Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.107620
Revised: April 28, 2025
Accepted: June 13, 2025
Published online: July 27, 2025
Processing time: 120 Days and 23.6 Hours
Current treatments for chronic hepatitis B (CHB) are lifelong, often accompanied by side effects and the risk of drug resistance, highlighting the urgent need for alternative therapies such as therapeutic vaccines. However, challenges such as selecting appropriate antigens and addressing multiple hepatitis B virus (HBV) genotypes hinder the development of these vaccines. One approach to over
Core Tip: Chronic hepatitis B (CHB) remains a significant public health concern worldwide where effective alternative therapies are still urgently needed. This review aims to explore the potential role of reverse vaccinology combined with immunoinformatics in advancing therapeutic vaccine design for CHB.
- Citation: Naully PG, Tan MI, El Khobar KE, Sukowati CHC, Giri-Rachman EA. Advancing therapeutic vaccines for chronic hepatitis B: Integrating reverse vaccinology and immunoinformatics. World J Hepatol 2025; 17(7): 107620
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/107620.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.107620
Chronic hepatitis B (CHB), the outcome of unresolved and persisted hepatitis B virus (HBV) infection, remains a significant public health concern due to its potential to cause cirrhosis, hepatocellular carcinoma (HCC), and hepatitis-related deaths[1,2]. As of 2024, an estimated 254 million people worldwide suffer from CHB, with a mortality rate higher than the previous year[1]. Hepatitis B vaccination for newborns prevents viral transmission from infected mothers to their children, however, the coverage of the recommended birth dose vaccine remains suboptimal and horizontal transmission still occurs in the community[3].
One contributing factor in the real time management of CHB is the limited effectiveness of current treatments. Current HBV treatment only resulted in partial cure, and not functional HBV cure which is defined as HBsAg loss and un
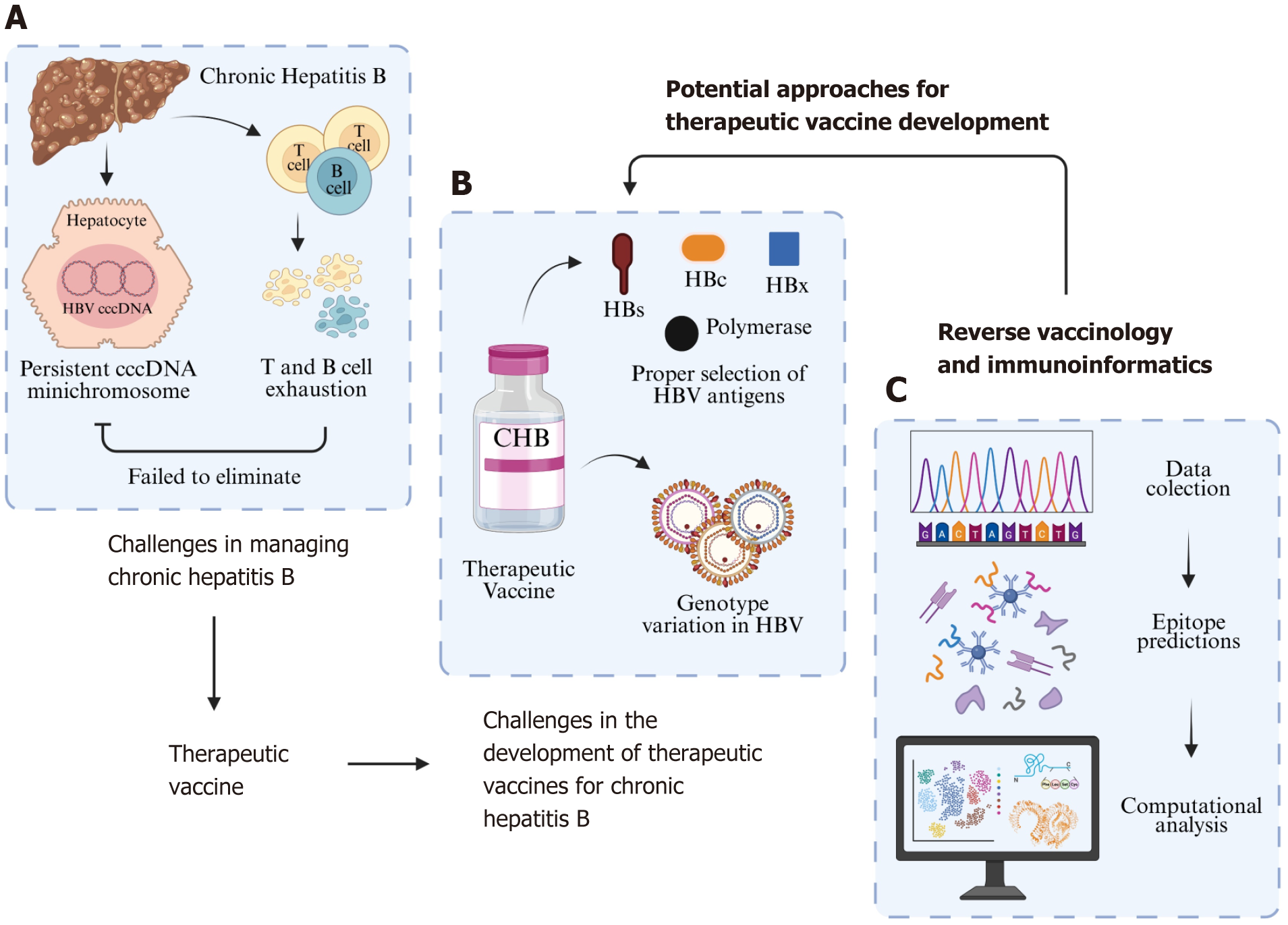
In CHB, there are complex issues involving the HBV covalently closed circular DNA (cccDNA) minichromosome and the host’s immune system (Figure 1)[8-11]. Upon infection, HBV genome is translocated to nucleus and converted from relaxed circular DNA (rcDNA) to cccDNA which acts as the transcription template for viral mRNAs and pregenomic RNAs[4,12,13]. This cccDNA interacts with both host histone proteins and HBV non-histone proteins, forming a mi
Ideally, the host’s immune response would be capable of eliminating this cccDNA minichromosome. However, HBV has developed several mechanisms to evade immune defenses[15,16]. Furthermore, excessive exposure to HBV antigens can result in exhaustion and dysfunction of the patient’s T and B cells[17-19]. NA drugs target the polymerase region to inhibit viral replication, resulting in reduce formation of both rcDNA and double-stranded linear DNA and subsequent viral DNA integration[20]. However, it has no effect on the rcDNA to cccDNA conversion, thus cannot completely suppress viral DNA synthesis and cccDNA replenishment[21]. On the other hand, interferon treatment may affect cccDNA in infected cells by decreasing its transcriptional activity by decreasing the acetylation of cccDNA-bound histones[4]. Combining existing NA therapy with new antiviral agents may resulted in suppression of HBV DNAs and RNAs and depletion of cccDNA pools, but if there is no HBsAg loss, stopping the therapy will lead to viral relapse[4,15].
In the early HBV infection phase (HBeAg positive), almost all (> 95%) HBsAg production is derived from cccDNA transcriptions, while in the late infection phase (HBeAg negative), the integrated HBV DNA produced more than half of the circulating HBsAg proteins, including the small HBsAg proteins and the subviral particles[2]. The circulating presence of large amounts of HBsAg affect the host immune response through (1) interference with neutralizing antibody as a decoy; (2) downregulation of antigen specific T-cell immunity; and (3) suppression of innate immunity by dysregulation of natural killer cell and dendritic cell functions, possibly resulting in exhaustion of the immune response[2,4].
To overcome the challenges of CHB, a treatment that is safe, can be administered over a short period, and enhances the immune system's ability to target HBV—particularly the cccDNA minichromosome—is needed. One potential solution is a therapeutic vaccine. Despite therapeutic vaccines for CHB being in development for roughly the past two decades, none have yet achieved a sterilizing cure, which is characterized by the elimination of the HBV cccDNA minichromosome. Several challenges exist in developing therapeutic vaccines for CHB, particularly at the stage of designing vaccine candidates (Figure 1). One major issue is antigen selection. Most therapeutic vaccines utilize the HBV surface antigen (HBs), following the success of prophylactic vaccines[22-25]. However, the use of HBs in therapeutic vaccines has proven less effective, as it can lead to T cell exhaustion[23,25-27]. Some studies have combined HBs with other HBV proteins, such as HBV core protein (HBc), HBV polymerase, and HBV X protein (HBx), but the results remain suboptimal[28-32].
The second challenge is the genetic diversity of HBV. HBV infection persistence is related to its complex life cycle, with high replicative capacity, establishment of viral reservoirs for HBV replication and antigen production, high viral burden, and impaired host innate and adaptive immune responses against HBV[2,33]. HBV has high replicative capacity, producing more than 100 billion virions per day. However, due to the absence of proofreading activity of the viral reverse transcriptase, HBV is prone to mutations. In addition, recombination events may also occur during viral replication, resulting in high viral genetic diversity. As such, HBV exists as viral quasispesies, a population of viral variants that are closely linked genetically, capable to adapt quickly to a variety of selective pressures[33]. Different HBV variants have been known to be associated with different clinical manifestations and responsiveness to CHB treatment[34].
Based on the differences on HBV genome, up to now, there are 10 different HBV genotypes, each with distinct geographical distributions[35]. Genotypes A to D exhibit more than 8% nucleotide variation across the entire genome, while genotypes E to H show around 4% variation in the HBs coding gene[36]. Genotypes C and D are particularly known for having a higher frequency of core promoter and pre-S mutations compared to genotypes A and B[37]. Ad
Therefore, new approaches are needed to achieve functional HBV cure. Restoring the host immune response, in combination with antiviral agents that targeted the cccDNA and integrated DNA, is now considered as a better approach to achieve sustained HBV control in CHB by eliminating the infected cells and/or block infection of new cells[2,15].
One potential solution to both issues is reverse vaccinology (RV). RV leverages computational techniques to identify antigens from the genetic data of pathogens[39]. Through RV, researchers can discover conserved antigenic regions or epitopes that are present across all HBV genotypes. Additionally, RV can be combined with immunoinformatics to predict the safety, immunogenicity, and immune responses of vaccine candidates, thereby saving time and reducing costs associated with laboratory testing (Figure 1)[40,41]. By integrating RV and immunoinformatics, only those vaccine candidates predicted to be safe, immunogenic, capable of inducing specific immune responses, and offering broad global population coverage will proceed to laboratory testing. Therefore, this article seeks to provide a deeper review of the potential role of RV and immunoinformatics in advancing the design of therapeutic vaccines for CHB.
The design of both prophylactic and therapeutic vaccines is traditionally initiated by cultivating the pathogen in a laboratory to identify its components[42,43]. Following this, potential vaccine candidates are selected by isolating each component of the pathogen. Once identified, these antigens must be mass-produced, either by growing the pathogen itself or through gene cloning[42]. The entire process is time-consuming, costly, and requires advanced laboratory equipment.
In contrast to the conventional method, RV offers several advantages. RV, also known as antigen- or epitope-based vaccinology, uses genomic and protein sequences from pathogens to identify antigens that can stimulate the host's immune response[44,45]. With RV and computational tools, researchers can predict epitopes recognized by T cells and B cells, which may serve as potential vaccine candidates without the need to handle the pathogen directly[39,41,46,47]. RV helps researchers identify key epitopes likely to trigger a strong immune response, reducing the need for extensive lab testing and lowering time and costs by focusing on the most promising candidates[40,41,48,49]. Additionally, RV helps identify conserved regions in the pathogen's genome or proteins, allowing for the design of vaccine candidates with broader protective capabilities[40,48-50]. The RV approach also facilitates the creation of multi-epitope vaccines, which combine epitopes from different parts of the pathogen, or even from different pathogens[41]. This is important because such vaccines can target multiple viral strains or different immune pathways simultaneously.
RV was first utilized in the 1990s to design a prophylactic vaccine candidate for Group B meningococcus[42]. At that time, the RV approach had a significant impact on Group B meningococcus vaccine development. In just a year and a half, 25 surface-exposed proteins were discovered, all capable of inducing antibodies[42,48]. This success led to the increasing use of RV in vaccine design, including therapeutic vaccines. The design of therapeutic vaccines using the RV approach begins with data collection, which can be either genomic or proteomic. When genomic data is used, gene prediction is required to identify coding sequences, which are then translated into protein sequences[51]. Data for RV can be obtained from sources like the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) or the UniProt Knowledgebase (https://www.uniprot.org/)[51-53].
After data collection, researchers select proteins that naturally interact with the immune system[40,51]. These proteins must be non-redundant, non-homologous, non-allergenic, and non-toxic[51]. To identify conserved protein sequences across different pathogen species, multiple sequence alignment can be performed using various software tools such as ClustalW[54], ClustalX[55], Clustal Omega[56], MUSCLE[57], FAMSA[58], MAGUS[59], or web servers like MAFFT (https://mafft.cbrc.jp/alignment/server/index.html)[60] and T-Coffee (https://tcoffee.crg.eu/)[61]. The alignment results can be visualized and edited using JalView[62] or BioEdit[63]. Sequence conservation and variability levels can be measured by performing entropy analysis with AVANA[64], which is based on Shannon's entropy concept.
The next step involves predicting epitopes from the identified conserved protein sequences[40,43,51]. Three types of epitopes that can be utilized as candidates for therapeutic vaccines: CTL epitopes, helper T lymphocyte (HTL) epitopes, and linear-B cell (LBC) epitopes. CTL epitopes promote long-term immunity by targeting pathogens and infected cells, HTL epitopes play a role in both humoral and cell-mediated immunity, and LBC epitopes stimulate antibody production in response to antigens[50,65]. For therapeutic vaccine candidate, epitopes must meet several key criteria, including being antigenic, immunogenic, non-allergenic, non-toxic, and providing broad population coverage[48]. In some cases, re
In the initial stages of therapeutic vaccine design, immunoinformatics plays a key role in epitope prediction. Epitopes chosen for therapeutic vaccine design should be capable of inducing an immune response, specifically a cellular immune response mediated by CTLs and HTLs, as therapeutic vaccines are administered to patients with previously infected cells[46,67]. Therefore, the selected epitopes need to exhibit binding affinity with multiple major histocompatibility complex (MHC) class I and II alleles[68]. Several tools are available for CTL epitope prediction, such as NetCTL1.2[69], NetMHCpan4.0[70], and CTLPred (Table 1)[71]. Among the many web servers capable of predicting CTL epitopes, NetCTL1.2 is one of the most widely used tools due to its advantages over other prediction tools. Several tools can be used for HTL epitope prediction, including NetMHCIIpan 4.0[70], ProPred[72], and MARIA[73] (Table 1). Unlike NetMHCIIpan 4.0 and ProPred, MARIA does not predict epitopes recognized by CTLs but rather those presented by MHC class II[73].
| Web server | Prediction methods | Website link | Ref. |
| Cytotoxic T lymphocyte epitope | |||
| NetCTL 1.2 | Uses an ANN approach to combine MHC class I peptide binding, proteasomal C-terminal cleavage, and TAP transport efficiency | https://services.healthtech.dtu.dk/services/NetCTL-1.2/ | Larsen et al[69], 2007 |
| NetMHCpan 4.1 | Predicts peptide binding to MHC molecules based on quantitative binding affinity and eluted ligands identified by mass spectrometry, using an ANN approach | https://services.healthtech.dtu.dk/services/NetMHCpan-4.1/ | Reynisson et al[70], 2020 |
| CTLPred | Predicts CTL epitopes based on T cell epitope patterns, utilizing both ANN and SVM approaches | http://crdd.osdd.net/raghava/ctlpred/ | Bhasin and Raghava[71], 2004 |
| Helper T lymphocyte epitope | |||
| NetMHCIIpan 4.0 | Predicts peptide binding to MHC II molecules (HLA-DR, HLA-DQ, HLA-DP) based on binding affinity and eluted ligands identified by mass spectrometry, using an ANN approach | https://services.healthtech.dtu.dk/services/NetMHCIIpan-4.0/ | Reynisson et al[70], 2020 |
| ProPred | Predicts MHC Class II (HLA-DR) binding regions within antigen sequences using QM | http://crdd.osdd.net/raghava/propred/ | Singh and Raghava[72], 2001 |
| MARIA | Predicts the likelihood of antigen presentation from a specific gene related to HLA class II alleles, using peptide sequences from mass spectrometry, antigen gene expression levels, and protease cleavage patterns with an ANN approach | https://maria.stanford.edu/ | Chen et al[73], 2019 |
| Linear B Cell epitope | |||
| ABCpred | Uses an RNN approach that considers peptide length to predict B cell epitopes within antigen sequences | http://crdd.osdd.net/raghava/abcpred/ | Saha and Raghava[114], 2006 |
| Bepipred Linear Epitope Prediction 2.0 | Uses a random forest algorithm trained on annotated epitopes from antibody-antigen protein structures | https://services.healthtech.dtu.dk/services/BepiPred-2.0/ | Jespersen et al[115], 2017 |
| BCEPS | Predicts linear B cell epitopes using an SVM approach based on the tertiary structure of antibody-antigen complexes | http://imbio.med.ucm.es/bceps/ | Ras-Carmona et al[116], 2021 |
| SEMA | Applies a transfer learning approach using a pre-trained deep learning model to predict conformational B cell epitopes based on primary antigen sequences and tertiary structures | https://sema.airi.net/ | Shashkova et al[117], 2022 |
| LBtope | Uses SVM and Ibk approaches on a large dataset of experimentally validated B cell epitopes and non-epitopes to predict linear B cell epitopes | https://webs.iiitd.edu.in/raghava/lbtope/index.php | Singh et al[118], 2013 |
| Bcepred | Predicts B cell epitopes using physicochemical properties, such as hydrophilicity, flexibility, accessibility, polarity, exposed surface, and turns | http://crdd.osdd.net/raghava/bcepred/ | Saha and Raghava[77], 2004 |
| COBEpro | Uses an SVM to predict short peptide fragments within query antigen sequences, calculating an epitope propensity score for each residue | https://scratch.proteomics.ics.uci.edu/ | Sweredoski and Baldi[119], 2009 |
| CLBTope | Combines alignment-based and alignment-free machine learning methods to predict B cell epitopes, using epitope and non-epitope sequence composition | https://webs.iiitd.edu.in/raghava/clbtope/ | Kumar et al[120], 2024 |
In addition to cellular immune responses, several studies report that epitopes used in therapeutic vaccine design should also be capable of inducing humoral immune responses[40,50,74,75]. Humoral response activation relies on the recognition of both linear (LBC) and conformational B-cell epitopes[76]. Initially, tools for predicting LBC epitopes, such as Bcepred[77], analyzed the sequential physicochemical properties of amino acids to make predictions. However, with the integration of machine learning algorithms data comparison across methods has improved (Table 1). This integration enhances prediction accuracy, producing more optimistic results and reducing misleading predictions[76]. Similarly, tools for predicting conformational B-cell epitopes have been developed to analyze the potential of epitopes to form different conformations, such as DiscoTope-3.0 (https://services.healthtech.dtu.dk/services/DiscoTope-3.0/)[78].
Predicted epitopes still require further selection before they can be used in therapeutic vaccine design. This selection process has become faster and easier with the use of immunoinformatics approaches. Numerous tools are now available to predict antigenicity, allergenicity, immunogenicity, toxicity, autoimmunity, and population coverage, each with varying levels of accuracy (Table 2). Additionally, tools have been developed to predict anti-inflammatory peptides and the ability to induce IFN-γ (Table 2), both of which are essential parameters for certain therapeutic vaccines[67,79]. In addition to the web servers listed in Tables 1 and 2, the Immune Epitope Database (IEDB) web server also provides tools to predict CTL epitopes (http://tools.iedb.org/mhci/), HTL epitopes (http://tools.iedb.org/mhcii/), and LBC epitopes (http://tools.iedb.org/bcell/) with a range of method options. The IEDB facilitates epitope prediction, particularly for CTL and HTL, as it includes human leukocyte antigen (HLA) MHC class I and II allele data, covering approximately 97% of the global population for CTL[80] and 99% for HTL[81]. Additionally, the server enables immunogenicity analysis and population coverage assessment for predicted CTL and HTL epitopes.
Immunoinformatics can also be used to predict the secondary and tertiary structures of vaccine candidates composed of selected epitopes. Several widely used tools are available for secondary structure prediction. These include PredictProtein (https://predictprotein.org/)[82]; Self Optimized Prediction Method with Alignment (https://npsa.lyon.inserm.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html)[83]; PSIPRED 4.0 (http://bioinf.cs.ucl.ac.uk/psipred/)[84]; and Jpred4 (https://www.compbio.dundee.ac.uk/jpred/)[85]. For tertiary structure prediction, tools such as Ite
After successfully modeling the therapeutic vaccine candidate in 3D, interactions and binding strength between the designed vaccine candidate and various immune-response receptors can be tested through molecular docking analysis. Selected epitopes can also undergo docking to identify their binding pockets on the target receptors. Molecular docking analysis can be performed using tools such as HDOCK (http://hdock.phys.hust.edu.cn/)[89], ClusPro 2.0 (https://cluspro.org/help.php)[90], SwissDock (https://www.swissdock.ch/)[91], and MolModa (https://durrantlab.com/molmoda)[92]. To further predict interactions and binding potential between the vaccine candidate and target receptors, molecular dynamics (MD) simulations can be applied. MD simulations add water molecules and simulate temperature increases, replicating in vivo conditions and providing detailed insights into vaccine-receptor interactions. Commonly used software for MD simulations includes AMBER[93] and GROMACS[94]. In the final stage of vaccine design, ensuring the candidate’s ability to induce an immune response is essential. Immunoinformatics can assist in this phase by enabling immune response analysis through the C-ImmSim (http: //150.146.2.1/C-IMMSIM/index.php) server, which simulates antigen recognition at both single-peptide and whole-proteome levels[95].
Since 2017, several studies have explored epitopes and designed therapeutic vaccines for CHB using RV and immunoinformatics approaches (Table 3). Zheng et al[96] were interested in exploring epitopes on HBV polymerase due to its lower expression level. Zheng et al[96] utilized a consensus sequence created from 13 amino acid sequences of polymerase from NCBI, representing 13 HBV (sub)genotypes (A, A1, A2, B, C, D, D3, D4, E, F2, F4, G, H). After predicting CTL and LBC epitopes, they selected these based on immunogenicity, conservation, toxicity, and population coverage, using immunoinformatics approaches. They identified four conserved CTL epitopes and two LBC epitopes that showed strong binding to HLA-A*0201 in molecular docking analysis. However, they only predicted CTL epitopes recognizing HLA-A*0201, which is prevalent among Caucasians but expressed in about 40% of the global population and is not dominant in regions with high HBV prevalence, such as Asia and Africa[97].
| Targeted antigens | Data type | Data source | Immunoinformatics applications | Ref. |
| HBV Polymerase | Proteomics | NCBI | Consensus sequence formation, epitope prediction, epitope selection (based on immunogenicity, toxicity, and conservation), population coverage calculation, and molecular docking analysis | Zheng et al[96], 2017 |
| HBV polymerase; HBx | Proteomics | HBVdb | Epitope prediction and epitope selection (based on conservation and immunogenicity) | de Beijer et al[98], 2020 |
| HBV polymerase | Proteomics | NCBI | Consensus sequence formation, epitope prediction, epitope selection (antigenicity, allergenicity, toxicity), population coverage calculation, physicochemical property prediction of vaccine candidates, secondary and tertiary structure prediction, molecular docking analysis, and molecular dynamics simulation | Ahmed et al[100], 2021 |
| HBc; HBx | Proteomics | NCBI | Epitope prediction and epitope selection (conservation and autoimmunity) | Saeed et al[101], 2023 |
| LHBs | Proteomics | NCBI | Epitope prediction, physicochemical property prediction, vaccine candidate antigenicity and allergenicity analysis, molecular docking analysis, molecular dynamics simulation, and immune response simulation | Zhu et al[104], 2024 |
In a different approach, de Beijer et al[98] predicted CTL epitopes from HBV polymerase and HBx using a larger dataset and more diverse HLA types. de Beijer et al[98] used 7489 amino acid sequences for polymerase and 8127 sequences for HBx from the HBVdb (https://hbvdb.lyon.inserm.fr/HBVdb/HBVdbIndex) database, which provides comprehensive genomic and proteomic data for HBV, covering genotypes A to H[99]. This study identified five novel HBx epitopes and 17 previously unreported polymerase epitopes[98]. Based on immunoinformatics analysis, all predicted epitopes were found to be conserved across eight HBV genotypes and demonstrated strong immunogenic potential. This was further verified through in vitro experiments, which showed that the predicted epitopes could bind to target HLA molecules and successfully induce IFN-γ production in peripheral blood mononuclear cells from individuals who had recovered from HBV infection[98].
Continuing the exploration of HBV polymerase, Ahmed et al[100] used a similar approach to design a vaccine candidate by combining several CTL, HTL, and LBC epitopes from polymerase. Unlike de Beijer et al[98], Ahmed et al[100] used a representative dataset of 148 HBV polymerase sequences from NCBI. They predicted CTL, HTL, and LBC epitopes using tools available on the IEDB web server. Epitopes in this study were selected based on antigenicity, allergenicity, and toxicity. Going a step further than previous studies, Ahmed et al[100] developed a multi-epitope polymerase vaccine candidate with an adjuvant, Mycobacterium tuberculosis 50S ribosomal protein L7/L12.
In addition to exploring HBV polymerase epitopes and vaccine design, other studies have targeted HBc and HBx epitopes for CHB therapy using immunoinformatics approaches. Saeed et al[101] gathered 237 HBx and 207 HBc amino acid sequences from NCBI, representing the 10 HBV genotypes. After epitope selection, they identified two LBC epitopes and 10 CTL epitopes from HBx, and 22 CTL epitopes from HBc, along with three HTL epitopes from HBx and 10 HTL epitopes from HBc, all with 100% conservation. The study also discovered several partially conserved epitopes (70%-90% conservation) in the C-terminal domain (CTD) of HBc, which contains four arginine-rich domains that can enhance pre-core gene transcription and either promote or inhibit host gene transcriptional activators[102]. Similarly, conserved epitopes in the CTD of HBx were found, where this domain includes two transactivation regions composed of several motifs, including a BH3-like motif that enables HBx to bind to anti-apoptotic proteins[103]. This information is highly valuable for designing CHB therapeutic vaccines, as it allows researchers to utilize specific epitopes from HBc and HBx rather than using the full-length proteins as primary vaccine components.
Although limited, there is a therapeutic CHB vaccine design using RV and immunoinformatics that has undergone in vitro and in vivo validation. In 2024, Zhu et al[104] predicted HTL and LBC epitopes from the LHBs protein and combined them with the immunoglobulin variable region of CTLA-4. Zhu et al[104] utilized amino acid sequences of LHBs and CTLA-4, obtained from NCBI, in their study. Selected LHBs epitopes were fused with the immunoglobulin variable region of CTLA-4 using a linker. Molecular docking analysis and MD simulation demonstrated that the vaccine candidate could strongly bind B7 molecules. These results were further corroborated by in vitro experiments, which showed that macrophages bound and engulfed the vaccine candidate more effectively than LHBs alone. ELISA and ELISPOT assays further indicated that the vaccine candidate could significantly stimulate HBV-specific T helper cell type 1 and type 2 responses. Western blot analysis also confirmed the good antigenicity of the vaccine candidate, as it effectively bound to specific antibodies in plasma from CHB patients. Immune simulation using the C-ImmSim server was further validated by in vivo experiments, where the levels of IFN-γ, IL-4, and total IgG in splenocyte culture supernatants were significantly higher in mice immunized with the vaccine candidate compared to those immunized with LHBs alone.
Although a perfect therapeutic vaccine design for CHB has yet to be achieved, various studies have demonstrated the crucial role of RV and immunoinformatics in addressing challenges in CHB vaccine design (Table 3). Through RV approaches, multiple studies have successfully identified numerous conserved epitopes in key antigens such as HBs[104], HBc[101], HBx[98,101], and polymerase[96,98,100] across all HBV genotypes, offering hope for developing a globally effective CHB therapeutic vaccine. The integration of immunoinformatics with RV has also proven to streamline the process of selecting optimal epitopes from different HBV antigens and combining them, for example, HBx with polymerase[98] or HBc with HBx[101], in vaccine candidate designs. Additionally, immunoinformatics enables resear
After exploring several studies that successfully identified epitopes and designed therapeutic vaccines for CHB, it is clear that there are still many opportunities for advancing CHB therapeutic vaccine design through the integration of RV and immunoinformatics approaches. While epitope prediction from HBc and HBx has been conducted, it has not yet incorporated large-scale genomic data, such as the comprehensive datasets available on NCBI. Saeed et al’s study[101] utilized only hundreds of protein sequences representing 10 genotypes. Increasing the amount of data in these studies would better reflect the amino acid sequence variations across genotypes. Sequence variations can lead to differing consensus amino acid sequences, which would influence predicted epitopes. Additionally, although HBc and HBx have the potential to influence the stability and transcription of the cccDNA minichromosome[8], their epitopes have yet to be utilized in therapeutic vaccine design. Targeting HBc and HBx could be a strategic approach to disrupt cccDNA mi
On the other hand, epitope prediction of HBV polymerase has been conducted using large datasets from HBVdb[98], although it covers only eight genotypes. Additionally, studies predicting CTL epitopes have used only a limited selection of HLA class I alleles[96,98]. While some research has employed reference HLA class II alleles from the IEDB for HTL epitope prediction[100], CHB-associated HLA class II alleles, such as HLA-DPA1*01: 03-DPB1*04: 02, HLA-DPA1*01: 03-DPB1*04: 01[105], have not yet been considered. The selection of HLA alleles in epitope prediction can significantly influence the resulting predicted epitopes. There is an opportunity to expand this research by utilizing resources like the AlleleFrequencies[106] web server (https://www.allelefrequencies.net/hla6006a.asp), which provides access to fre
In prior studies, epitope selection has typically been based on factors such as antigenicity, allergenicity, immunogenicity, toxicity, autoimmunity, and conservancy[96,98,100,101,104]. However, additional parameters could enhance epitope selection to better address CHB, such as the ability to induce IFN-γ and being classified as anti-inflammatory peptides[107]. Additionally, epitopes can be filtered based on physicochemical properties, which are predictable using the ProtParam[108] web server (https://web.expasy.org/protparam/). Although previous studies have used this tool to assess physicochemical properties for vaccine candidates[104], it can also assist in epitope selection. For instance, HBx is a highly hydrophobic protein, indicating that its predicted epitopes may also be hydrophobic. Incorporating these epitopes fully into a therapeutic vaccine could increase the vaccine candidate’s overall hydrophobicity, though ideally, it should be hydrophilic. By selecting epitopes based on physicochemical properties, researchers could enhance the hydrophilicity of the vaccine candidate.
Another opportunity is to advance CHB therapeutic vaccine design through the optimal selection of adjuvants. To date, only a few studies have included adjuvants in CHB therapeutic vaccine design[100]. Immunoinformatics can facilitate this selection process, with tools such as the VaxinPad[109] web server (http://crdd.osdd.net/raghava/vaxinpad/) for peptide vaccine adjuvant design. In addition to enhancing immune responses, studies have shown that adjuvants in CHB therapeutic vaccines may help mitigate T and B cell exhaustion[17]. A recent study by Sacher et al[110], however, showed that amino-acid formulation efficiently stabilized hepatitis B therapeutic vaccine to break immune tolerance in AAV-HBV mice. It can be one of strategical approaches for therapeutic vaccines designed with RV and immunoinformatics.
Although previous studies have shown that RV and immunoinformatics can help address challenges related to antigen or epitope selection and HBV genotype diversity, these approaches have limitations, posing new challenges in CHB therapeutic vaccine design. RV can only identify epitopes encoded by the genome, while other macromolecules, such as carbohydrates and lipids, may also serve as antigens[111]. Vaccine candidates designed using RV and immunoinformatics still require laboratory validation and verification through comprehensive in vitro and in vivo studies. Predictions made with immunoinformatics depend on data quality and algorithm sophistication, so they cannot fully replace experimental research methods[112]. In addition, missing information between the relevance between HBV genotypes, DNA mutations, and clinical manifestations[113] might complicate the selection of the genomic and proteomic datasets for the vaccine design that might be useful for different populations of the patients.
As discussed in the previous section, available tools employ different predictive approaches, leading to variations in data accuracy. Additionally, these tools have other limitations. For example, the C-ImmSim web server can only predict immune responses in individuals with normal immune function[95]. It cannot make predictions for patients with altered immune statuses, such as those with immunodeficiency from HIV or diabetes. To date, only a few studies have dem
Future research should focus on improving the accuracy and reliability of computational tools by incorporating more comprehensive, high-quality genomic and proteomic datasets from diverse HBV genotypes. Advances in machine learning and artificial intelligence could further enhance epitope prediction and immune response modeling, leading to more robust vaccine candidates. Continued development of these technologies, combined with rigorous in vitro and in vivo validation followed by robust clinical trials, the maintenance of the stability and immunogenicity of the protein, will be essential to overcome current limitations and bring effective therapeutic vaccines for CHB closer to clinical application.
This article highlights the significant potential of RV and immunoinformatics in advancing the development of therapeutic vaccines for CHB. By enabling the identification of conserved epitopes across diverse HBV genotypes and streamlining the vaccine design process, these approaches offer promising strategies to address longstanding challenges in CHB treatment. Although their application has yielded encouraging in silico results, challenges related to data quality and experimental validation remain. Future efforts should prioritize enhancing computational accuracy through improved datasets and advanced algorithms, alongside comprehensive in vitro and in vivo validation, to successfully translate promising in silico findings into effective clinical interventions.
We would like to thank Husna Nugrahapraja and Reza Aditama for the discussion about reverse vaccinology and immunoinformatics.
| 1. | World Health Organization. Global hepatitis report 2024: action for access in low- and middle-income countries. Apr 9, 2024. [cited 26 March 2025]. Available from: https://www.who.int/publications/i/item/9789240091672. |
| 2. | Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76:1249-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 3. | Mironova M, Ghany MG. Hepatitis B Vaccine: Four Decades on. Vaccines (Basel). 2024;12:439. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Lim SG, Baumert TF, Boni C, Gane E, Levrero M, Lok AS, Maini MK, Terrault NA, Zoulim F. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat Rev Gastroenterol Hepatol. 2023;20:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 5. | Aguilar-Rubido JC, Klundert MAA van de, Michel ML. Chronic Hepatitis B therapies: challenges and opportunities. Biotecnol Apl. 2019;36:1401-1410. |
| 6. | Lau KCK, Burak KW, Coffin CS. Impact of Hepatitis B Virus Genetic Variation, Integration, and Lymphotropism in Antiviral Treatment and Oncogenesis. Microorganisms. 2020;8:1470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Pan Y, Xia H, He Y, Zeng S, Shen Z, Huang W. The progress of molecules and strategies for the treatment of HBV infection. Front Cell Infect Microbiol. 2023;13:1128807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Singh P, Kairuz D, Arbuthnot P, Bloom K. Silencing hepatitis B virus covalently closed circular DNA: The potential of an epigenetic therapy approach. World J Gastroenterol. 2021;27:3182-3207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 9. | Dandri M. Epigenetic modulation in chronic hepatitis B virus infection. Semin Immunopathol. 2020;42:173-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Wang Z, Wang W, Wang L. Epigenetic regulation of covalently closed circular DNA minichromosome in hepatitis B virus infection. Biophys Rep. 2020;6:115-126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Zeisel MB, Guerrieri F, Levrero M. Host Epigenetic Alterations and Hepatitis B Virus-Associated Hepatocellular Carcinoma. J Clin Med. 2021;10:1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Gao W, Hu J. Formation of hepatitis B virus covalently closed circular DNA: removal of genome-linked protein. J Virol. 2007;81:6164-6174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Sohn JA, Litwin S, Seeger C. Mechanism for CCC DNA synthesis in hepadnaviruses. PLoS One. 2009;4:e8093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Li X, Zhao J, Yuan Q, Xia N. Detection of HBV Covalently Closed Circular DNA. Viruses. 2017;9:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 16. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 764] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 17. | Horng JH, Lin WH, Wu CR, Lin YY, Wu LL, Chen DS, Chen PJ. HBV X protein-based therapeutic vaccine accelerates viral antigen clearance by mobilizing monocyte infiltration into the liver in HBV carrier mice. J Biomed Sci. 2020;27:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Lai MW, Hsu CW, Lin CL, Chien RN, Lin WR, Chang CS, Liang KH, Yeh CT. Multiple doses of hepatitis B recombinant vaccine for chronic hepatitis B patients with low surface antigen levels: a pilot study. Hepatol Int. 2018;12:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Yoon SK, Seo YB, Im SJ, Bae SH, Song MJ, You CR, Jang JW, Yang SH, Suh YS, Song JS, Kim BM, Kim CY, Jeong SH, Sung YC. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015;35:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Zoulim F, Chen PJ, Dandri M, Kennedy PT, Seeger C. Hepatitis B virus DNA integration: Implications for diagnostics, therapy, and outcome. J Hepatol. 2024;81:1087-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 21. | Zoulim F, Testoni B. Eliminating cccDNA to cure hepatitis B virus infection. J Hepatol. 2023;78:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 22. | Wang W, Zhou X, Bian Y, Wang S, Chai Q, Guo Z, Wang Z, Zhu P, Peng H, Yan X, Li W, Fu YX, Zhu M. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15:406-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 23. | Fontaine H, Kahi S, Chazallon C, Bourgine M, Varaut A, Buffet C, Godon O, Meritet JF, Saïdi Y, Michel ML, Scott-Algara D, Aboulker JP, Pol S; ANRS HB02 study group. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial--ANRS HB02 VAC-ADN. Gut. 2015;64:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Reynolds TD, Buonocore L, Rose NF, Rose JK, Robek MD. Virus-Like Vesicle-Based Therapeutic Vaccine Vectors for Chronic Hepatitis B Virus Infection. J Virol. 2015;89:10407-10415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Yang FQ, Rao GR, Wang GQ, Li YQ, Xie Y, Zhang ZQ, Deng CL, Mao Q, Li J, Zhao W, Wang MR, Han T, Chen SJ, Pan C, Tan DM, Shang J, Zhang MX, Zhang YX, Yang JM, Chen GM. Phase IIb trial of in vivo electroporation mediated dual-plasmid hepatitis B virus DNA vaccine in chronic hepatitis B patients under lamivudine therapy. World J Gastroenterol. 2017;23:306-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Lee YB, Lee JH, Kim YJ, Yoon JH, Lee HS. The effect of therapeutic vaccination for the treatment of chronic hepatitis B virus infection. J Med Virol. 2015;87:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Xu DZ, Wang XY, Shen XL, Gong GZ, Ren H, Guo LM, Sun AM, Xu M, Li LJ, Guo XH, Zhen Z, Wang HF, Gong HY, Xu C, Jiang N, Pan C, Gong ZJ, Zhang JM, Shang J, Xu J, Xie Q, Wu TF, Huang WX, Li YG, Xu J, Yuan ZH, Wang B, Zhao K, Wen YM; YIC Efficacy Trial Study Team. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol. 2013;59:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Spellman M, Martin J. 751 Treatment of chronic hepatitis b infection with dv-601, a therapeutic vaccine. J Hepatol. 2011;54:S302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lim Y, Mutimer D, Heo J, Tak WY, Rosenberg W, Kukjang B, Kim Y, Forton D, Tasker S, Georges B. PS-078-A phase 1b evaluation of HepTcell HBV-specific immunotherapy in nuc-controlled, eAg negative chronic HBV infection. J Hepatol. 2019;70:E50-E51. [DOI] [Full Text] |
| 30. | Luo J, Li J, Chen RL, Nie L, Huang J, Liu ZW, Luo L, Yan XJ. Autologus dendritic cell vaccine for chronic hepatitis B carriers: a pilot, open label, clinical trial in human volunteers. Vaccine. 2010;28:2497-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Wei MJ, Pan XN, Wei KP, Li XH, Liu XL, Zhang XM, Jiang YL, Zhang CY, Shen JK. Efficacy of HBV-pulsed DCs in combination with entecavir in patients with chronic hepatitis B infection. Int Immunopharmacol. 2015;27:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Zoulim F, Fournier C, Habersetzer F, Sprinzl M, Pol S, Coffin CS, Leroy V, Ma M, Wedemeyer H, Lohse AW, Thimme R, Lugardon K, Martin P, Bastien B, Sansas B, Adda N, Halluard C, Bendjama K, Brandely M, Inchauspé G. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother. 2020;16:388-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | do Lago BV, Bezerra CS, Moreira DA, Parente TE, Portilho MM, Pessôa R, Sanabani SS, Villar LM. Genetic diversity of hepatitis B virus quasispecies in different biological compartments reveals distinct genotypes. Sci Rep. 2023;13:17023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Wang SH, Yeh SH, Chen PJ. Unique Features of Hepatitis B Virus-Related Hepatocellular Carcinoma in Pathogenesis and Clinical Significance. Cancers (Basel). 2021;13:2454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Mulyanto. Viral Hepatitis in Indonesia: Past, Present, and Future. Euroasian J Hepatogastroenterol. 2016;6:65-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Bartholomeusz A, Schaefer S. Hepatitis B virus genotypes: comparison of genotyping methods. Rev Med Virol. 2004;14:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med. 2015;5:a021436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 38. | Zhang ZH, Wu CC, Chen XW, Li X, Li J, Lu MJ. Genetic variation of hepatitis B virus and its significance for pathogenesis. World J Gastroenterol. 2016;22:126-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (3)] |
| 39. | Sunita, Sajid A, Singh Y, Shukla P. Computational tools for modern vaccine development. Hum Vaccin Immunother. 2020;16:723-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Russo G, Crispino E, Maleki A, Di Salvatore V, Stanco F, Pappalardo F. Beyond the state of the art of reverse vaccinology: predicting vaccine efficacy with the universal immune system simulator for influenza. BMC Bioinformatics. 2023;24:231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Pratiwi SE, Ysrafil Y, Mardhia M, Mahyarudin M, Ilmiawan MI, Trianto HF, Liana DF, Amia Y. A novel therapeutic multiepitope vaccine based on oncoprotein E6 and E7 of HPV 16 and 18: An in silico approach. Bioimpacts. 2024;14:27846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Rappuoli R. Reverse vaccinology. Curr Opin Microbiol. 2000;3:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 496] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 43. | Kanampalliwar AM. Reverse Vaccinology and Its Applications. Methods Mol Biol. 2020;2131:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Sun X, Ma H, Wang X, Bao Z, Tang S, Yi C, Sun B. Broadly neutralizing antibodies to combat influenza virus infection. Antiviral Res. 2024;221:105785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 45. | Hizbullah, Nazir Z, Afridi SG, Shah M, Shams S, Khan A. Reverse vaccinology and subtractive genomics-based putative vaccine targets identification for Burkholderia pseudomallei Bp1651. Microb Pathog. 2018;125:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Sanami S, Azadegan-Dehkordi F, Rafieian-Kopaei M, Salehi M, Ghasemi-Dehnoo M, Mahooti M, Alizadeh M, Bagheri N. Design of a multi-epitope vaccine against cervical cancer using immunoinformatics approaches. Sci Rep. 2021;11:12397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Prado LCDS, Giacchetto Felice A, Rodrigues TCV, Tiwari S, Andrade BS, Kato RB, Oliveira CJF, Silva MV, Barh D, Azevedo VAC, Jaiswal AK, Soares SC. New putative therapeutic targets against Serratia marcescens using reverse vaccinology and subtractive genomics. J Biomol Struct Dyn. 2022;40:10106-10121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Zeniye A, Teshome I. Reverse Vaccinology Approach Against Viruses: A Review. Austin J Vet Sci & Anim Husb. 2024;11:1143. |
| 49. | Herrera LRM. Reverse Vaccinology Approach in Constructing a Multi-Epitope Vaccine Against Cancer-Testis Antigens Expressed in Non-Small Cell Lung Cancer. Asian Pac J Cancer Prev. 2021;22:1495-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Khan S, Aziz S, Waqas M, Kakar MA, Ahmad S. Targeted vaccine development against Bilophila wadsworthia to curb colon diseases: A multiepitope approach based on reverse vaccinology and computational analysis. Int J Biol Macromol. 2023;250:126002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Goodswen SJ, Kennedy PJ, Ellis JT. A guide to current methodology and usage of reverse vaccinology towards in silico vaccine discovery. FEMS Microbiol Rev. 2023;47:fuad004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 52. | UniProt Consortium. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023;51:D523-D531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 3223] [Article Influence: 1611.5] [Reference Citation Analysis (0)] |
| 53. | NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018;46:D8-D13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 909] [Cited by in RCA: 969] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 54. | Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-4680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47226] [Cited by in RCA: 45081] [Article Influence: 1454.2] [Reference Citation Analysis (0)] |
| 55. | Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876-4882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29832] [Cited by in RCA: 27638] [Article Influence: 987.1] [Reference Citation Analysis (0)] |
| 56. | Sievers F, Higgins DG. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018;27:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 1256] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 57. | Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28603] [Cited by in RCA: 30031] [Article Influence: 1430.0] [Reference Citation Analysis (0)] |
| 58. | Deorowicz S, Debudaj-Grabysz A, Gudyś A. FAMSA: Fast and accurate multiple sequence alignment of huge protein families. Sci Rep. 2016;6:33964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 59. | Smirnov V. Recursive MAGUS: Scalable and accurate multiple sequence alignment. PLoS Comput Biol. 2021;17:e1008950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2654] [Cited by in RCA: 4551] [Article Influence: 910.2] [Reference Citation Analysis (0)] |
| 61. | Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5210] [Cited by in RCA: 5008] [Article Influence: 200.3] [Reference Citation Analysis (0)] |
| 62. | Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8558] [Cited by in RCA: 7285] [Article Influence: 455.3] [Reference Citation Analysis (0)] |
| 63. | Hall T. BioEdit : a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95-98. |
| 64. | Miotto O, Tan TW, Brusic V. Rule-based knowledge aggregation for large-scale protein sequence analysis of influenza A viruses. BMC Bioinformatics. 2008;9 Suppl 1:S7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Zhang L. Multi-epitope vaccines: a promising strategy against tumors and viral infections. Cell Mol Immunol. 2018;15:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 66. | La Manna S, Di Natale C, Florio D, Marasco D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int J Mol Sci. 2018;19:2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 67. | Guo N, Niu Z, Yan Z, Liu W, Shi L, Li C, Yao Y, Shi L. Immunoinformatics Design and In Vivo Immunogenicity Evaluation of a Conserved CTL Multi-Epitope Vaccine Targeting HPV16 E5, E6, and E7 Proteins. Vaccines (Basel). 2024;12:392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 68. | Soria-Guerra RE, Nieto-Gomez R, Govea-Alonso DO, Rosales-Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inform. 2015;53:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 69. | Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O, Nielsen M. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics. 2007;8:424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 653] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 70. | Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48:W449-W454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 958] [Cited by in RCA: 1206] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 71. | Bhasin M, Raghava GP. Prediction of CTL epitopes using QM, SVM and ANN techniques. Vaccine. 2004;22:3195-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 72. | Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 645] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 73. | Chen B, Khodadoust MS, Olsson N, Wagar LE, Fast E, Liu CL, Muftuoglu Y, Sworder BJ, Diehn M, Levy R, Davis MM, Elias JE, Altman RB, Alizadeh AA. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol. 2019;37:1332-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 74. | Masum MHU, Wajed S, Hossain MI, Moumi NR, Talukder A, Rahman MM. An mRNA vaccine for pancreatic cancer designed by applying in silico immunoinformatics and reverse vaccinology approaches. PLoS One. 2024;19:e0305413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 75. | Priyamvada P, Ramaiah S. Pan-genome and reverse vaccinology approaches to design multi-epitope vaccine against Epstein-Barr virus associated with colorectal cancer. Immunol Res. 2023;71:887-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 76. | Rawat SS, Keshri AK, Kaur R, Prasad A. Immunoinformatics Approaches for Vaccine Design: A Fast and Secure Strategy for Successful Vaccine Development. Vaccines (Basel). 2023;11:221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 77. | Saha S, Raghava GPS. BcePred: Prediction of Continuous B-Cell Epitopes in Antigenic Sequences Using Physico-chemical Properties. In: Nicosia G, Cutello V, Bentley PJ, Timmis J, editors. Artificial Immune Systems. ICARIS 2004. Lecture Notes in Computer Science; 2004; Berlin: Springer, 2004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 78. | Høie MH, Gade FS, Johansen JM, Würtzen C, Winther O, Nielsen M, Marcatili P. DiscoTope-3.0: improved B-cell epitope prediction using inverse folding latent representations. Front Immunol. 2024;15:1322712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 79. | Lathwal A, Kumar R, Raghava GPS. In-silico identification of subunit vaccine candidates against lung cancer-associated oncogenic viruses. Comput Biol Med. 2021;130:104215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A. 2013;110:E2046-E2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 81. | Greenbaum J, Sidney J, Chung J, Brander C, Peters B, Sette A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. 2011;63:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 82. | Yachdav G, Kloppmann E, Kajan L, Hecht M, Goldberg T, Hamp T, Hönigschmid P, Schafferhans A, Roos M, Bernhofer M, Richter L, Ashkenazy H, Punta M, Schlessinger A, Bromberg Y, Schneider R, Vriend G, Sander C, Ben-Tal N, Rost B. PredictProtein--an open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014;42:W337-W343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 83. | Geourjon C, Deléage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 810] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 84. | Buchan DWA, Moffat L, Lau A, Kandathil SM, Jones DT. Deep learning for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2024;52:W287-W293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 85. | Drozdetskiy A, Cole C, Procter J, Barton GJ. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 2015;43:W389-W394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1232] [Cited by in RCA: 1315] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 86. | Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3616] [Cited by in RCA: 3901] [Article Influence: 229.5] [Reference Citation Analysis (0)] |
| 87. | Du Z, Su H, Wang W, Ye L, Wei H, Peng Z, Anishchenko I, Baker D, Yang J. The trRosetta server for fast and accurate protein structure prediction. Nat Protoc. 2021;16:5634-5651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 366] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 88. | Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24593] [Cited by in RCA: 22684] [Article Influence: 5671.0] [Reference Citation Analysis (0)] |
| 89. | Yan Y, Zhang D, Zhou P, Li B, Huang SY. HDOCK: a web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017;45:W365-W373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 813] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 90. | Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Beglov D, Vajda S. The ClusPro web server for protein-protein docking. Nat Protoc. 2017;12:255-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 2081] [Article Influence: 260.1] [Reference Citation Analysis (0)] |
| 91. | Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270-W277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1027] [Cited by in RCA: 1285] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 92. | Kochnev Y, Ahmed M, Maldonado AM, Durrant JD. MolModa: accessible and secure molecular docking in a web browser. Nucleic Acids Res. 2024;52:W498-W506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 93. | Case DA, Aktulga HM, Belfon K, Ben-Shalom IY, Berryman JT, Brozell SR, Cerutti DS, Cheatham TE, III, Cisneros GA, Cruzeiro VWD, Darden TA, Duke RE, Giambasu G, Gilson MK, Gohlke H, Goetz AW, Harris R, Izadi S, Izmailov SA, Kasavajhala K, Kaymak MC, King E, Kovalenko A, Kurtzman T, Lee TS, LeGrand S, Li P, Lin C, Liu J, Luchko T, Luo R, Machado M, Man V, Manathunga M, Merz KM, Miao Y, Mikhailovskii O, Monard G, Nguyen H, O’Hearn KA, Onufriev A, Pan F, Pantano S, Qi R, Rahnamoun A, Roe DR, Roitberg A, Sagui C, Schott-Verdugo S, Shajan A, Shen J, Simmerling CL, Skrynnikov NR, Smith J, Swails J, Walker RC, Wang J, Wang J, Wei H, Wolf RM, Wu X, Xiong Y, Xue Y, York DM, Zhao S, Kollman PA. Amber 2022. [cited 26 March 2025]. Available from: https://ambermd.org/doc12/Amber22.pdf. |
| 94. | Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19-25. [DOI] [Full Text] |
| 95. | Rapin N, Lund O, Bernaschi M, Castiglione F. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS One. 2010;5:e9862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 663] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 96. | Zheng J, Lin X, Wang X, Zheng L, Lan S, Jin S, Ou Z, Wu J. In Silico Analysis of Epitope-Based Vaccine Candidates against Hepatitis B Virus Polymerase Protein. Viruses. 2017;9:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 98. | de Beijer MTA, Jansen DTSL, Dou Y, van Esch WJE, Mok JY, Maas MJP, Brasser G, de Man RA, Woltman AM, Buschow SI. Discovery and Selection of Hepatitis B Virus-Derived T Cell Epitopes for Global Immunotherapy Based on Viral Indispensability, Conservation, and HLA-Binding Strength. J Virol. 2020;94:e01663-e01619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 99. | Hayer J, Jadeau F, Deléage G, Kay A, Zoulim F, Combet C. HBVdb: a knowledge database for Hepatitis B Virus. Nucleic Acids Res. 2013;41:D566-D570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 100. | 100 Ahmed RA, Almofti YA, Abd-elrahman KA. Structural Analysis of the Polymerase Protein for Multiepitopes Vaccine Prediction against Hepatitis B Virus. Biosci Biotech Res Asia. 2021;18:125-146. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Saeed U, Piracha ZZ, Alrokayan S, Hussain T, Almajhdi FN, Waheed Y. Immunoinformatics and Evaluation of Peptide Vaccines Derived from Global Hepatitis B Viral HBx and HBc Proteins Critical for Covalently Closed Circular DNA Integrity. Microorganisms. 2023;11:2826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 102. | Kwon JA, Rho HM. Hepatitis B viral core protein activates the hepatitis B viral enhancer II/pregenomic promoter through the nuclear factor kappaB binding site. Biochem Cell Biol. 2002;80:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Jiang T, Liu M, Wu J, Shi Y. Structural and biochemical analysis of Bcl-2 interaction with the hepatitis B virus protein HBx. Proc Natl Acad Sci U S A. 2016;113:2074-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Zhu Y, Yu M, Aisikaer M, Zhang C, He Y, Chen Z, Yang Y, Han R, Li Z, Zhang F, Ding J, Lu X. Contriving a novel of CHB therapeutic vaccine based on IgV_CTLA-4 and L protein via immunoinformatics approach. J Biomol Struct Dyn. 2024;42:6323-6341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 105. | Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, Puseenam A, Sura T, Daigo Y, Chayama K, Chantratita W, Nakamura Y, Matsuda K. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 106. | Gonzalez-Galarza FF, McCabe A, Santos EJMD, Jones J, Takeshita L, Ortega-Rivera ND, Cid-Pavon GMD, Ramsbottom K, Ghattaoraya G, Alfirevic A, Middleton D, Jones AR. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020;48:D783-D788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 316] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 107. | Chaudhuri D, Datta J, Majumder S, Giri K. In silico designing of peptide based vaccine for Hepatitis viruses using reverse vaccinology approach. Infect Genet Evol. 2020;84:104388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM, editor. The Proteomics Protocols Handbook. Springer Protocols Handbooks. Totowa: Humana Press, 2005. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3963] [Cited by in RCA: 4256] [Article Influence: 212.8] [Reference Citation Analysis (0)] |
| 109. | Nagpal G, Chaudhary K, Agrawal P, Raghava GPS. Computer-aided prediction of antigen presenting cell modulators for designing peptide-based vaccine adjuvants. J Transl Med. 2018;16:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 110. | Sacherl J, Kosinska AD, Kemter K, Kächele M, Laumen SC, Kerth HA, Öz EA, Wolff LS, Su J, Essbauer S, Sutter G, Scholz M, Singethan K, Altrichter J, Protzer U. Efficient stabilization of therapeutic hepatitis B vaccine components by amino-acid formulation maintains its potential to break immune tolerance. JHEP Rep. 2023;5:100603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 111. | Cocorullo M, Chiarelli LR, Stelitano G. Improving Protection to Prevent Bacterial Infections: Preliminary Applications of Reverse Vaccinology against the Main Cystic Fibrosis Pathogens. Vaccines (Basel). 2023;11:1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 112. | Oli AN, Obialor WO, Ifeanyichukwu MO, Odimegwu DC, Okoyeh JN, Emechebe GO, Adejumo SA, Ibeanu GC. Immunoinformatics and Vaccine Development: An Overview. Immunotargets Ther. 2020;9:13-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 113. | Sukowati CHC, Jayanti S, Turyadi T, Muljono DH, Tiribelli C. Hepatitis B virus genotypes in precision medicine of hepatitis B-related hepatocellular carcinoma: Where we are now. World J Gastrointest Oncol. 2024;16:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 114. | Saha S, Raghava GP. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins. 2006;65:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 1124] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 115. | Jespersen MC, Peters B, Nielsen M, Marcatili P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017;45:W24-W29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 1039] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 116. | Ras-Carmona A, Pelaez-Prestel HF, Lafuente EM, Reche PA. BCEPS: A Web Server to Predict Linear B Cell Epitopes with Enhanced Immunogenicity and Cross-Reactivity. Cells. 2021;10:2744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 117. | Shashkova TI, Umerenkov D, Salnikov M, Strashnov PV, Konstantinova AV, Lebed I, Shcherbinin DN, Asatryan MN, Kardymon OL, Ivanisenko NV. SEMA: Antigen B-cell conformational epitope prediction using deep transfer learning. Front Immunol. 2022;13:960985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 118. | Singh H, Ansari HR, Raghava GP. Improved method for linear B-cell epitope prediction using antigen's primary sequence. PLoS One. 2013;8:e62216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 119. | Sweredoski MJ, Baldi P. COBEpro: a novel system for predicting continuous B-cell epitopes. Protein Eng Des Sel. 2009;22:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 120. | Kumar N, Tripathi S, Sharma N, Patiyal S, Devi NL, Raghava GPS. A method for predicting linear and conformational B-cell epitopes in an antigen from its primary sequence. Comput Biol Med. 2024;170:108083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 121. | Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics. 2007;8:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1096] [Cited by in RCA: 1851] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 122. | Rahman MS, Rahman MK, Saha S, Kaykobad M, Rahman MS. Antigenic: An improved prediction model of protective antigens. Artif Intell Med. 2019;94:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 123. | Magnan CN, Zeller M, Kayala MA, Vigil A, Randall A, Felgner PL, Baldi P. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics. 2010;26:2936-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 124. | Rathore AS, Choudhury S, Arora A, Tijare P, Raghava GPS. ToxinPred 3.0: An improved method for predicting the toxicity of peptides. Comput Biol Med. 2024;179:108926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 38] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 125. | Pan X, Zuallaert J, Wang X, Shen HB, Campos EP, Marushchak DO, De Neve W. ToxDL: deep learning using primary structure and domain embeddings for assessing protein toxicity. Bioinformatics. 2021;36:5159-5168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 126. | Wei L, Ye X, Sakurai T, Mu Z, Wei L. ToxIBTL: prediction of peptide toxicity based on information bottleneck and transfer learning. Bioinformatics. 2022;38:1514-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 127. | Calis JJ, Maybeno M, Greenbaum JA, Weiskopf D, De Silva AD, Sette A, Keşmir C, Peters B. Properties of MHC class I presented peptides that enhance immunogenicity. PLoS Comput Biol. 2013;9:e1003266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 613] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 128. | Wang H, Hao X, He Y, Fan L. AbImmPred: An immunogenicity prediction method for therapeutic antibodies using AntiBERTy-based sequence features. PLoS One. 2024;19:e0296737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 129. | Li G, Iyer B, Prasath VBS, Ni Y, Salomonis N. DeepImmuno: deep learning-empowered prediction and generation of immunogenic peptides for T-cell immunity. Brief Bioinform. 2021;22:bbab160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 130. | Dimitrov I, Naneva L, Doytchinova I, Bangov I. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics. 2014;30:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 131. | Dimitrov I, Bangov I, Flower DR, Doytchinova I. AllerTOP v.2--a server for in silico prediction of allergens. J Mol Model. 2014;20:2278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 799] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 132. | Nguyen MN, Krutz NL, Limviphuvadh V, Lopata AL, Gerberick GF, Maurer-Stroh S. AllerCatPro 2.0: a web server for predicting protein allergenicity potential. Nucleic Acids Res. 2022;50:W36-W43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 133. | Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics. 2006;7:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 376] [Cited by in RCA: 599] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 134. | Garg A, Kumari B, Kumar R, Kumar M. miPepBase: A Database of Experimentally Verified Peptides Involved in Molecular Mimicry. Front Microbiol. 2017;8:2053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Manavalan B, Shin TH, Kim MO, Lee G. AIPpred: Sequence-Based Prediction of Anti-inflammatory Peptides Using Random Forest. Front Pharmacol. 2018;9:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 136. | Khatun MS, Hasan MM, Kurata H. PreAIP: Computational Prediction of Anti-inflammatory Peptides by Integrating Multiple Complementary Features. Front Genet. 2019;10:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 137. | Han J, Kong T, Liu J. PepNet: an interpretable neural network for anti-inflammatory and antimicrobial peptides prediction using a pre-trained protein language model. Commun Biol. 2024;7:1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 138. | Dhanda SK, Vir P, Raghava GP. Designing of interferon-gamma inducing MHC class-II binders. Biol Direct. 2013;8:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 561] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 139. | Manavalan B, Shin TH, Kim MO, Lee G. PIP-EL: A New Ensemble Learning Method for Improved Proinflammatory Peptide Predictions. Front Immunol. 2018;9:1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |