Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.107668
Revised: May 4, 2025
Accepted: June 25, 2025
Published online: July 27, 2025
Processing time: 119 Days and 11.3 Hours
The search for reliable biomarkers to predict metabolic dysfunction-associated steatotic liver disease (MASLD) remains a key research focus. Traditional anthropometric parameters, such as triglycerides, glucose, and waist circumference (WC), have proven to be robust tools for diagnosing, stratifying, and predicting health outcomes. These measures facilitate early detection, personalized treatment strategies, and long-term risk assessment in metabolic health. The triglyceride-glucose (TyG) index and related parameters, particularly the TyG-WC index, are gaining recognition as reliable biomarkers for MASLD, with consistently high diagnostic accuracy across diverse populations. The TyG-WC index is associated with MASLD and an increased likelihood of all-cause, cardiovascular, and diabetes-related mortality, highlighting its importance in stratification and patient management. This opinion review summarizes key findings on the TyG-WC index across different MASLD populations and provides nutritional recommendations aimed at reducing this index. The TyG-WC index stands out as a practical and scalable biomarker for identifying and stratifying the risk of MASLD, particularly in resource-limited environments where access to advanced diagnostic tools is restricted. However, before the TyG-WC index can be integrated into routine clinical practice, rigorous, longitudinal studies involving ethnically diverse cohorts must validate its prognostic per
Core Tip: The search for reliable biomarkers for metabolic dysfunction-associated steatotic liver disease (MASLD) remains a critical priority. Among non-invasive and accessible tools, the triglyceride-glucose-waist circumference index (TyG-WC) has emerged as a particularly robust marker, demonstrating superior diagnostic performance compared with other indices. Its utility extends beyond MASLD, offering valuable insights into cardiovascular risk and other extrahepatic manifestations. Given its simplicity and affordability, the TyG-WC index holds significant promise, particularly in low-resource settings. Nonetheless, longitudinal studies are needed to validate its predictive capacity and guide clinical implementation. Integrating this index within clinical and research frameworks, alongside investigations into the gut microbiota, multiomic profiling, and artificial intelligence, may unlock new pathways for improving MASLD diagnosis and management.
- Citation: Priego-Parra BA, Román-Calleja BM, Gallego-Duran R, Gracia-Sancho J, Velarde Ruiz-Velasco JA, Remes-Troche JM. Triglyceride-glucose-waist circumference index: A powerful tool for metabolic dysfunction-associated steatotic liver disease. World J Hepatol 2025; 17(7): 107668
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/107668.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.107668
In recent years, the terminology surrounding liver diseases has evolved, shifting from non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated fatty liver disease (MAFLD)[1] and metabolic dysfunction-associated steatotic liver disease (MASLD)[2]. These newer terms emphasize the integration of liver steatosis within the broader context of metabolic syndrome.
MASLD is the leading cause of chronic liver disease worldwide[3], with projections that the number of cases will continue to rise over the next few years[4], representing a silent pandemic that imposes significant costs on healthcare systems, contributes to long-term complications, decreases quality of life, and carries considerable stigma[5,6].
Characterized by lipid accumulation in the liver, MASLD is a sex-dimorphic disease[7], that presents with distinct “metabotypes”, leading to significant heterogeneity in its clinical presentation and associated risks[8,9]. While liver biopsy remains the diagnostic gold standard, its invasive nature, inherent limitations, and associated risks underscore the urgent need for reliable non-invasive biomarkers[10].
Epidemiological studies indicate that up to 99% of individuals diagnosed with NAFLD meet the criteria for MA
MAFLD has been proposed as a more effective nomenclature for identifying individuals with a pro-inflammatory metabolic profile who are at an increased risk of disease progression and complications[15,16]. Compared with NAFLD and MASLD, MAFLD has been associated with a higher risk of cardiovascular disease[17], chronic kidney disease[18], and liver-related adverse outcomes[16,19]. However, this increased risk may be attributed to the broader inclusion criteria of MAFLD, which allow for the presence of other hepatic comorbidities and alcohol use[20].
Conversely, MASLD is a more inclusive classification regarding metabolic risk factors, facilitating earlier diagnosis and improving the recognition of lean individuals and those at heightened risk for diabetes[21]. On the other hand, from a nomenclatural perspective, the term “fatty” does not universally carry the same stigma and may be more comprehensible to the general public than “steatotic”[22], raising concerns about the global applicability of the revised terminology. Furthermore, MASLD could lead to overdiagnosis, underscoring the need for extensive educational campaigns targeting both healthcare professionals and the general population.
Steatosis indices have supported the diagnosis of early-stage NAFLD in patients with preserved liver function. However, these patients do not meet the criteria for inclusion in the MAFLD or MASLD frameworks, highlighting a gap in the current diagnostic systems[23].
A notable proposal is to change the “D” in “disease” to “disorders,” which would better reflect the spectrum of the condition, rather than a single disease entity[20]. Additionally, the term “combinatorial MASLD” has been proposed to encompass individuals with MASLD and other causes of liver disease, potentially improving overall clinical outcomes[24,25].
Moving forward, a critical aspect to consider is the number, type, and potential combinations of metabolic criteria, along with specific genetic polymorphisms[26], epigenetic changes[27,28], hormonal status[29], microbiota signatures[30,31], sex-specific microbial heterogeneity[32], social determinants of health[33,34], and endocrine conditions, such as polycystic ovary syndrome[35,36], gout[37] and hypothyroidism[38,39], as these factors may influence disease pro
Some essential strategies for integrating the MASLD nomenclature into healthcare systems involve education and training, standardization of diagnostic protocols, incorporation into national and international coding frameworks, public health awareness initiatives, and tailored adaptations for resource-limited settings, as highlighted by Iruzubieta et al[20].
The pathophysiology of MASLD is multifactorial and remains incompletely understood. It involves a complex and dynamic interplay of genetic, epigenetic, environmental, metabolic, immunological, and cellular processes. Genetic susceptibility plays a central role, with variants in PNPLA3 (I148M), TM6SF2 (E167K), MBOAT7, GCKR, HSD17B13, and CDKN1A associated with altered lipid metabolism, increased hepatic fat accumulation, and fibrogenesis[40–42]. Epi
Environmental factors are increasingly recognized as important contributors to MASLD. Chronic exposure to microplastics, heavy metals, pesticides, and air pollutants, such as particulate matter (PM) 2.5, PM10, nitrogen dioxide, and nitrogen oxides, is associated with systemic and hepatic stress[47,48]. Dietary influences, including high consumption of fructose, food additives, preservatives, emulsifiers, and artificial sweeteners, may further exacerbate hepatic steatosis and inflammation[49]. Disruption of the gut–liver axis, largely driven by intestinal microbiota dysbiosis, increases intestinal permeability and promotes endotoxemia. This facilitates the translocation of microbial products and enhances hepatic inflammation through mediators, such as neurotensin and advanced glycation end products[50].
Metabolic dysregulation is a hallmark of MASLD, encompassing dysregulated lipid metabolism[51], peripheral leptin resistance[52], adiposopathy[53], intrahepatic hypothyroidism[54], bile acid abnormalities[55,56], and altered systemic amino acid profiles[57]. At the cellular level, a variety of mechanisms, including changes in the antioxidant system[58], stellate cell dysfunction[59], cell senescence[60], mitochondrial and lysosomal dysfunction[61], ferroptosis[62] and apoptosis mediated by Bcl-2 family proteins[63], contribute to hepatocellular injury and fibrosis. The convergence of these pathways results in a maladaptive interplay between inflammatory and metabolic processes, which accelerates disease progression and increases clinical severity in MASLD.
The transition from MASLD to metabolic dysfunction-associated steatohepatitis is driven by insulin resistance in key metabolic tissues, such as adipose tissue, skeletal muscle, and the pancreas, culminating in hepatocyte injury (apoptosis, necrosis, pyroptosis), pro-inflammatory cytokine release, and fibrogenesis, with progression to cirrhosis and hepatocellular carcinoma[64]. Central to this pathophysiology is visceral adipose tissue (VAT), which amplifies metabolic dysfunction through dysregulated lipolysis and chronic inflammation. VAT releases excessive free fatty acids (FFAs) into the portal circulation, overwhelming hepatic β-oxidation capacity and driving re-esterification into triglycerides, thereby exacerbating hepatic steatosis[65]. Concurrently, VAT-derived inflammatory mediators (e.g., tumor necrosis factor-alpha, interleukin-6) and adipokines (e.g., reduced adiponectin) impair insulin signaling pathways, worsening systemic insulin resistance. This creates a vicious cycle: Hepatic de novo lipogenesis and gluconeogenesis are upregulated, while skeletal muscle glucose uptake is suppressed, further elevating circulating glucose and insulin levels[66].
The metabolic sequelae of these disruptions are profound. Hypertriglyceridemia, elevated FFA flux, and impaired glucose homeostasis reflect both hepatic and peripheral insulin resistance. Clinically, these disturbances correlate with visceral adiposity [elevated waist circumference (WC)] and hyperinsulinemia, hallmarks of heightened cardiometabolic risk. Prolonged insulin resistance and chronic inflammation activate hepatic stellate cells, promoting collagen deposition and fibrosis[67]. Over time, this maladaptive cascade perpetuates inflammation, dysbiosis, systemic hyperglycemia, compensatory hyperinsulinemia, and end-organ damage, creating a bidirectional link between hepatic and extrahepatic metabolic dysfunction.
Beyond hepatic pathology, MASLD has been associated with cerebrovascular changes[68] and cognitive impairment[69], likely driven by chronic inflammation and dysfunction of the microbiota-liver-brain axis[70]. Cognitive impairment in MASLD patients may further hinder adherence to treatment regimens, reduce physical activity engagement, and exacerbate fatigue, ultimately worsening metabolic dysfunction. Moreover, visceral fat (VF) may exacerbate brain dys
The possible combination of mechanisms and individual environmental exposure leads to significant heterogeneity in the clinical presentation of MASLD, making a “one-size-fits-all” approach to therapy impossible and highlighting the urgency of advancing precision medicine to tailor interventions based on individual pathophysiological profiles[72].
MASLD is diagnosed based on the presence of hepatic steatosis along with at least one metabolic criterion[2]. However, diagnosing steatosis presents challenges, as liver biopsy—the gold standard—has inherent limitations, and commonly used imaging techniques, such as ultrasound, magnetic resonance imaging, and controlled attenuation parameter, are not always accessible, particularly in developing countries. This underscores the need for non-invasive diagnostic methods, which offer significant opportunities across various clinical settings.
A wide range of non-invasive biomarkers has been developed for MASLD[73,74], including serum markers, scoring systems, genetic and epigenetic[75] biomarkers, and imaging-based tools. These biomarkers provide a promising al
While composite scoring systems, such as the fatty liver index[76], hepatic steatosis index[77], SteatoTest[78], NAFLD liver fat score[79], and triglyceride-glucose (TyG) index models, are widely recognized, emerging data suggest that the triglyceride-waist index variants: (1) TyG-body mass index; (2) TyG-waist-to-height ratio; (3) TyG-high-density li
| Index/biomarker | Abbreviation | Formula | Ref. |
| Body mass index | BMI | Weight (kg)/height² (m²) | Ma et al[83] |
| Dallas steatosis index | DSI | McHenry et al[84], McHenry et al[85] | |
| Fatty liver index | FLI | [e0953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC - 15.745]/[1 + e0953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC - 15.745) × 100] | Bedogni et al[76] |
| Fibroblast growth 21 | FGF-21 | Molecular kits | Gallego-Durán et al[86], Keskin et al[87] |
| Framingham steatosis index | FSI | -7.981 + 0.011 × age - (0.146 × sex, female = 1, male = 0) + 0.173 × BMI + 0.007 × TG + (0.593 × hypertension, yes = 1, no = 0) + (0.789 × diabetes, yes = 1, no = 0) + (1.1 × ALT/AST ratio ≥ 1.33, yes = 1, no = 0) | Long et al[88] |
| Hepatic steatosis index | HSI | 8 × ALT/AST ratio + BMI (+ 2, if diabetes mellitus; + 2, if woman) | Lee et al[77], Chung et al[89] |
| Homeostatic model assessment | HOMA | Fasting glucose × fasting insulin/405 | Matthews et al[90] |
| Leukocyte cell-derived chemotaxin 2 | LECT2 | Molecular kits | Suzuki et al[91] |
| Lipid accumulation product | LAP | WC and fasting plasma TG: LAP = (WC – 65) × TG for men, and LAP = (WC – 58) × TG for women | Ebrahimi et al[92], Li et al[93] |
| Micro RNA | MiR-122-5p, miR-151a-3p, miR-126-5p and miR-21-5p | Molecular kits | Tobaruela-Resola et al[75] |
| N3 MASH for MASLD | N3-MASH | N3-MASH = 5.278 × log10(CK-18) + 4.423 × log10(CXCL10) + 1.833 × BMI – 21.652 | Zhang et al[94] |
| N7 MASH for MASLD | N7-MASH | N7-MASH = 3.639 × log10(CK-18) + 4.811 × log10(CXCL10) + 2.911 × log10(squalene epoxidase) + 0.050 × ALT + 0.869 × glycated hemoglobin – 0.117 × AST + 1.193 × BMI – 33.023 | Zhang et al[94] |
| Triglyceride-glucose index | TyG | Ln (TG × glucose/2) | Simental-Mendía et al[95], Zou et al[96] |
| TyG-BMI | TyG-BMI | BMI × TyG index | Khamseh et al[81], Zou et al[96] |
| TyG-HDL cholesterol | TyG-HDL-c | TyG/HDL cholesterol | |
| TyG-WC | TyG-WC | TyG index × WC | Khamseh et al[81] |
| TyG-waist-to-height ratio | TyG-WHtr | TyG index × WC/height | Zhang et al[82] |
| TyG-weight-adjusted-waist index | TyG-WWI | TyG index × WC/weight | Zhang et al[82] |
| Uric acid | UA | Plasma/urine concentrations | Chang et al[97], Fukuda et al[98] |
| Uric acid to creatinine ratio | UACR | UA/creatinine | Choi et al[99], Wang et al[100] |
| Visceral adiposity index | VAI | WC/(39.68 + 1.88 × BMI) × (TG/1.03) × (1.31/HDL) | Amato et al[101], Liu et al[102] |
| Visceral fat | VF | Measured by bioelectrical impedance/magnetic resonance imaging | Lee et al[103] |
| Waist circumference | WC | Measuring tape | Pustjens et al[104] |
| Waist-to-height ratio | WHtr | WC/height | Sheng et al[105] |
| Waist to hip ratio | WHr | WC/hip circumference as measured by measuring tape | Song et al[106] |
| Weight-adjusted-waist index | WWI | WC/weight | Park et al[107], Lian et al[108] |
| Zhejiang university index | ZJU | BMI + fasting plasma glucose + TG + 3 × ALT/AST ratio (+ 2, if woman) | Wang et al[109], Ma et al[110] |
The TyG-WC index has been widely recognized as a superior diagnostic biomarker for NAFLD, MAFLD, and MASLD across different populations. In Japan, Sheng et al[111] demonstrated its diagnostic strength with an area under the receiver operating characteristic (AUROC) curve of 0.88, a finding consistent with studies from South Korea, where Han et al[112] (AUROC = 0.94), Song et al[113] (AUROC = 0.83), and Kim et al[114] (AUROC = 0.86) reported similar accuracy. Research from Iran (Khamseh et al[81], AUROC = 0.69, and Forouzesh et al[115], AUROC = 0.77), Turkey (Demirci and Sezer[116], AUROC = 0.82) and China (Yu et al[117], AUROC = 0.87, and Xue et al[118], AUROC = 0.83) further supports its diagnostic utility. In the United States, Li et al[119] reported an AUROC of 0.80 in non-obese NAFLD individuals, whereas Wu et al[120] highlighted its broad applicability in the general population. Mexican studies by Mijangos-Trejo et al[80] and Priego-Parra et al[121] found AUROCs of 0.81 and 0.84, respectively, for detecting liver steatosis and MASLD.
He et al[122] and Zhang et al[94] identified non-linear associations between TyG-WC and MASLD, which suggests that minor fluctuations in TyG-WC values might not uniformly influence the prevalence or severity of MASLD across its entire spectrum. Instead, the findings imply that genetic predisposition, epigenetics, lifestyle factors, and comorbid conditions could serve as more critical determinants of disease progression or clinical manifestations. Yang et al[123] recently described the TyG-WC as the best indicator for MASLD screening in middle-aged and elderly Americans. These collective findings support the strong capacity of TyG-WC for MASLD detection.
Critically, the TyG-WC has shown associations with all-cause mortality, cardiovascular mortality, diabetes-related mortality[124], and the prediction of major adverse cardiovascular events in MASLD patients with heart failure[125]. Therefore, its values could be useful not only for diagnosing MASLD but also for identifying individuals at higher cardiovascular risk, potentially influencing staging and long-term prognosis.
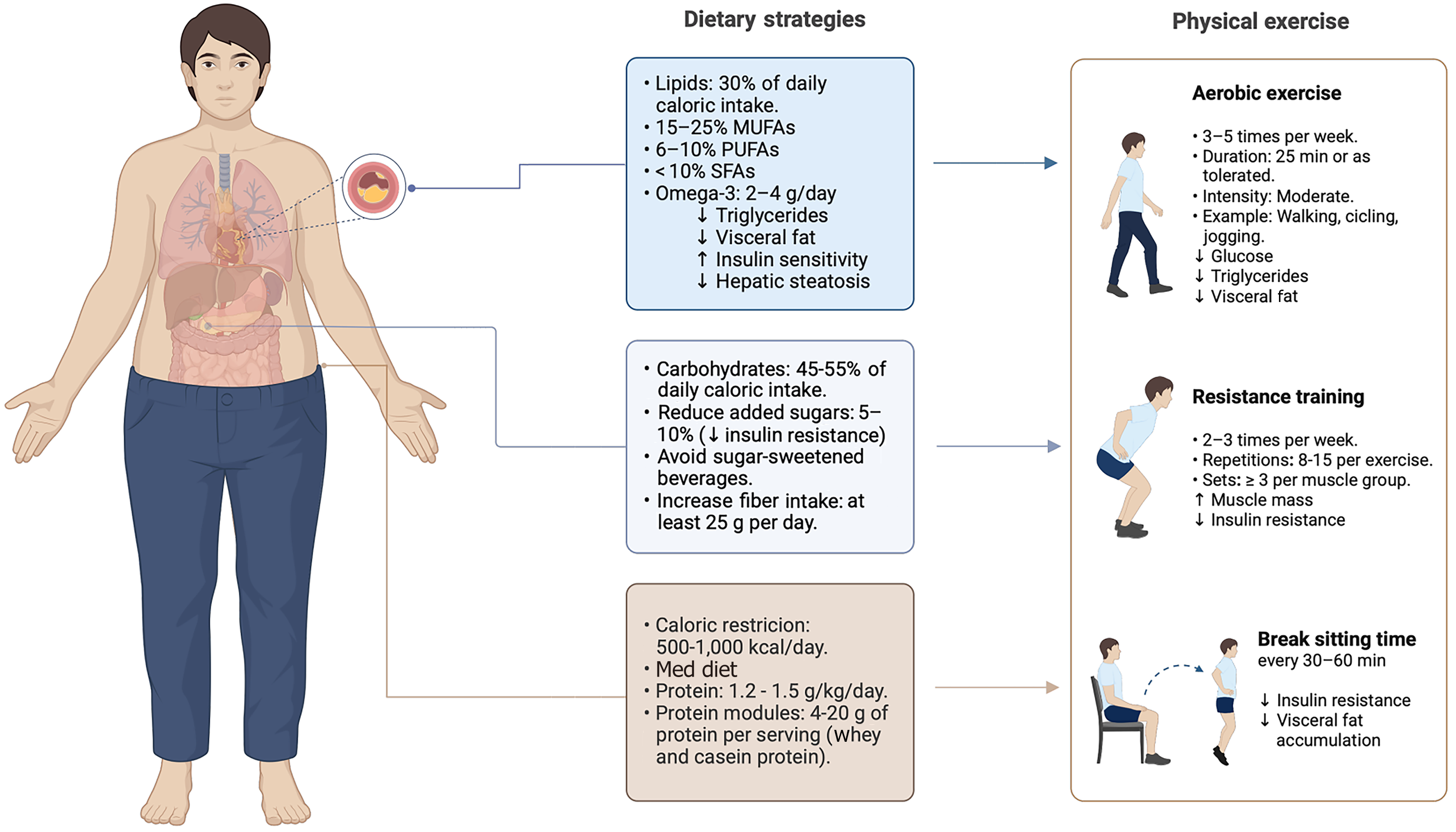
Nutritional therapy is the first-line treatment for MASLD. Caloric restriction between 500–1000 kcal/day is widely recognized as a primary approach for achieving weight loss of 5%-10% of total body weight and reducing fat mass, both of which significantly improve hepatic steatosis and fibrosis[126]. Moreover, the Mediterranean diet remains the most well-established dietary pattern for MASLD management, as it has consistently demonstrated beneficial effects on key disease-related outcomes[127,128].
While these general dietary patterns provide a solid foundation for improving metabolic health, a more targeted approach is needed to directly modulate the TyG-WC components and further optimize disease management. Although no clinical trials have yet evaluated diet-induced changes in TyG-WC as a primary outcome, the strong pathoph
Carbohydrate quality directly influences TyG-WC modulation. Reducing the intake of refined carbohydrates from added sugars, refined grains, and fructose-rich foods is essential for improving triglyceride and glucose metabolism. Although carbohydrates should constitute 45%–55% of total energy intake, added sugars should be limited to 5%–10%[128]. In particular, liquid sugars, such as soft drinks, fruit juices, syrups, and sweetened yogurts, have been associated with higher glucose and triglyceride levels, increased WC, and metabolic dysfunction. Their rapid intestinal absorption facilitates hepatic fructose transport and fat storage. Moreover, they induce lower satiety than solid sugar sources, promoting excessive energy intake and impairing metabolic regulation[128]. These effects contribute to elevations in both fasting glucose and triglyceride levels, directly impacting two components of the TyG-WC.
Not all carbohydrate sources have the same metabolic impact. Carbohydrates from fruits, legumes, and whole grains are associated with reductions in WC and lower cardiometabolic risk, likely due to their higher fiber content, which slows glucose absorption and modulates metabolic responses. To better assess carbohydrate quality, a ≥ 1 g fiber per 10 g carbohydrate ratio has been recommended[129]. Despite the general recommendation of consuming at least 25 g of fiber per day, meeting this target is often challenging in clinical practice. To bridge this gap, fiber supplementation of 5–10 g/day may be beneficial. Soluble fiber, in particular, has been strongly associated with lower circulating lipid levels[130] with effects on cognition.
Protein plays a crucial role in MASLD, contributing 20%–25% of total energy expenditure or 1.2–1.5 g/kg/day, with higher intakes (1.4–1.5 g/kg/day) recommended for individuals following a hypocaloric diet or presenting with abdominal obesity[126,131]. High-protein diets (30%–35% of total energy intake) have shown benefits in glycemic and lipid control, particularly triglyceride levels. While some evidence suggests a potential reduction in WC, findings remain unclear[131]. To meet protein requirements and support metabolic outcomes, a balanced combination of plant-based and animal-based proteins is advised.
Conversely, diets providing < 1 g/kg/day are not recommended, as they have been linked to greater insulin resistance, MASLD progression, sarcopenic obesity, and an increased risk of cardiovascular disease and all-cause mortality[132]. A balanced intake of both plant-based and animal-based proteins is necessary. Plant proteins contribute vitamins, minerals, and fiber, whereas animal proteins provide all essential amino acids. When dietary intake is insufficient, protein modules can be considered as supplementation; however, formulas designed for body mass gain are not recommended in weight-loss strategies. In this regard, whey protein and casein exert greater effects on satiety than other protein sources, potentially supporting weight loss by enhancing appetite regulation and reducing overall energy intake[131].
Once carbohydrate and protein requirements are determined, the remaining total energy intake is covered by lipids, contributing approximately 30% of total energy intake, while saturated fat intake should not exceed 7%–10%[133]. This recommendation can be achieved by using cooking methods, such as boiling, roasting, steaming, or cooking with spray oil, while avoiding fried and ultra-processed foods containing more than 17 g of fat per 100 g. Limiting saturated fat intake and favoring unsaturated fat sources may improve triglyceride levels and reduce VF accumulation, thus supporting favorable changes in TyG-WC index parameters.
Monounsaturated fatty acids, found in olive oil and avocado, should contribute between 10%-20% of total energy expenditure, while polyunsaturated fatty acids (PUFAs), including omega-3, account for 6%-10%. Among PUFAs, omega-3 has shown beneficial effects on MASLD, as a daily intake of 2–4 g improves transaminases, hepatic steatosis, and hypertriglyceridemia, and reduces VF. However, exceeding 3 g/day may cause gastrointestinal discomfort and a perceived fishy taste.
Omega-3 supplements contain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). EPA has anti-inflammatory effects and improves cardiovascular function, while DHA is essential for the structure and function of cell membranes, particularly in the brain and liver. The EPA/DHA ratio varies among supplements, so dosage should consider individual tolerance, especially in MASLD patients treated with glucagon-like peptide-1, thyroid hormone receptor beta receptor agonists, or fibroblast growth factor analogs, who often experience gastrointestinal symptoms[134]. The proposed nutritional strategies, by targeting the individual components of the TyG-WC, may collectively contribute to its improvement.
Beyond dietary modifications, physical exercise plays a key role in optimizing TyG-WC components and other surrogate markers[135], by improving insulin sensitivity, enhancing fatty acid utilization, reducing triglyceride levels, and promoting VF loss. Both aerobic and resistance training provide metabolic benefits, with evidence suggesting that their combination offers the greatest impact[136–138].
Aerobic exercise is recommended 3–5 times per week at moderate intensity, including activities such as swimming, walking, and running, as it enhances glucose metabolism and promote VF reduction[139]. Resistance training is crucial for individuals with altered TyG-WC parameters, given that skeletal muscle plays a key role in glucose disposal and lipid oxidation. Low muscle mass is linked to higher insulin resistance and triglyceride levels, worsening TyG-WC index scores[140]. To mitigate these effects, resistance training should be performed 2–3 times per week, with at least three sets of 12 repetitions targeting all major muscle groups, improving insulin sensitivity and metabolic function.
For individuals with low physical conditioning, walking is a safe and effective option, starting with 25 minutes, or as much as tolerated, progressively increasing duration and intensity to maximize its impact on TyG-WC parameters. In cases where mobility is limited, stationary cycling or seated movements may serve as viable alternatives.
Prolonged sedentary behavior negatively influences TyG-WC components by promoting insulin resistance and VF accumulation, both of which contribute to increased fasting glucose and WC. Although reducing sitting time alone has shown modest effects on insulin sensitivity, slight improvements in fasting insulin have been reported[141]. Encouraging regular movement breaks, such as standing up or walking every 30-60 minutes for 5 minutes[142,143] and incorporating daily activities like climbing stairs or performing light upper-body exercises can help improve metabolic health.
Hence, physical activity complements dietary strategies by targeting all three components of the TyG-WC index simultaneously. Figure 1 summarizes these key recommendations, highlighting the role of macronutrient distribution, fiber intake, and structured physical activity in improving triglyceride levels, glucose metabolism, and WC/VF.
TyG-WC index relevance extends beyond classic metabolic disorders, being associated with conditions, such as hyperuricemia[144], arthritis[145], periodontitis[146], osteoporosis[147], gallstone risk[148], renal lithiasis[149], psoriasis[150], lower extremity peripheral artery disease[151], serum creatinine levels and glomerular filtration rate[152], de
It is crucial to recognize that the cut-off points and diagnostic accuracy of the TyG-WC index differ significantly between sexes[121]. A significant limitation of the current literature is the absence of longitudinal studies, which are crucial for establishing causal relationships and assessing the temporal validity of the TyG-WC as a predictive tool. The predominance of cross-sectional studies restricts the ability to evaluate the index’s predictive accuracy over time. Furthermore, heterogeneity in study designs—including variations in measurement techniques and the lack of standardized pro
A notable concern is the observed variability in diagnostic performance of the TyG-WC index across diverse populations. For instance, the reported AUROC score of 0.69 in a cohort from Iran suggests suboptimal performance in that population. Such a low AUROC may indicate reduced diagnostic accuracy, which could be attributed to several factors, including genetic differences, regional lifestyle variations, dietary habits, and environmental influences. This variability underscores the critical need for population-specific validation and the establishment of tailored cut-off values to improve the diagnostic accuracy of the TyG-WC index in different demographic and clinical settings.
To address these limitations, large-scale, multi-ethnic, longitudinal studies are essential to establish robust, population-specific thresholds and validate the clinical utility of the TyG and TyG-WC indices. Additionally, the impact of confounding factors—such as genetic predispositions, age, hormonal status (particularly in women), comorbid con
Given that triglyceride levels, fasting glucose, and WC are markers of underlying metabolic dysfunction and inflammatory processes, the TyG-WC index represents a promising, low-cost tool for identifying individuals at risk for MASLD. However, its clinical integration is contingent on further rigorous validation and refinement across diverse populations. This article provides an opinion-based review of the current evidence and should not be interpreted as a clinical guideline or definitive recommendation. High-quality, large-scale studies are needed to establish the TyG-WC index as a reliable tool for routine clinical practice.
The TyG-WC index holds considerable promise as a non-invasive, cost-effective marker for identifying individuals at risk of MASLD, particularly when integrated with advanced analytical approaches, such as multiomics and artificial intelligence. However, substantial inter-population variability and methodological limitations underscore the need for rigorous, longitudinal validation. Establishing standardized, population-specific cut-offs and addressing confounding variables will be critical to unlocking its full clinical potential. Current evidence is encouraging but insufficient to support widespread clinical adoption. Future research should prioritize large-scale, multi-ethnic studies and harmonized protocols to enhance comparability and reliability. Until such data are available, the TyG-WC index should be regarded as a complementary tool within a broader diagnostic framework, supporting but not substituting established approaches in the effort to improve MASLD detection and management.
| 1. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2211] [Article Influence: 442.2] [Reference Citation Analysis (1)] |
| 2. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1563] [Cited by in RCA: 1320] [Article Influence: 660.0] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1444] [Article Influence: 722.0] [Reference Citation Analysis (2)] |
| 4. | Kan C, Zhang K, Wang Y, Zhang X, Liu C, Ma Y, Hou N, Huang N, Han F, Sun X. Global burden and future trends of metabolic dysfunction-associated Steatotic liver disease: 1990-2021 to 2045. Ann Hepatol. 2025;30:101898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Alqahtani SA, Alswat K, Yilmaz Y, Keklikkiran C, Funuyet-Salas J, Romero-Gómez M, Fan JG, Zheng MH, El-Kassas M, Castera L, Liu CJ, Wai-Sun Wong V, Zelber-Sagi S, Allen AM, Lam B, Treeprasertsuk S, Hameed S, Takahashi H, Kawaguchi T, Schattenberg JM, Duseja A, Newsome PN, Francque S, Spearman CW, Castellanos Fernández MI, Burra P, Roberts SK, Chan WK, Arrese M, Silva M, Rinella M, Singal AK, Gordon S, Fuchs M, Alkhouri N, Cusi K, Loomba R, Ranagan J, Eskridge W, Kautz A, Ong JP, Kugelmas M, Eguchi Y, Diago M, Yu ML, Gerber L, Fornaresio L, Nader F, Henry L, Racila A, Golabi P, Stepanova M, Carrieri P, Lazarus JV; Global NASH Council. Global survey of stigma among physicians and patients with nonalcoholic fatty liver disease. J Hepatol. 2024;80:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 6. | Younossi ZM, AlQahtani SA, Funuyet-Salas J, Romero-Gómez M, Yilmaz Y, Keklikkiran C, Alswat K, Yu ML, Liu CJ, Fan JG, Zheng MH, Burra P, Francque SM, Castera L, Schattenberg JM, Newsome PN, Allen AM, El-Kassas M, Treeprasertsuk S, Hameed S, Wai-Sun Wong V, Zelber-Sagi S, Takahashi H, Kawaguchi T, Castellanos Fernández MI, Duseja A, Arrese M, Rinella M, Singal AK, Gordon SC, Fuchs M, Eskridge W, Alkhouri N, Cusi K, Loomba R, Ranagan J, Kautz A, Ong JP, Kugelmas M, Eguchi Y, Diago M, Gerber L, Lam B, Fornaresio L, Nader F, Spearman CW, Roberts SK, Chan WK, Silva M, Racila A, Golabi P, Ananchuensook P, Henry L, Stepanova M, Carrieri P, Lazarus JV; Global NASH Council. The impact of stigma on quality of life and liver disease burden among patients with nonalcoholic fatty liver disease. JHEP Rep. 2024;6:101066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 7. | Cherubini A, Della Torre S, Pelusi S, Valenti L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol Med. 2024;30:1126-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 8. | Luukkonen PK. Subtypes of MASLD confer distinct clinical trajectories. J Hepatol. 2025;82:1138-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Ryu G, Yoon EL, Kim W, Jun DW. Molecular Clustering of Metabolic Dysfunction-Associated Steatotic Liver Disease Based on Transcriptome Analysis. Diagnostics (Basel). 2025;15:342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Chowdhury AB, Mehta KJ. Liver biopsy for assessment of chronic liver diseases: a synopsis. Clin Exp Med. 2023;23:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 11. | Song R, Li Z, Zhang Y, Tan J, Chen Z. Comparison of NAFLD, MAFLD and MASLD characteristics and mortality outcomes in United States adults. Liver Int. 2024;44:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 12. | Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80:e54-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 218] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 13. | Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. 2024;80:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 14. | Ratziu V, Boursier J; AFEF Group for the Study of Liver Fibrosis. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. J Hepatol. 2024;80:e51-e52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 62] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 15. | Pan Z, Eslam M. MAFLD or MASLD: Let the evidence decide again. Ann Hepatol. 2024;29:101521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Ramírez-Mejía MM, Jiménez-Gutiérrez C, Eslam M, George J, Méndez-Sánchez N. Breaking new ground: MASLD vs. MAFLD-which holds the key for risk stratification? Hepatol Int. 2024;18:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 17. | Pan Z, Shiha G, Esmat G, Méndez-Sánchez N, Eslam M. MAFLD predicts cardiovascular disease risk better than MASLD. Liver Int. 2024;44:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 18. | Pan Z, Derbala M, AlNaamani K, Ghazinian H, Fan JG, Eslam M. MAFLD criteria are better than MASLD criteria at predicting the risk of chronic kidney disease. Ann Hepatol. 2024;29:101512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Reference Citation Analysis (0)] |
| 19. | Fouad Y, Sanai F, Alboraie M, Zheng MH. What the New Definition of MASLD Left Behind: Dual Etiology With Viral Hepatitis. Clin Gastroenterol Hepatol. 2024;22:1751-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Iruzubieta P, Jimenez-gonzalez C, Cabezas J, Crespo J. From NAFLD to MASLD: transforming steatotic liver disease diagnosis and management. Metab Target Organ Damage. 2025;5. [DOI] [Full Text] |
| 21. | Zhou XD, Lonardo A, Pan CQ, Shapiro MD, Zheng MH; WMU MAFLD Clinical Research Working Group. Clinical features and long-term outcomes of patients diagnosed with MASLD, MAFLD, or both. J Hepatol. 2024;81:e157-e159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |
| 22. | Alboraie M, Fouad Y, Eslam M. Letter to the Editor: MAFLD versus MASLD-Which is more appropriate from a global perspective? Hepatology. 2024;80:E42-E43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 23. | Elsabaawy M, Naguib M, Abuamer A, Shaban A. Comparative application of MAFLD and MASLD diagnostic criteria on NAFLD patients: insights from a single-center cohort. Clin Exp Med. 2025;25:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Wang CC, Cheng YM, Kao JH. Letter to the Editor: Statement of steatotic liver disease-A great leap toward the global standardization. Hepatology. 2024;79:E7-E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Gambardella ML, Abenavoli L. Metabolic Dysfunction-Associated Steatotic Liver Disease vs. Metabolic Dysfunction-Associated Fatty Liver Disease: Which Option is the Better Choice? Br J Hosp Med (Lond). 2025;86:1-5. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Piras IS, DiStefano JK. Comprehensive meta-analysis reveals distinct gene expression signatures of MASLD progression. Life Sci Alliance. 2024;7:e202302517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Li Y, Li L, Zhang Y, Lu J, Tang X, Bi C, Qu Y, Chai J. Clinical and pathological characteristics of metabolic dysfunction-associated steatotic liver disease and the key role of epigenetic regulation: implications for molecular mechanism and treatment. Ther Adv Endocrinol Metab. 2025;16:20420188251321602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Schütz F, Longo L, Keingeski MB, Filippi-Chiela E, Uribe-Cruz C, Álvares-da-Silva MR. Lipophagy and epigenetic alterations are related to metabolic dysfunction-associated steatotic liver disease progression in an experimental model. World J Hepatol. 2024;16:1468-1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Duan H, Gong M, Yuan G, Wang Z. Sex Hormone: A Potential Target at Treating Female Metabolic Dysfunction-Associated Steatotic Liver Disease? J Clin Exp Hepatol. 2025;15:102459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Haag M, Winter S, Kemas AM, Tevini J, Feldman A, Eder SK, Felder TK, Datz C, Paulweber B, Liebisch G, Burk O, Lauschke VM, Aigner E, Schwab M. Circulating metabolite signatures indicate differential gut-liver crosstalk in lean and obese MASLD. JCI Insight. 2025;10:e180943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Nychas E, Marfil-Sánchez A, Chen X, Mirhakkak M, Li H, Jia W, Xu A, Nielsen HB, Nieuwdorp M, Loomba R, Ni Y, Panagiotou G. Discovery of robust and highly specific microbiome signatures of non-alcoholic fatty liver disease. Microbiome. 2025;13:10. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Pirola CJ, Landa MS, Schuman M, García SI, Salatino A, Sookoian S. Metabolic dysfunction-associated steatotic liver disease exhibits sex-specific microbial heterogeneity within intestinal compartments. Clin Mol Hepatol. 2025;31:179-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Abu-Rumaileh M, Dhoop S, Pace J, Qapaja T, Martinez ME, Tincopa M, Loomba R. Social Determinants of Health Associated With Metabolic Dysfunction-Associated Steatotic Liver Disease Prevalence and Severity: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 34. | Faulkner CS, Aboona MB, Surendra L, Rangan P, Ng CH, Huang DQ, Muthiah M, Kim D, Fallon MB, Noureddin M, Chen VL, Kardashian A, Wijarnpreecha K. Neighborhood Social Determinants of Health are Associated With Metabolic Dysfunction-associated Steatotic Liver Disease Outcomes. Clin Gastroenterol Hepatol. 2024;S1542-3565(24)01068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 35. | Arvanitakis K, Chatzikalil E, Kalopitas G, Patoulias D, Popovic DS, Metallidis S, Kotsa K, Germanidis G, Koufakis T. Metabolic Dysfunction-Associated Steatotic Liver Disease and Polycystic Ovary Syndrome: A Complex Interplay. J Clin Med. 2024;13:4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 36. | Chen H, Simons PIHG, Brouwers MCGJ. Is cardiovascular disease in PCOS driven by MASLD? Trends Endocrinol Metab. 2025;36:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Liu S, Li F, Cai Y, Sun L, Ren L, Yin M, Cui H, Pan Y, Gang X, Wang G. Gout drives metabolic dysfunction-associated steatotic liver disease through gut microbiota and inflammatory mediators. Sci Rep. 2025;15:9395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Mantovani A, Csermely A, Bilson J, Borella N, Enrico S, Pecoraro B, Shtembari E, Morandin R, Polyzos SA, Valenti L, Tilg H, Byrne CD, Targher G. Association between primary hypothyroidism and metabolic dysfunction-associated steatotic liver disease: an updated meta-analysis. Gut. 2024;73:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Reytor-González C, Annunziata G, Campuzano-Donoso M, Morales-López T, Basantes-Tituaña C, Fascì-Spurio F, Verde L, Muscogiuri G, Barrea L, Frias-Toral E, Simancas-Racines D. Endocrinologist's crucial role in metabolic dysfunction-associated steatotic liver disease: a comprehensive review. Minerva Endocrinol (Torino). 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Trépo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. J Hepatol. 2020;72:1196-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 274] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 41. | Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 691] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 42. | Sookoian S, Rotman Y, Valenti L. Genetics of Metabolic Dysfunction-associated Steatotic Liver Disease: The State of the Art Update. Clin Gastroenterol Hepatol. 2024;22:2177-2187.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 43. | Lee YH, Lee J, Jeong J, Park K, Baik B, Kwon Y, Kim K, Khim KW, Ji H, Lee JY, Kim K, Kim JW, Dao T, Kim M, Lee TY, Yang YR, Yoon H, Ryu D, Hwang S, Lee H, Nam D, Kim WK, Park NH, Yun H, Choi JH. Hepatic miR-93 promotes the pathogenesis of metabolic dysfunction-associated steatotic liver disease by suppressing SIRT1. Metabolism. 2025;169:156266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Antoniou E, Oikonomou E, Theofilis P, Lambadiari V, Kassi E, Chasikidis C, Zisimos K, Andreou K, Kalogera V, Katsarou O, Foti E, Kleopa E, Striki A, Siasos G. MicroRNAs in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Systematic Review of Clinical Studies. Microrna. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Hu M, Huang H, Jia M, Xu M, Chen M, Wu J, Gu S, Liang H, Zhou H, Gong Y. A panel of miRNAs in the serum extracellular vesicles serve as novel diagnostic biomarkers for MASLD. Biomed J. 2025;100838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Zhang X, Chen T, Li Z, Wan L, Zhou Z, Xu Y, Yan D, Zhao W, Chen H. NORAD exacerbates metabolic dysfunction-associated steatotic liver disease development via the miR-511-3p/Rock2 axis and inhibits ubiquitin-mediated degradation of ROCK2. Metabolism. 2025;164:156111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 47. | Ran S, Zhang J, Tian F, Qian ZM, Wei S, Wang Y, Chen G, Zhang J, Arnold LD, McMillin SE, Lin H. Association of metabolic signatures of air pollution with MASLD: Observational and Mendelian randomization study. J Hepatol. 2025;82:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Reference Citation Analysis (0)] |
| 48. | An G, Song J, Ying W, Lim W. Overview of the hazardous impacts of metabolism-disrupting chemicals on the progression of fatty liver diseases. Mol Cell Toxicol. 2025;21:387-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 49. | Jarmakiewicz-Czaja S, Sokal-Dembowska A, Filip R. Effects of Selected Food Additives on the Gut Microbiome and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Medicina (Kaunas). 2025;61:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Corrao S, Calvo L, Granà W, Scibetta S, Mirarchi L, Amodeo S, Falcone F, Argano C. Metabolic dysfunction-associated steatotic liver disease: A pathophysiology and clinical framework to face the present and the future. Nutr Metab Cardiovasc Dis. 2025;35:103702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Seidemann L, Lippold CP, Rohm CM, Eckel JC, Schicht G, Matz-Soja M, Berg T, Seehofer D, Damm G. Sex hormones differently regulate lipid metabolism genes in primary human hepatocytes. BMC Endocr Disord. 2024;24:135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 52. | De la Cruz-Color L, Dominguez-Rosales JA, Maldonado-González M, Ruíz-Madrigal B, Sánchez Muñoz MP, Zaragoza-Guerra VA, Espinoza-Padilla VH, Ruelas-Cinco EDC, Ramírez-Meza SM, Torres Baranda JR, González-Gutiérrez MDR, Hernandez Nazara ZH. Evidence That Peripheral Leptin Resistance in Omental Adipose Tissue and Liver Correlates with MASLD in Humans. Int J Mol Sci. 2024;25:6420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | Shah A, Davarci O, Chaftari P, Avenatti E. Obesity as a Disease: A Primer on Clinical and Physiological Insights. Methodist Debakey Cardiovasc J. 2025;21:4-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Kuchay MS, Isaacs S, Misra A. Intrahepatic hypothyroidism in MASLD: Role of liver-specific thyromimetics including resmetirom. Diabetes Metab Syndr. 2024;18:103034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 55. | Li T, Fu C, Tang Z, Li C, Hua D, Liu B, Tao Z, Yang J, Zhang L, Cheng T, Wang S, Ning G, Gu Y. Disentangling Organ-Specific Roles of Farnesoid X Receptor in Bile Acid and Glucolipid Metabolism. Liver Int. 2025;45:e70027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Liu Z, Gao H, Yan W, Li S, Jiang W, Wang Y, Jiang Y, You C. Clinical application of bile acid profile combined with lipid indices in metabolic dysfunction-associated steatotic liver disease. Clin Chim Acta. 2025;570:120217. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 57. | Mino M, Kakazu E, Sano A, Tsuruoka M, Matsubara H, Kakisaka K, Kogure T, Sekine K, Aoki Y, Imamura M, Matsuda M, Yamazoe T, Mori T, Yoshio S, Inoue J, Masamune A, Kanto T. Comprehensive analysis of peripheral blood free amino acids in MASLD: the impact of glycine-serine-threonine metabolism. Amino Acids. 2024;57:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Svobodová G, Horní M, Velecká E, Boušová I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: a review. Arch Toxicol. 2025;99:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 59. | Kim HY, Rosenthal SB, Liu X, Miciano C, Hou X, Miller M, Buchanan J, Poirion OB, Chilin-Fuentes D, Han C, Housseini M, Carvalho-Gontijo Weber R, Sakane S, Lee W, Zhao H, Diggle K, Preissl S, Glass CK, Ren B, Wang A, Brenner DA, Kisseleva T. Multi-modal analysis of human hepatic stellate cells identifies novel therapeutic targets for metabolic dysfunction-associated steatotic liver disease. J Hepatol. 2025;82:882-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Sanfeliu-Redondo D, Gibert-Ramos A, Gracia-Sancho J. Cell senescence in liver diseases: pathological mechanism and theranostic opportunity. Nat Rev Gastroenterol Hepatol. 2024;21:477-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 61. | Mischitelli M, Poggiogalle E, Tozzi G, Ferri F, Parisse S, Meloni B, Morrone A, Sabbadini A, Salem M, Gangitano E, De Santis A, d'Amati G, Gnessi L, Donini LM, Ginanni Corradini S. Reduced Intra- and Extracellular Circulating Postprandial Lysosomal Acid Lipase Activity in Patients with MASLD. Metabolites. 2024;14:725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | El-Sehrawy AAMA, Rashid TA, Ullah MI, Uthirapathy S, Ganesan S, Singh A, Devi A, Joshi KK, Jasim AS, Kadhim AJ. Cutting edge: ferroptosis in metabolic dysfunction-associated steatotic liver disease (MASLD) pathogenesis and therapy. Funct Integr Genomics. 2025;25:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Li Y, Yang P, Ye J, Xu Q, Wu J, Wang Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024;23:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 64. | Bo T, Gao L, Yao Z, Shao S, Wang X, Proud CG, Zhao J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024;36:947-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 65. | Kumar A, Arora A, Sharma P, Jan S, Ara I. Visceral Fat and Diabetes: Associations With Liver Fibrosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. J Clin Exp Hepatol. 2025;15:102378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 66. | Armandi A, Rosso C, Caviglia GP, Bugianesi E. An updated overview on hepatocellular carcinoma in patients with Metabolic dysfunction-Associated Steatotic Liver Disease: Trends, pathophysiology and risk-based surveillance. Metabolism. 2025;162:156080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 67. | Hardwick JP, Song BJ, Rote P, Leahy C, Lee YK, Wolf AR, Diegisser D, Garcia V. The CYP4/20-HETE/GPR75 axis in the progression metabolic dysfunction-associated steatosis liver disease (MASLD) to chronic liver disease. Front Physiol. 2024;15:1497297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 68. | Seidel F, Vreeken D, Custers E, Wiesmann M, Özsezen S, van Duyvenvoorde W, Caspers M, Menke A, Morrison MC, Verschuren L, Duering M, Hazebroek EJ, Kiliaan AJ, Kleemann R. Metabolic dysfunction-associated steatotic liver disease is associated with effects on cerebral perfusion and white matter integrity. Heliyon. 2024;10:e38516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Vataja E, Viitanen M, Rinne JO, Lehtisalo J, Erlund I, Ngandu T, Koskinen S, Åberg F, Jula A, Ekblad L. Metabolic dysfunction-associated steatotic liver disease as a predictor of cognitive performance: An 11-year population-based follow-up study. Dig Liver Dis. 2025;57:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Mikkelsen ACD, Kjærgaard K, Schapira AHV, Mookerjee RP, Thomsen KL. The liver-brain axis in metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol Hepatol. 2025;10:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 71. | Jin Y, Tang R, Wu L, Xu K, Chen X, Zhu Y, Shi J, Li J. Cognitive Impairment in MASLD is associated with Amygdala-Related Connectivity Dysfunction in the Prefrontal and Sensory Cortex. J Integr Neurosci. 2024;23:215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Priego-Parra BA, Gallego-Durán R, Román-Calleja BM, Velasco Velarde-Ruiz JA, Romero-Gómez M, Gracia-Sancho J. Advancing precision medicine in metabolic dysfunction-associated steatotic liver disease. Trends Endocrinol Metab. 2025;S1043-2760(25)00052. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Abdelhameed F, Kite C, Lagojda L, Dallaway A, Chatha KK, Chaggar SS, Dalamaga M, Kassi E, Kyrou I, Randeva HS. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review From NAFLD to MAFLD and MASLD. Curr Obes Rep. 2024;13:510-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 48] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 74. | Kouvari M, Chrysohoou C, Damigou E, Barkas F, Kravvariti E, Liberopoulos E, Tsioufis C, Sfikakis PP, Pitsavos C, Panagiotakos D, Mantzoros CS; ATTICA Study group. Non-invasive tools for liver steatosis and steatohepatitis predict incidence of diabetes, cardiovascular disease and mortality 20 years later: The ATTICA cohort study (2002-2022). Clin Nutr. 2024;43:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 75. | Tobaruela-Resola AL, Milagro FI, Elorz M, Benito-Boillos A, Herrero JI, Mogna-Peláez P, Tur JA, Martínez JA, Abete I, Zulet MÁ. Circulating miR-122-5p, miR-151a-3p, miR-126-5p and miR-21-5p as potential predictive biomarkers for Metabolic Dysfunction-Associated Steatotic Liver Disease assessment. J Physiol Biochem. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 76. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2039] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 77. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1067] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 78. | Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y, Munteanu M, Mercadier A, Manns M, Albrecht J. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 79. | Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L, Orho-Melander M, Yki-Järvinen H. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 601] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 80. | Mijangos-Trejo A, Gómez-Mendoza R, Ramos-Ostos MH, Castro-Narro G, Uribe M, Juárez-Hernández E, López-Méndez I. Diagnostic Accuracy of the Triglyceride-Glucose Index (TyG), TyG Body Mass Index, and TyG Waist Circumference Index for Liver Steatosis Detection. Diagnostics (Basel). 2024;14:762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 81. | Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. Triglyceride Glucose Index and Related Parameters (Triglyceride Glucose-Body Mass Index and Triglyceride Glucose-Waist Circumference) Identify Nonalcoholic Fatty Liver and Liver Fibrosis in Individuals with Overweight/Obesity. Metab Syndr Relat Disord. 2021;19:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 82. | Zhang Y, Wu J, Li T, Qu Y, Wang Y. Association of triglyceride-glucose related indices with mortality among individuals with MASLD combined with prediabetes or diabetes. Cardiovasc Diabetol. 2025;24:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 83. | Ma X, Chang L, Li S, Gu Y, Wan J, Sang H, Ding L, Liu M, He Q. Genetic associations of birthweight, childhood, and adult BMI with metabolic dysfunction-associated steatotic liver disease: a Mendelian randomization. BMC Gastroenterol. 2024;24:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | McHenry S, Park Y, Browning JD, Sayuk G, Davidson NO. Dallas Steatosis Index Identifies Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2020;18:2073-2080.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 85. | McHenry S, Park Y, Davidson NO. Validation of the Dallas Steatosis Index to Predict Nonalcoholic Fatty Liver Disease in the UK Biobank Population. Clin Gastroenterol Hepatol. 2022;20:2638-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Gallego-Durán R, Ampuero J, Maya-Miles D, Pastor-Ramírez H, Montero-Vallejo R, Rivera-Esteban J, Álvarez-Amor L, Pareja MJ, Rico MC, Millán R, Robles-Frías MJ, Aller R, Rojas Á, Muñoz-Hernández R, Gil-Gómez A, Gato S, García-Lozano M, Arias-Loste MT, Abad J, Calleja JL, Andrade RJ, Crespo J, González-Rodríguez Á, García-Monzón C, Andreola F, Pericás JM, Jalan R, Martín-Bermudo F, Romero-Gómez M. Fibroblast growth factor 21 is a hepatokine involved in MASLD progression. United European Gastroenterol J. 2024;12:1056-1068. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Keskin M, Arsoy HA, Kara O, Sarandol E, Beyaz A, Koca N, Yilmaz Y. Metabolic and Hepatic Profiles of Non-Obese and Obese Metabolic Dysfunction-Associated Steatotic Liver Disease in Adolescents: The Role of FibroScan Parameters,Fibroblast Growth Factor-21, and Cytokeratin-18. Turk J Gastroenterol. 2025;36:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Long MT, Pedley A, Colantonio LD, Massaro JM, Hoffmann U, Muntner P, Fox CS. Development and Validation of the Framingham Steatosis Index to Identify Persons With Hepatic Steatosis. Clin Gastroenterol Hepatol. 2016;14:1172-1180.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 89. | Chung J, Park HS, Kim YJ, Yu MH, Park S, Jung SI. Association of Hepatic Steatosis Index with Nonalcoholic Fatty Liver Disease Diagnosed by Non-Enhanced CT in a Screening Population. Diagnostics (Basel). 2021;11:2168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24513] [Article Influence: 612.8] [Reference Citation Analysis (1)] |
| 91. | Suzuki K, Tsujiguchi H, Hara A, Takeshita Y, Goto H, Nakano Y, Yamamoto R, Takayama H, Tajima A, Yamashita T, Honda M, Nakamura H, Takamura T. Hepatokine leukocyte cell-derived chemotaxin 2 as a biomarker of insulin resistance, liver enzymes, and metabolic dysfunction-associated steatotic liver disease in the general population. J Diabetes Investig. 2025;16:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Ebrahimi M, Seyedi SA, Nabipoorashrafi SA, Rabizadeh S, Sarzaeim M, Yadegar A, Mohammadi F, Bahri RA, Pakravan P, Shafiekhani P, Nakhjavani M, Esteghamati A. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Lipids Health Dis. 2023;22:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 93. | Li H, Zhang Y, Luo H, Lin R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front Endocrinol (Lausanne). 2022;13:977625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 94. | Zhang X, Zheng MH, Liu D, Lin Y, Song SJ, Chu ES, Liu D, Singh S, Berman M, Lau HC, Gou H, Wong GL, Zhang N, Yuan HY, Loomba R, Wong VW, Yu J. A blood-based biomarker panel for non-invasive diagnosis of metabolic dysfunction-associated steatohepatitis. Cell Metab. 2025;37:59-68.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 95. | Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 1271] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 96. | Zou H, Xie J, Ma X, Xie Y. The Value of TyG-Related Indices in Evaluating MASLD and Significant Liver Fibrosis in MASLD. Can J Gastroenterol Hepatol. 2025;2025:5871321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | Chang Z, Liu Z. Serum uric acid as a biomarker for metabolic dysfunction-associated steatotic liver disease: insights from ultrasound elastography in a Chinese cohort. BMC Gastroenterol. 2025;25:94. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 98. | Fukuda T, Akihisa T, Okamoto T, Fukaishi T, Kawakami A, Tanaka M, Yamada T, Monzen K. Association of uric acid levels with the development of metabolic dysfunction-associated and metabolic and alcohol-related/associated steatotic liver disease: a study on Japanese participants undergoing health checkups. Endocr J. 2025;72:671-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 99. | Choi J, Joe H, Oh JE, Cho YJ, Shin HS, Heo NH. The correlation between NAFLD and serum uric acid to serum creatinine ratio. PLoS One. 2023;18:e0288666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 100. | Wang R, Xue F, Wang L, Shi G, Qian G, Yang N, Chen X. Serum uric acid to creatinine ratio is associated with higher prevalence of NAFLD detected by FibroScan in the United States. J Clin Lab Anal. 2022;36:e24590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 101. | Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A; AlkaMeSy Study Group. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 778] [Cited by in RCA: 1162] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 102. | Liu H, Deng M, Luo G, Chen J. Associations between Chinese Visceral Adiposity Index and the Risk of Metabolic Dysfunction-associated Steatotic Liver Disease and Liver Fibrosis: A Large Cross-sectional Study. Iran J Med Sci. 2025;50:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 103. | Lee YC, Lee YH, Chuang PN, Kuo CS, Lu CW, Yang KC. The utility of visceral fat level measured by bioelectrical impedance analysis in predicting metabolic syndrome. Obes Res Clin Pract. 2020;14:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Pustjens J, van Kleef LA, Janssen HLA, de Knegt RJ, Brouwer WP. Differential prevalence and prognostic value of metabolic syndrome components among patients with MASLD. JHEP Rep. 2024;6:101193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 105. | Sheng G, Xie Q, Wang R, Hu C, Zhong M, Zou Y. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 2021;21:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 106. | Song K, Seol EG, Yang H, Jeon S, Shin HJ, Chae HW, Kim EK, Kwon YJ, Lee JW. Bioelectrical impedance parameters add incremental value to waist-to-hip ratio for prediction of metabolic dysfunction associated steatotic liver disease in youth with overweight and obesity. Front Endocrinol (Lausanne). 2024;15:1385002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 107. | Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8:16753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 108. | Lian YE, Wang Y, Yang Y, Chen J. Weight-adjusted waist circumference index with hepatic steatosis and fibrosis in adult females: a cross-sectional, nationally representative study (NHANES 2017-2020). BMC Gastroenterol. 2025;25:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 109. | Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, Li Y. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep. 2015;5:16494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 110. | Ma X, Zou H, Zhan J, Gao J, Xie Y. Assessment of the clinical value of five noninvasive predictors of metabolic dysfunction-associated steatotic liver disease in Han Chinese adults. Eur J Gastroenterol Hepatol. 2024;36:1209-1219. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 111. | Sheng G, Lu S, Xie Q, Peng N, Kuang M, Zou Y. The usefulness of obesity and lipid-related indices to predict the presence of Non-alcoholic fatty liver disease. Lipids Health Dis. 2021;20:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 112. | Han AL, Lee HK, Shin SR. Diagnostic Performance of Insulin Resistance Indices for Identifying Metabolic Dysfunction-Associated Fatty Liver Disease. Metab Syndr Relat Disord. 2024;22:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 113. | Song S, Son DH, Baik SJ, Cho WJ, Lee YJ. Triglyceride Glucose-Waist Circumference (TyG-WC) Is a Reliable Marker to Predict Non-Alcoholic Fatty Liver Disease. Biomedicines. 2022;10:2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 114. | Kim AH, Son DH, Lee YJ. Modified triglyceride-glucose index indices are reliable markers for predicting risk of metabolic dysfunction-associated fatty liver disease: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1308265. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 115. | Forouzesh P, Kheirouri S, Alizadeh M. Predicting hepatic steatosis degree in metabolic dysfunction-associated steatotic liver disease using obesity and lipid-related indices. Sci Rep. 2025;15:8612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 116. | Demirci S, Sezer S. Fatty Liver Index vs. Biochemical-Anthropometric Indices: Diagnosing Metabolic Dysfunction-Associated Steatotic Liver Disease with Non-Invasive Tools. Diagnostics (Basel). 2025;15:565. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 117. | Yu R, Xie W, Peng H, Lu L, Yin S, Xu S, Hu Z, Peng XE. Diagnostic value of triglyceride-glucose index and related parameters in metabolism-associated fatty liver disease in a Chinese population: a cross-sectional study. BMJ Open. 2023;13:e075413. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 118. | Xue Y, Xu J, Li M, Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters. Front Endocrinol (Lausanne). 2022;13:951689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 119. | Li S, Feng L, Ding J, Zhou W, Yuan T, Mao J. Triglyceride glucose-waist circumference: the optimum index to screen nonalcoholic fatty liver disease in non-obese adults. BMC Gastroenterol. 2023;23:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 120. | Wu Z, Huang K, Bao S, Zhang X, Li J, Kong W, Shi Y, Xie Y. The association of triglyceride-glucose-waist circumference with metabolic associated fatty liver disease and the severity of liver steatosis and fibrosis in American adults: a population-based study. Scand J Gastroenterol. 2024;59:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 121. | Priego-Parra BA, Reyes-Diaz SA, Ordaz-Alvarez HR, Bernal-Reyes R, Icaza-Chávez ME, Martínez-Vázquez SE, Amieva-Balmori M, Vivanco-Cid H, Velasco JAV, Gracia-Sancho J, Remes-Troche JM. Diagnostic performance of sixteen biomarkers for MASLD: A study in a Mexican cohort. Clin Res Hepatol Gastroenterol. 2024;48:102400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Reference Citation Analysis (0)] |
| 122. | He X, Huang X, Qian Y, Sun T. A non-linear relationship between triglyceride glucose waist circumference and nonalcoholic fatty liver disease in a Japanese population: a secondary analysis. Front Endocrinol (Lausanne). 2023;14:1188214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 123. | Yang Y, Luo Y, Shi J, Yin Y, Du X, Guo J, Zhuang H. The triglyceride glucose-waist circumference is the best indicator for screening non-alcoholic fatty liver disease in middle-aged and elderly people. Nutr Hosp. 2025;43:476-484. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 124. | Chen Q, Hu P, Hou X, Sun Y, Jiao M, Peng L, Dai Z, Yin X, Liu R, Li Y, Zhu C. Association between triglyceride-glucose related indices and mortality among individuals with non-alcoholic fatty liver disease or metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. 2024;23:232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 125. | Xie Q, Liu C, Liu F, Zhang X, Zhang Z, An X, Yang Y, Li X. Predictive Effect of Alternative Insulin Resistance Indexes on Adverse Cardiovascular Events in Patients with Metabolic Syndrome with Heart Failure. Diabetes Metab Syndr Obes. 2024;17:2347-2356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 126. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 337] [Article Influence: 337.0] [Reference Citation Analysis (1)] |
| 127. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1168] [Article Influence: 584.0] [Reference Citation Analysis (1)] |
| 128. | Yan RR, Louie JCY. Sugar guidelines should be evidence-based and contain simple and easily actionable messages. Front Nutr. 2023;10:1227377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 129. | Campos V, Tappy L, Bally L, Sievenpiper JL, Lê KA. Importance of Carbohydrate Quality: What Does It Mean and How to Measure It? J Nutr. 2022;152:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 130. | Ghavami A, Ziaei R, Talebi S, Barghchi H, Nattagh-Eshtivani E, Moradi S, Rahbarinejad P, Mohammadi H, Ghasemi-Tehrani H, Marx W, Askari G. Soluble Fiber Supplementation and Serum Lipid Profile: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2023;14:465-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 131. | Ellinger S, Amini AM, Haardt J, Lehmann A, Schmidt A, Bischoff-Ferrari HA, Buyken AE, Kroke A, Kühn T, Louis S, Lorkowski S, Nimptsch K, Schulze MB, Schwingshackl L, Siener R, Stangl GI, Volkert D, Zittermann A, Watzl B, Egert S; German Nutrition Society. Protein intake and body weight, fat mass and waist circumference: an umbrella review of systematic reviews for the evidence-based guideline on protein intake of the German Nutrition Society. Eur J Nutr. 2024;63:3-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 132. | Charatcharoenwitthaya P, Tansakul E, Chaiyasoot K, Bandidniyamanon W, Charatcharoenwitthaya N. Dietary Composition and Its Association with Newly Diagnosed Nonalcoholic Fatty Liver Disease and Insulin Resistance. Nutrients. 2021;13:4438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 133. | Kargulewicz A, Stankowiak-Kulpa H, Grzymisławski M. Dietary recommendations for patients with nonalcoholic fatty liver disease. Prz Gastroenterol. 2014;9:18-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 134. | Huang X, Wu M, Huang B, Zhang Y. Gastrointestinal adverse events associated with GLP-1 receptor agonists in metabolic dysfunction-associated steatotic liver disease (MASLD): a systematic review and meta-analysis. Front Med (Lausanne). 2025;12:1509947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 135. | Cuthbertson DJ, Keating SE, Pugh CJA, Owen PJ, Kemp GJ, Umpleby M, Geyer NG, Chinchilli VM, Stine JG. Exercise improves surrogate measures of liver histological response in metabolic dysfunction-associated steatotic liver disease. Liver Int. 2024;44:2368-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 136. | Fu L, Zhang W, Ao Y, Zheng Z, Hu H. Efficacy of aerobic and resistance exercises in improving visceral adipose in patients with non-alcoholic fatty liver: a meta-analysis of randomized controlled trials. Z Gastroenterol. 2022;60:1644-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 926] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 138. | van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018;18:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 139. | Armstrong A, Jungbluth Rodriguez K, Sabag A, Mavros Y, Parker HM, Keating SE, Johnson NA. Effect of aerobic exercise on waist circumference in adults with overweight or obesity: A systematic review and meta-analysis. Obes Rev. 2022;23:e13446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 140. | Viswanath A, Fouda S, Fernandez CJ, Pappachan JM. Metabolic-associated fatty liver disease and sarcopenia: A double whammy. World J Hepatol. 2024;16:152-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 141. | Sjöros T, Laine S, Garthwaite T, Vähä-Ypyä H, Löyttyniemi E, Koivumäki M, Houttu N, Laitinen K, Kalliokoski KK, Sievänen H, Vasankari T, Knuuti J, Heinonen IHA. Reducing Sedentary Time and Whole-Body Insulin Sensitivity in Metabolic Syndrome: A 6-Month Randomized Controlled Trial. Med Sci Sports Exerc. 2023;55:342-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 142. | Whitaker KM, Pereira MA, Jacobs DR Jr, Sidney S, Odegaard AO. Sedentary Behavior, Physical Activity, and Abdominal Adipose Tissue Deposition. Med Sci Sports Exerc. 2017;49:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 143. | Duran AT, Friel CP, Serafini MA, Ensari I, Cheung YK, Diaz KM. Breaking Up Prolonged Sitting to Improve Cardiometabolic Risk: Dose-Response Analysis of a Randomized Crossover Trial. Med Sci Sports Exerc. 2023;55:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 144. | Gou R, Dou D, Tian M, Chang X, Zhao Y, Meng X, Li G. Association between triglyceride glucose index and hyperuricemia: a new evidence from China and the United States. Front Endocrinol (Lausanne). 2024;15:1403858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 145. | Zhang X, Tang H, Chen J, Chen J, Zhou H, Qi T, Wang D, Zeng H, Yu F. Association between different triglyceride-glucose index combinations with obesity indicators and arthritis: results from two nationally representative population-based study. Eur J Med Res. 2024;29:389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 146. | Lee HJ, Lee JW, Kim S, Kwon YJ. Comparison of the triglyceride glucose index and modified triglyceride glucose indices in assessing periodontitis in Korean adults. J Periodontal Res. 2023;58:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 147. | Tian N, Chen S, Han H, Jin J, Li Z. Association between triglyceride glucose index and total bone mineral density: a cross-sectional study from NHANES 2011-2018. Sci Rep. 2024;14:4208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 148. | Fu C, Li X, Wang Y, Chen J, Yang Y, Liu K. Association between triglyceride glucose index-related indices with gallstone disease among US adults. Lipids Health Dis. 2024;23:203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 149. | Yu H, Wu J. Associations of triglyceride glucose-related parameters with kidney stones: a cross-sectional study from NHANES 2007-2020. Transl Androl Urol. 2025;14:379-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 150. | Huang D, Ma R, Zhong X, Jiang Y, Lu J, Li Y, Shi Y. Positive association between different triglyceride glucose index-related indicators and psoriasis: evidence from NHANES. Front Immunol. 2023;14:1325557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 151. | Miao Y, Wang Y, Wang Y, Yan P, Chen Z, Wan Q. The Association Between Triglyceride-Glucose Index and Its Combination with Obesity Indicators and Lower Extremity Peripheral Artery Disease in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2024;17:2607-2617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 152. | Li L, Xu Z, Jiang L, Zhuang L, Huang J, Liu D, Wu Q. Triglyceride-Glucose Index and Its Correlates: Associations with Serum Creatinine and Estimated Glomerular Filtration Rate in a Cross-Sectional Study from CHARLS 2011-2015. Metab Syndr Relat Disord. 2024;22:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 153. | Liu X, Li J, He D, Zhang D, Liu X. Association between different triglyceride glucose index-related indicators and depression in premenopausal and postmenopausal women: NHANES, 2013-2016. J Affect Disord. 2024;360:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 154. | Zhang X, Wang Y, Yang X, Li Y, Gui J, Mei Y, Liu H, Guo LL, Li J, Lei Y, Li X, Sun L, Yang L, Yuan T, Wang C, Zhang D, Li J, Liu M, Hua Y, Zhang L. Obesity and lipid indices as predictors of depressive symptoms in middle-aged and elderly Chinese: insights from a nationwide cohort study. BMC Psychiatry. 2024;24:351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 155. | Yin H, Guo L, Zhu W, Li W, Zhou Y, Wei W, Liang M. Association of the triglyceride-glucose index and its related parameters with frailty. Lipids Health Dis. 2024;23:150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 156. | Wang C, Wang J, Li X, Zhou P, Zhao X, Xin A, Liao G, Huang Y, Zhang Y. Associations between triglyceride-glucose index combined with waist circumference and heart failure in individuals with different body mass indices: a cross-sectional study using NHANES 2011-2020 data. Lipids Health Dis. 2025;24:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 157. | Jia Y, He Z, Liu F, Li J, Liang F, Huang K, Chen J, Cao J, Li H, Shen C, Yu L, Liu X, Hu D, Huang J, Zhao Y, Liu Y, Lu X, Gu D, Chen S. Dietary intake changes the associations between long-term exposure to fine particulate matter and the surrogate indicators of insulin resistance. Environ Int. 2024;186:108626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |