disease, and hepatobiliary malignancies seen in PFIC2. Post-transplant outcomes demonstrate encouraging survival rates, with approximately 85% patient survival and 77% graft survival. LT is generally curative for PFIC2 and PFIC3, with minimal risk of extrahepatic complications. However, outcomes in PFIC1 are more variable due to persistent post-transplant issues such as diarrhea, steatosis, and growth impairment, often linked to increased bile acid secretion; these may necessitate additional interventions such as bile acid resins or biliary diversion.
Living donor liver transplantation has proven equally effective and safe, even when donors are genetically related, and serves as a timely alternative in regions with limited access to deceased donor organs. While LT carries the general risks of infection and rejection, PFIC-specific challenges include worsening extrahepatic symptoms in PFIC1 and potential disease recurrence in PFIC2 due to anti-BSEP antibodies, which may require targeted immunosuppressive therapies such as plasmapheresis or rituximab. Ultimately, optimal outcomes in PFIC require an individualized, multidisciplinary approach based on the subtype, specific gene mutations, and severity of clinical manifestations[35].
Odevixibat is a selective inhibitor of the IBAT, also known as the apical sodium-dependent bile acid transporter (ASBT). In July 2021, Odevixibat was the first approved IBAT inhibitor in children with PFIC. The EU approved it for the treatment of PFIC in patients aged ≥ 6 months and in the United States for the treatment of pruritus in patients aged ≥ 3 months with PFIC[36,37].
Pharmacodynamics: The concept behind inhibiting the IBAT stems from the fact that 95%-97% of bile acids produced by the liver are reabsorbed in the terminal ileum and return to the liver via the enterohepatic circulation. Bile acids are absorbed through the ASBT or the IBAT at the ileal enterocyte’s apical border.
Bile acids activate bile acid activated receptors, such as FXR, which regulate bile acid synthesis and metabolism. FXR activation decreases the expression of ASBT, reducing bile acid absorption in the ileum, and inhibits bile acid synthesis in the liver by suppressing the enzyme CYP7A1 (the rate limiting step of bile acid synthesis). Additionally, FXR reduces bile acid reuptake into hepatocytes and promotes bile acid excretion through increased BSEP expression[31]. Odevixibat is an oral small-molecule inhibitor that blocks bile acid absorption in the terminal ileum, promoting bile acid excretion in feces. Reduced bile acid levels lead to less FXR activation, increasing CYP7A1 activity and promoting more cholesterol conversion into bile acids. This process also enhances LDL receptor activity, lowering blood cholesterol levels[38].
Odevixibat is an IBAT inhibitor used for PFIC treatment and is available in four strengths. The smaller capsules (400 µg and 1200 µg) are swallowed whole, while the larger capsules (200 µg and 600 µg) can be opened, with the oral pellets inside sprinkled onto soft food for easier administration[37].
If PFIC is highly suspected or confirmed, clinicians may begin treatment with a starting dose of 40 µg/kg/day for 12 weeks while awaiting genetic testing results. If there is clinical or biochemical improvement, treatment continues. A successful response has been defined by a reduction of over 70% in serum bile acids or a decrease of at least 1 point in the pruritus score. Various tools, such as the PRUCISION tool[39], are available to assess pruritus, and it is recommended to quantify the pruritus before starting treatment to track the response. If there is response to treatment, odevixibat could be maintained at the same dose. If there is no improvement, the dose can be increased to 120 µg/kg/day for another 12 weeks[36].
Odevixibat has been generally well tolerated in pediatric patients with cholestatic liver diseases. In the phase III PEDFIC 1 trial, 33.3% of patients on odevixibat (40 or 120 µg/kg/day) reported adverse events, including diarrhea and elevated liver enzymes, compared to 15.0% of those on placebo. Similar results were found in the PEDFIC 2 extension study, where 29% of patients experienced adverse events, primarily related to elevated bilirubin and liver enzymes. No patients developed vitamin deficiencies resistant to supplementation, and serious adverse events were rare[37].
Pharmacokinetics: Odevixibat has minimal absorption in pediatric patients with PFIC. Plasma concentrations are usually below detectable limits when administered at 40 or 120 µg/kg daily. The drug’s absorption is unaffected by food, and it is primarily excreted through feces (82.9%), with minimal clearance via urine. Odevixibat has a half-life of around 2.5 hours, is highly bound to plasma proteins (> 99%), and undergoes minimal metabolism[37].
Odevixibat may reduce the absorption of fat-soluble vitamins, and monitoring of fat-soluble vitamin levels is recommended. It may also affect the absorption of fat-soluble medications. Staggered administration is recommended to avoid binding with bile acid-binding resins in the gut[37].
Therapeutic trials: PEDFIC 1 trial: Odevixibat has demonstrated efficacy in reducing pruritus and serum bile acids in children with PFIC in the phase III PEDFIC 1 trial. In this study, children with PFIC type 1 or 2 showed significant improvements in pruritus and serum bile acid levels compared to placebo.
PEDFIC 2 trial: Long-term benefits were observed in the ongoing PEDFIC 2 trial, with reduction in pruritus and improvement in growth parameters and quality of life over 48 weeks.
Pooled analysis PEDFIC 1 and 2: A pooled analysis of data from both PEDFIC 1 and 2 further supported the efficacy of odevixibat, with sustained improvements in serum bile acids, pruritus, growth, and sleep seen as early as four weeks into treatment and maintained for up to 48 weeks[36,37].
Additional studies: A case report published in 2023 described a 6-year-old female patient presenting with chronic cholestatic jaundice and severe pruritus, who was ultimately diagnosed with PFIC9 due to a homozygous mutation in the ZFYVE19 gene. Despite preserved hepatic synthetic function, laboratory evaluation revealed markedly elevated serum bile acids and transaminases, alongside treatment-refractory pruritus. The introduction of odevixibat led to a rapid and sustained improvement in pruritus and a progressive reduction in bile acid levels. This case represents the first published evidence of odevixibat’s efficacy in PFIC9 and highlights its potential as a therapeutic option in rare, non-classical PFIC subtypes[39].
A multicenter, prospective real-world study was undertaken to assess the therapeutic impact of odevixibat across a spectrum of PFIC subtypes. The study enrolled 24 pediatric patients with genetically confirmed PFIC, encompassing both classic and rare variants, including PFIC-4, PFIC-5, PFIC-6, and PFIC-9. All patients received odevixibat at an initial dose of 40 µg/kg/day, with the option to escalate to 120 µg/kg/day based on clinical need. Pruritus severity was evaluated monthly using the Physician Global Impression of Symptom scale, and serum bile acid (sBA) levels were monitored to determine biochemical response. Notably, one-third of the cohort consisted of individuals with rare PFIC subtypes, allowing for direct assessment of treatment efficacy in this underrepresented group. The meaningful clinical and biochemical improvements observed in these patients provide compelling evidence supporting the efficacy of odevixibat across the full genetic and phenotypic spectrum of PFIC[40].
A case series reported the use of odevixibat as an adjunctive therapy for refractory pruritus in three pediatric patients with rare genetic variants of cholestatic liver disease. The cohort included children with heterozygous mutations in AKR1D1, ABCB4, and combined PKHD1/PKHD2 variants, all of which were classified as variants of uncertain significance. Two patients (with AKR1D1 and ABCB4 variants) experienced significant clinical improvement in pruritus and substantial reductions in serum bile acid levels, with the ABCB4 case also notable for concurrent sclerosing cholangitis and Crohn’s disease—the first report of odevixibat use in such a context. The third patient, with progressive fibrocystic liver disease, showed limited response, likely due to the severity of disease progression. Odevixibat was well tolerated across all cases, with no reported adverse effects. These findings support the potential role of odevixibat as a safe and effective adjunct in managing refractory pruritus associated with atypical or genetically undefined cholestatic liver disorders, particularly when introduced early in the disease course[41].
Ongoing research: The phase III PEDFIC 2 trial continues to assess the long-term safety and efficacy of odevixibat in pediatrics and adults. Additionally, two other phase III trials are currently underway: ASSERT study is complete but the open label extension is still ongoing, studying the long-term efficacy of odevixibat in children and adults with Alagille syndrome, and another trial phase III BOLD trial focusing on children with biliary atresia who have undergone a Kasai procedure.
These trials aim to further expand the therapeutic potential of odevixibat for other cholestatic liver diseases[37].
Maralixibat is a selective IBAT inhibitor, similar to odevixibat, that reduces bile acid reabsorption in the ileum, promoting its fecal excretion. It received Food and Drug Administration (FDA) approval in September 2021 for treating cholestatic pruritus in Alagille syndrome (ALGS) in patients aged 1 year and older. It received FDA and European Medicine Agency approval in late 2024 for children with PFIC based on the data from the phase III MARCH study. The randomized trial involved 93 patients with type 1, 2, 3, 4 and 6 PFIC[42].
Pharmacodynamics: Maralixibat works by inhibiting the ASBT/IBAT in the ileum, reducing bile acid reabsorption and enhancing fecal excretion. This mechanism helps alleviate bile acid–induced liver injury and associated complications.
In the INDIGO trial, significant clinical benefit was observed in patients with non-truncating BSEP (nt-BSEP) mutations, who retain partial transporter function. Among 19 such patients, seven achieved ≥ 75% reduction in sBA, accompanied by notable improvements in pruritus, growth parameters, and quality of life. Remarkably, all responders remained transplant-free for over five years. Conversely, patients with truncating BSEP mutations (t-BSEP) or FIC1 deficiency did not experience significant clinical benefit, emphasizing the need for residual BSEP activity for maralixibat to be effective[43].
Maralixibat is formulated as an oral solution and should be administered 30 minutes before the first meal of the day. Treatment is initiated at 190 µg/kg once daily, with the dose increased to 380 µg/kg once daily after one week, as tolerated. The maximum recommended daily dose is 28.5 mg[44].
In the ALGS clinical development program (median treatment duration: 32.3 months), maralixibat was generally well tolerated. The most frequently reported adverse events were mild to moderate gastrointestinal symptoms, including diarrhea, abdominal pain, and vomiting, primarily occurring within the first four weeks of therapy and resolving spontaneously. Serious adverse events were observed in 33% of patients, though only 3.5% were considered treatment-related. Mild elevations in liver enzymes were noted but typically resolved without requiring discontinuation. Importantly, no patients discontinued treatment due to gastrointestinal side effects[44].
Pharmacokinetics: Maralixibat exhibits minimal systemic absorption, and pharmacokinetic parameters are not reliably measurable at doses below 20 mg. In pediatric ALGS patients, plasma concentrations were typically below the quantifiable limit (0.25 ng/mL). Following oral doses of 30 mg, 45 mg, and 100 mg, maralixibat displayed approximately dose-proportional exposure under fasting conditions. The drug is 91% bound to plasma proteins, undergoes minimal meta
Therapeutic trials: ICONIC Trial: In children with ALGS, maralixibat significantly reduced serum bile acids and pruritus. Improvements were sustained up to 204 weeks, with additional benefits in growth, xanthomas, and quality of life.
INDIGO trial: In PFIC2 patients, 7 out of 25 showed ≥ 70% bile acid reduction and ≥ 1-point improvement in pruritus. All responders remained transplant-free after 4.5 years, with sustained improvements in liver biochemistry and growth.
Ongoing research: The MARCH-PFIC trial is evaluating maralixibat in a broader PFIC population to support potential approval in additional subtypes. Additionally, long-term data will help determine maralixibat’s ability to delay or prevent liver transplantation and compare its outcomes to alternatives like odevixibat[44].
Aldafermin is a synthetic analogue of FGF19, an enterokine produced in the ileum in response to bile acid-induced activation of the FXR. Once secreted, FGF19 enters the portal circulation and acts on the liver to inhibit CYP7A1, the rate-limiting enzyme in classical bile acid synthesis. This negative feedback mechanism is essential for maintaining bile acid homeostasis and preventing hepatotoxic accumulation. Aldafermin preserves this regulatory function while eliminating the tumorigenic potential observed with native FGF19 in preclinical models, thereby enhancing its safety profile.
In preclinical and early-phase clinical studies involving adults, aldafermin has demonstrated the ability to lower serum bile acid concentrations and improve hepatic inflammation and fibrosis. Although data in pediatric populations with PFIC remain limited, the pharmacological mechanism of aldafermin supports its potential utility, particularly in PFIC subtypes characterized by bile acid synthesis dysregulation and intact FXR signalling[31].
Obeticholic acid, a semi-synthetic FXR agonist, represents a promising therapeutic approach in the management of cholestatic liver diseases. FXR is a nuclear receptor predominantly expressed in the liver and intestines that plays a central role in regulating bile acid metabolism. Upon activation by bile acids—particularly chenodeoxycholic acid—FXR downregulates CYP7A1 and CYP8B1 to reduce bile acid synthesis and upregulates BSEP (ABCB11), enhancing bile acid excretion into the bile canaliculi. Clinically, FXR agonists such as obeticholic acid have shown efficacy in adult studies by lowering bile acid synthesis, improving cholestasis, and attenuating hepatic inflammation and fibrosis, particularly in patients with primary biliary cholangitis (PBC).
In the context of progressive familial intrahepatic cholestasis (PFIC), the therapeutic potential of FXR agonists depends largely on the underlying genetic subtype. In PFIC2, which is caused by mutations in ABCB11 Leading to non-functional BSEP, the response to FXR activation may be limited. In contrast, patients with PFIC4 or non-classical variants—where bile acid transporter function is at least partially retained—may derive greater benefit from FXR-targeted therapies. Although pediatric-specific clinical data remain sparse, ongoing research and clinical trials are actively investigating the utility of FXR agonists in pediatric cholestatic conditions[31].
PFIC represents a heterogeneous group of autosomal recessive genetic disorders caused by mutations in genes encoding proteins essential for bile acid transport. While classically inherited in a recessive manner, recent case reports have identified symptomatic individuals with heterozygous variants[45,46], suggesting a broader spectrum of inheritance patterns. The underlying defect in bile acid transport leads to cholestasis, hepatocellular injury due to the toxic accumulation of bile acids within hepatocytes, and malabsorption of fats and fat-soluble vitamins, resulting in significant nutritional deficiencies[25,26].
Historically, three main types of PFIC were described; however, advances in genetic research have expanded this classification to 13 distinct subtypes. These subtypes vary significantly in terms of age of onset, clinical presentation, biochemical profile, histopathological features, and associated systemic involvement. For example, PFIC type 2 typically presents in early infancy with a severe, progressive course and carries an increased long-term risk of hepatocellular carcinoma. In contrast, types 3 and 7 may have a later onset with milder, intermittent symptoms. While some PFIC variants manifest with isolated hepatic involvement, others can present with extrahepatic features such as sensorineural hearing loss, cardiomyopathy, skeletal anomalies, and renal dysfunction. Biochemically, PFIC is often suspected in patients presenting with cholestasis and normal GGT levels, although certain subtypes (e.g., types 3, 6, 8, and 9) are characterized by elevated GGT[14].
To date, no definitive curative medical therapy exists for PFIC. Current management focuses on supportive care, including nutritional supplementation to address deficiencies in fat and fat-soluble vitamins. Pharmacologic treatments such as UDCA and rifampicin are used off-label to enhance bile acid secretion or alter bile acid metabolism, respectively. In patients who do not respond to medical therapy, surgical interventions such as partial biliary diversion or ileal bypass may be employed to disrupt enterohepatic circulation and reduce bile acid accumulation, often serving as a bridge to liver transplantation[26,27]. Liver transplantation remains the definitive therapy in cases of refractory pruritus or end-stage liver disease, although it carries a risk of disease recurrence, particularly in PFIC type 2 due to the formation of anti-BSEP antibodies[38].
Recently, novel therapies targeting bile acid reabsorption have emerged. Bile acid transporter inhibitors, such as odevixibat and maralixibat, acting by blocking the ASBT in the terminal ileum, thereby reducing enterohepatic recirculation of bile acids. Odevixibat has received FDA approval for use as a first-line treatment across all PFIC types in July 2021, while maralixibat was approved in 2023. Both agents have shown promising results in reducing pruritus and serum bile acids and are generally well tolerated with minimal adverse effects.
Emerging clinical reports and real-world evidence further support the therapeutic potential of odevixibat in paediatric cholestatic liver diseases beyond classical PFIC types. Documented real life cases have demonstrated meaningful improvements in pruritus and serum bile acid levels in children with rare and genetically diverse PFIC subtypes, including PFIC9 and other atypical variants. A multicentre prospective study confirmed consistent clinical and biochemical responses across a broad PFIC spectrum, while a case series highlighted its benefit as an adjunctive treatment in patients with refractory cholestasis due to uncertain or complex genetic diagnoses[39-41].
Further research is ongoing to establish their long-term safety and effectiveness in managing PFIC[35,36,43,44].
Emerging therapies targeting bile acid regulation have shown potential in the management of PFIC. Aldafermin, a non-tumorigenic analogue of FGF19, suppresses bile acid synthesis via CYP7A1 inhibition and has demonstrated favorable effects in adults, including reductions in serum bile acids and liver inflammation. Although pediatric data are lacking, its mechanism supports potential use in PFIC subtypes with preserved FXR function. Similarly, obeticholic acid, a semi-synthetic FXR agonist, modulates bile acid metabolism by reducing synthesis and enhancing excretion. While its efficacy may vary by genetic subtype, particularly in cases with partial transporter function, ongoing research continues to explore its applicability in pediatric cholestatic liver diseases[31]. PFIC is a heterogenous autosomal recessive progressive cholestatic liver disease with varying genotype and phenotype. Identifying the underlying genetic disorder allows better understanding of the clinical phenotype and associated complications. Management is aimed at nutritional support, trying to make bile more hydrophilic by administering UDCA and control of pruritis. Surgical biliary diversion has proved to reduce bile acid levels and improve pruritis in some patients. New hope exists with IBAT receptor inhibitors, however more data is still required. The ultimate treatment for end stage liver disease and persistent pruritis is liver transplantation.
PFIC is a heterogenous autosomal recessive progressive cholestatic liver disease with varying genotype and phenotype. Identifying the underlying genetic disorder allows better understanding of the clinical phenotype and associated complications. Management is aimed at nutritional support, trying to make bile more hydrophilic by administering UDCA and control of pruritis. Surgical biliary diversion has proved to reduce bile acid levels and improve pruritis in some patients. New hope exists with IBAT receptor inhibitors, however more data is still required. The ultimate treatment for end stage liver disease and persistent pruritis is liver transplantation.
| 1. | Hundt M, Basit H, John S. Physiology, Bile Secretion. 2022 Sep 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 2. | Henkel SA, Squires JH, Ayers M, Ganoza A, Mckiernan P, Squires JE. Expanding etiology of progressive familial intrahepatic cholestasis. World J Hepatol. 2019;11:450-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 3. | Amer S, Hajira A. A Comprehensive Review of Progressive Familial Intrahepatic Cholestasis (PFIC): Genetic Disorders of Hepatocanalicular Transporters. Gastroenterology Res. 2014;7:39-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | A Siddiqi I, Tadi P. Progressive Familial Intrahepatic Cholestasis. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 5. | Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, Oude Elferink RP. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S26-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Sohail MI, Dönmez-Cakil Y, Szöllősi D, Stockner T, Chiba P. The Bile Salt Export Pump: Molecular Structure, Study Models and Small-Molecule Drugs for the Treatment of Inherited BSEP Deficiencies. Int J Mol Sci. 2021;22:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Wei CS, Becher N, Friis JB, Ott P, Vogel I, Grønbæk H. New tight junction protein 2 variant causing progressive familial intrahepatic cholestasis type 4 in adults: A case report. World J Gastroenterol. 2020;26:550-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Sakhuja P, Goyal S. Progressive familial intrahepatic cholestasis. [cited 3 March 2025]. Available from: PathologyOutlines.com. |
| 10. | Grammatikopoulos T. Familial Intrahepatic Cholestasis. In: Guandalini S, Dhawan A, (editors). Textbook of Pediatric Gastroenterology, Hepatology and Nutrition. Cham: Springer, 2022. [DOI] [Full Text] |
| 11. | Vinayagamoorthy V, Srivastava A, Sarma MS. Newer variants of progressive familial intrahepatic cholestasis. World J Hepatol. 2021;13:2024-2038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Online Mendelian Inheritance in Man (OMIM). Cholestasis, progressive familial intrahepatic, 6; PFIC6 (MIM #619484) [Internet]. [cited 3 March, 2025]. Available from: https://www.omim.org/entry/619484. |
| 13. | Xie S, Wei S, Ma X, Wang R, He T, Zhang Z, Yang J, Wang J, Chang L, Jing M, Li H, Zhou X, Zhao Y. Genetic alterations and molecular mechanisms underlying hereditary intrahepatic cholestasis. Front Pharmacol. 2023;14:1173542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Vitale G, Sciveres M, Mandato C, d'Adamo AP, Di Giorgio A. Genotypes and different clinical variants between children and adults in progressive familial intrahepatic cholestasis: a state-of-the-art review. Orphanet J Rare Dis. 2025;20:80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Bull LN, Ellmers R, Foskett P, Strautnieks S, Sambrotta M, Czubkowski P, Jankowska I, Wagner B, Deheragoda M, Thompson RJ. Cholestasis Due to USP53 Deficiency. J Pediatr Gastroenterol Nutr. 2021;72:667-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Kazmierczak M, Harris SL, Kazmierczak P, Shah P, Starovoytov V, Ohlemiller KK, Schwander M. Progressive Hearing Loss in Mice Carrying a Mutation in Usp53. J Neurosci. 2015;35:15582-15598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Online Mendelian Inheritance in Man (OMIM). Progressive familial intrahepatic cholestasis 8; PFIC8 (MIM #619662) [Internet]. [cited 2 March 2025]. Available from: https://omim.org/entry/619662. |
| 18. | Stalke A, Sgodda M, Cantz T, Skawran B, Lainka E, Hartleben B, Baumann U, Pfister ED. KIF12 Variants and Disturbed Hepatocyte Polarity in Children with a Phenotypic Spectrum of Cholestatic Liver Disease. J Pediatr. 2022;240:284-291.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Vitale G, Mattiaccio A, Conti A, Turco L, Seri M, Piscaglia F, Morelli MC. Genetics in Familial Intrahepatic Cholestasis: Clinical Patterns and Development of Liver and Biliary Cancers: A Review of the Literature. Cancers (Basel). 2022;14:3421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Ben Sabbahia D, Atrasssi M, Bennani N, Benmoussa A, Abkari A. ZFYVE19 gene mutation: A novel variant of progressive familial intrahepatic cholestasis. JPGN Rep. 2024;5:552-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Online Mendelian Inheritance in Man (OMIM). Cholestasis, progressive familial intrahepatic, 11; PFIC11 (MIM #619874) [Internet]. [cited 3 March 2025]. Available from: https://omim.org/entry/619874. |
| 22. | Pan Q, Luo G, Qu J, Chen S, Zhang X, Zhao N, Ding J, Yang H, Li M, Li L, Cheng Y, Li X, Xie Q, Li Q, Zhou X, Zou H, Fan S, Zou L, Liu W, Deng G, Cai SY, Boyer JL, Chai J. A homozygous R148W mutation in Semaphorin 7A causes progressive familial intrahepatic cholestasis. EMBO Mol Med. 2021;13:e14563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Online Mendelian Inheritance in Man (OMIM). Cholestasis, progressive familial intrahepatic, 12; PFIC12 (MIM #620010) [Internet]. [cited 4 March 2025]. Available from: https://omim.org/entry/620010. |
| 24. | Online Mendelian Inheritance in Man (OMIM). Cholestasis, progressive familial intrahepatic, 13; PFIC13 (MIM #620962) [Internet]. [cited 4 March 2025]. Available from: https://www.omim.org/entry/620962. |
| 25. | Chen HL, Wu SH, Hsu SH, Liou BY, Chen HL, Chang MH. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Jacquemin E, Hermans D, Myara A, Habes D, Debray D, Hadchouel M, Sokal EM, Bernard O. Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis. Hepatology. 1997;25:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Stapelbroek JM, van Erpecum KJ, Klomp LW, Houwen RH. Liver disease associated with canalicular transport defects: current and future therapies. J Hepatol. 2010;52:258-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R, Gustafsson U, Sahlin S, Einarsson C, Trauner M. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 30. | Children’s Antimicrobial Management Program Pharmacist. Rifampicin monograph – paediatric [Internet]. 2023. [cited 7 April 2025]. Available from: https://r.search.yahoo.com/_ylt=AwrFPkwUqkZoCOUI1gtXNyoA;_ylu=Y29sbwNiZjEEcG9zAzQEdnRpZAMEc2VjA3Ny/RV=2/RE=1750671124/RO=10/RU=https%3a%2f%2fwww.fionastanley.health.wa.gov.au%2f-%2fmedia%2fFiles%2fHospitals%2fPCH%2fGeneral-documents%2fHealth-professionals%2fChAMP-Monographs%2fRifampicin.pdf/RK=2/RS=oFsWx14TnvbhpoocaN3moLbDN_A-. |
| 31. | Kriegermeier A, Green R. Pediatric Cholestatic Liver Disease: Review of Bile Acid Metabolism and Discussion of Current and Emerging Therapies. Front Med (Lausanne). 2020;7:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology. 1988;95:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 188] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Bustorff-Silva J, Sbraggia Neto L, Olímpio H, de Alcantara RV, Matsushima E, De Tommaso AM, Brandão MA, Hessel G. Partial internal biliary diversion through a cholecystojejunocolonic anastomosis--a novel surgical approach for patients with progressive familial intrahepatic cholestasis: a preliminary report. J Pediatr Surg. 2007;42:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Mochizuki K, Obatake M, Takatsuki M, Nakatomi A, Hayashi T, Okudaira S, Eguchi S. Partial internal biliary diversion for patients with progressive familial intrahepatic cholestasis type 1. Pediatr Surg Int. 2012;28:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Kaliciński PJ, Ismail H, Jankowska I, Kamiński A, Pawłowska J, Drewniak T, Markiewicz M, Szymczak M. Surgical treatment of progressive familial intrahepatic cholestasis: comparison of partial external biliary diversion and ileal bypass. Eur J Pediatr Surg. 2003;13:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Porwal M, Kumar A, Rastogi V, Maheshwari KK, Verma A. Odevixibat: A Review of a Bioactive Compound for the Treatment of Pruritus Approved by the FDA. Curr Drug Res Rev. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Deeks ED. Odevixibat: First Approval. Drugs. 2021;81:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Flattmann FE, Mohiuddin FS, Singh A, Tandon A, Lockett SJ, Hirsch JD, Mosieri CN, Kaye AM, Varrassi G, Ahmadzadeh S, Shekoohi S, Kaye AD. Odevixibat: A Novel Bile Salt Inhibitor Treatment for Pruritus in Progressive Familial Intrahepatic Cholestasis. Cureus. 2024;16:e56886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Pepe A, Colucci A, Carucci M, Nazzaro L, Bucci C, Ranucci G, Di Giorgio A, Vajro P, Mandato C. Case Report: Add-on treatment with odevixibat in a new subtype of progressive familial intrahepatic cholestasis broadens the therapeutic horizon of genetic cholestasis. Front Pediatr. 2023;11:1061535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 40. | Di Giorgio A, Sciveres M, Fuoti M, Calvo P, Cananzi M, Lleo A, Gatti S, Indolfi G, Madeo A, Mandato C, Nuti F, Zanchi C, Carioli G, Ghirardi A, Nicastro E, D'Antiga L. Real-world experience with odevixibat in children with progressive familial intrahepatic cholestasis. JHEP Rep. 2025;7:101309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 41. | Goel A, Tucker B, Casale LS, Grammatikopoulos T. Odevixibat as an adjunctive treatment for refractory pruritus in rare variants of cholestatic liver disease. JPGN Rep. 2024;5:296-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Miethke AG, Moukarzel A, Porta G, Covarrubias Esquer J, Czubkowski P, Ordonez F, Mosca A, Aqul AA, Squires RH, Sokal E, D'Agostino D, Baumann U, D'Antiga L, Kasi N, Laborde N, Arikan C, Lin CH, Gilmour S, Mittal N, Chiou FK, Horslen SP, Huber WD, Jaecklin T, Nunes T, Lascau A, Longpre L, Mogul DB, Garner W, Vig P, Hupertz VF, Gonzalez-Peralta RP, Ekong U, Hartley J, Laverdure N, Ovchinsky N, Thompson RJ. Maralixibat in progressive familial intrahepatic cholestasis (MARCH-PFIC): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2024;9:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Loomes KM, Squires RH, Kelly D, Rajwal S, Soufi N, Lachaux A, Jankowska I, Mack C, Setchell KDR, Karthikeyan P, Kennedy C, Dorenbaum A, Desai NK, Garner W, Jaecklin T, Vig P, Miethke A, Thompson RJ. Maralixibat for the treatment of PFIC: Long-term, IBAT inhibition in an open-label, Phase 2 study. Hepatol Commun. 2022;6:2379-2390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 44. | Shirley M. Maralixibat: First Approval. Drugs. 2022;82:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 45. | Pandey D, Chandnani S, Gandhi H, Vishal M, Rathi PM. A Novel Compound Heterozygous Mutation in Progressive Familial Intrahepatic Cholestasis (PFIC) 4: A Rare Case Report With Literature Review. Cureus. 2024;16:e73927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Bai J, Li L, Liu H, Liu S, Bai L, Ning H, Song W, Zou H, Wang X, Chen Y, Zheng S, Duan Z. A novel compound heterozygous mutation in ABCB4 gene in a pedigree with progressive familial intrahepatic cholestasis 3: a case report. Ann Transl Med. 2021;9:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |