Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.107223
Revised: April 23, 2025
Accepted: June 9, 2025
Published online: July 27, 2025
Processing time: 129 Days and 10.1 Hours
Primary biliary cholangitis (PBC) is a chronic autoimmune cholestatic liver di
Core Tip: Primary biliary cholangitis (PBC) is a chronic, progressive, cholestatic autoimmune liver disease predominantly affecting middle-aged women. Novel therapeutic strategies have emerged in recent years, providing promising treatment options for more refractory disease. This comprehensive review provides an overview of existing and new treatment therapies for PBC.
- Citation: Mitchell NE, Chan SY, Jerez Diaz D, Ansari N, Lee J, Twohig P. Evolving therapeutic landscape of primary biliary cholangitis: A review. World J Hepatol 2025; 17(7): 107223
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/107223.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.107223
Primary biliary cholangitis (PBC) is a chronic and progressive autoimmune cholestatic liver disease with a variable course. If left untreated, it is associated with a high risk of progression to cirrhosis and other related complications and may ultimately require liver transplantation.
PBC is thought to be secondary to a combination of predisposing genetic and environmental triggers, most commonly affecting women in their fifth and sixth decade of life. Clinically, it can be asymptomatic or present with a multitude of symptoms, which most commonly include fatigue and pruritis. The serologic hallmark of PBC is a positive anti-mitochondrial antibody (AMA), which is present in approximately 90%-95% of patients with PBC[1].
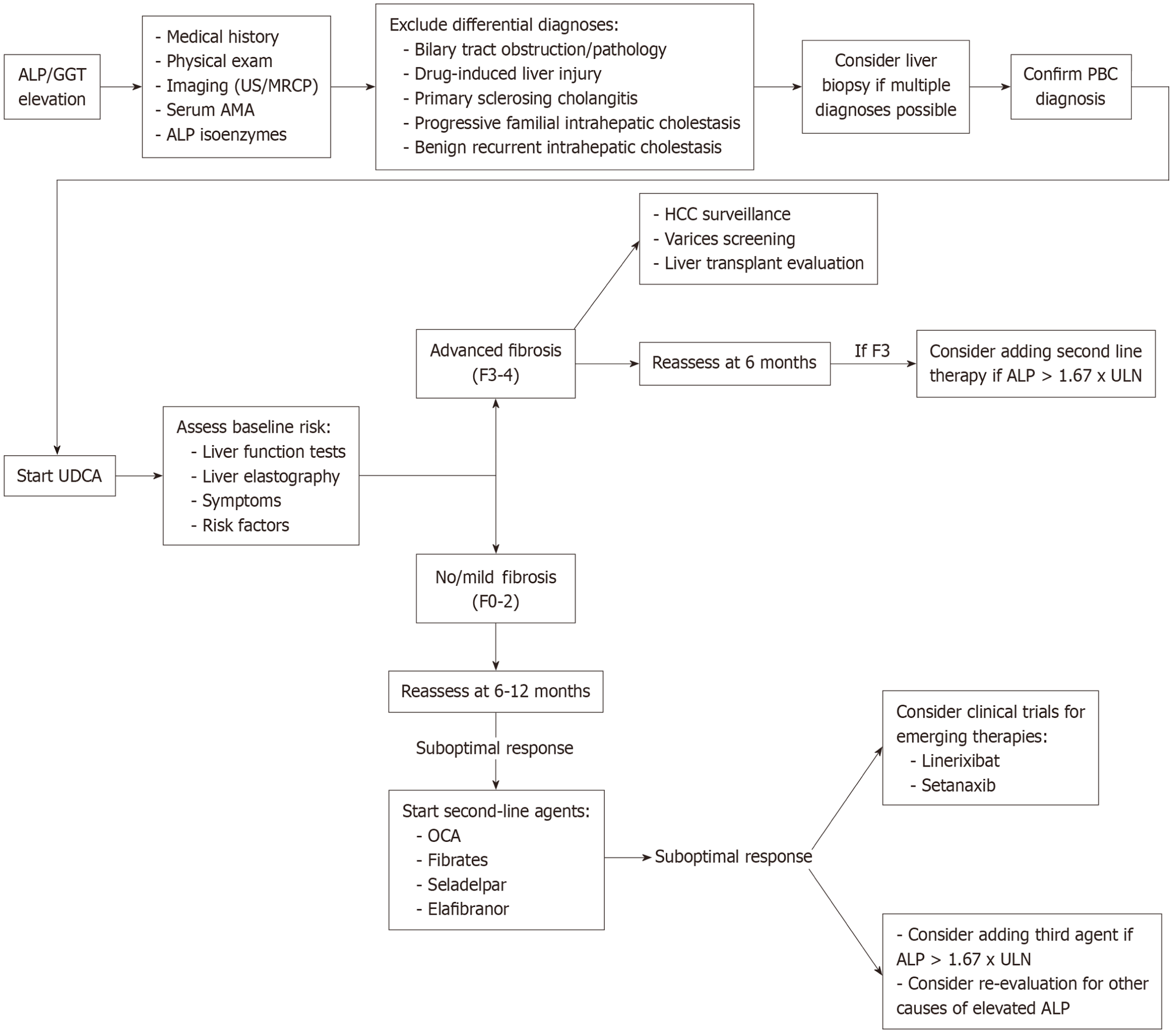
A dysbiosis of the intestinal microbiome has increasingly been recognized as a potential pathogenic factor for development of the disease. Cholangiocytes, the cells lining bile ducts, are proposed to be the main target of an aberrant immune response, which leads to impaired bile duct function and disease progression. Bile acids have also been recognized as playing a critical role in disease development. Therefore, bile acid-based therapies, including ursodeoxycholic acid (UDCA) and obeticholic acid (OCA), have been the cornerstones of treatment for PBC[2]. However, several novel therapeutic drugs have recently been developed, including elafibranor and seladelpar. Here, we will review the role of bile-acid based therapies, recently approved medications by the United States Food and Drug Administration (FDA) and discuss novel therapeutic targets and strategies in the treatment of PBC. Table 1 provides a comprehensive overview of pharmacologic agents for PBC, including dosing, mechanism of action, adverse effects and major contraindications. Figure 1 proposes a current treatment paradigm for PBC.
| Drug | Typical dose | Mechanism of action | Key clinical effects | Contraindications/major cautions | Notable adverse effects |
| Obeticholic acid | 5-10 mg once daily (dose adjustments may be required in cirrhosis) | Farnesoid X receptor agonist; decreases bile acid synthesis and promotes bile flow | Improves ALP and other liver enzymes; May reduce fibrosis progression; Used if inadequate response or intolerance to UDCA | Decompensated cirrhosis (Child-Pugh B or C) without appropriate dose adjustment may precipitate hepatic decompensation; Complete biliary obstruction Exercise caution in severe renal impairment | Pruritus (often significant); Fatigue; Hepatic decompensation in advanced cirrhosis; May decrease HDL cholesterol |
| Bezafibrate | 400 mg/day | Primarily a peroxisome proliferator–activated receptor alpha (PPAR-α) agonist (with some activity on PPAR-δ/γ); reduces triglycerides and may enhance bile acid transporter function | Improves LFTs; May alleviate pruritus in certain patients; Enhances GLOBE and UK-PBC scores when added to UDCA | Significant renal impairment: Fibrates generally require dose adjustment or avoidance; Possible impact on gallbladder disease risk (due to changes in biliary lipid composition); Monitor for interactions with statins (risk of myopathy) | Elevated creatinine or creatine kinase levels (myopathy risk, especially with concomitant statin therapy); Gastrointestinal disturbances; Possible gallstone formation |
| Fenofibrate | 100-200 mg/day | PPAR-α agonist; reduces triglyceride, hepatic inflammation and cholestasis | Lowers ALP, GGT, AST, ALT, bilirubin; Potentially decreases disease progression in UDCA nonresponders; May offer survival benefits when used in combination with UDCA | Severe renal impairment requires dose adjustment or avoidance; Active liver disease outside the context of PBC may be exacerbated; Concomitant statin therapy increases risk of myopathy/rhabdomyolysis (use caution) | Gastrointestinal upset; Possible myopathy (especially when combined with statins); Transient elevations in liver enzymes |
| Seladelpar | 2-10 mg once daily | PPAR-δ agonist; modulates bile acid metabolism and inflammation, potentially reduces IL-31 Levels (thereby alleviating pruritus) | Substantial reduction in ALP, transaminases, and bilirubin; Demonstrated efficacy in improving pruritus; Favorable safety profile in multiple Phase II/III clinical trials | Not yet widely approved in all jurisdictions; Use with caution in advanced cirrhosis until further data are available | Generally well tolerated; Transient aminotransferase elevations reported at higher doses in early studies; Few drug–drug interactions reported to date |
| Elafibranor | 80 mg once daily | Dual PPAR-α/δ agonist; reduces ALP, mitigates inflammation, and may beneficially influence lipid metabolism | Lowers ALP, GGT, CRP, IgM; May reduce progression in UDCA-insufficient responders; Potential improvement in pruritus | Decompensated cirrhosis (contraindicated); Use caution in hepatic impairment; Monitor for potential drug–drug interactions (especially those affecting lipid pathways) | Mild gastrointestinal symptoms (most common); Transient CPK elevations; Rare interactions with statins; Generally well tolerated in clinical trials |
| Linerixibat | Investigational doses: 20-180 mg once daily; 40-90 mg twice daily | Apical Sodium-Dependent Bile Acid Transporter inhibitor; disrupts enterohepatic circulation of bile acids to alleviate pruritus | May reduce pruritus severity in moderate-to-severe cases; Decreases total serum bile acids and fibroblast growth factor 19 (FGF-19), increases 7α-hydroxy-4-cholesten-3-one (C4); Potential improvement in sleep quality if pruritus improves | Investigational; no formal contraindications established yet; Higher doses often cause gastrointestinal adverse events; Caution in patients with chronic diarrhea or malabsorption | Diarrhea (up to approximately 38%–68% with higher doses); Abdominal pain; Study discontinuations often due to gastrointestinal intolerance at higher doses |
| Setanaxib | 400 mg once or twice daily (investigational) | Selective NOX 1/4 inhibitor; attenuates NADPH oxidase–mediated reactive oxygen species (ROS) generation, potentially reversing cholestatic fibrosis and inflammation | May reduce GGT and ALP; May improve fatigue (PBC-40 scores); Anti-fibrotic potential | Investigational; limited data regarding formal contraindications; Should be used with caution in advanced cirrhosis | Gastrointestinal disturbances; Further safety and efficacy data pending |
First-line treatment for PBC is UDCA; it is recommended for a total daily dose of 13 to 15 mg/kg in divided doses. It was first proposed as a novel therapeutic modality in 1987. A multicenter, controlled trial in 1991 investigated ursodiol demonstrating efficacy and safety in the treatment of PBC[3]. More recent studies have suggested that UDCA response is associated with significant reductions in rates of liver-related death or liver transplantation by preventing progression to cirrhosis[4]. UDCA is proposed to work by reducing hepatocyte damage caused by accumulation of hydrophobic bile acids, though the exact mechanism of action in PBC is not entirely clear[5].
Treatment response is primarily biochemical, with serum alkaline phosphatase (ALP) and total bilirubin used as the primary markers[6]. The recommended time frame to monitor these laboratory values is monthly for three months then every six months thereafter. Most patients are expected to note improvement in these liver tests within a few weeks, and most improvement usually occurs within the first six to nine months. A variety of published quantitative criteria exist to assess an adequate biochemical response to UDCA, including the Rochester, Barcelona, and Paris-I/II criteria. The majority suggest a goal ALP ≤ 1.5 to 2 times the upper limit of normal and a total bilirubin goal of around 1.0 mg/dL after 6 to 12 months of therapy[7]. Some studies have suggested that use of albumin and bilirubin levels may provide a more accurate prognosis compared to ALP[8]. Long-term, UDCA has been associated with reduction in the risk of developing varices and serum low-density lipoprotein (LDL) levels. However, it is important to inform patients that UDCA is not known to improve symptoms associated with PBC, specifically fatigue, pruritus, and progression of associated bone disease[7]. Patients with PBC who are treated with UDCA early in their disease course have an excellent prognosis. Adverse events because of insufficient treatment response to medical therapy include advanced fibrosis, cirrhosis and its complications, such as esophageal varices and ascites, and ultimately, liver-related death. For patients who are diagnosed later in their disease course, the emergence of non-invasive methods, such as elastography, have replaced more invasive assessments of fibrosis, such as liver biopsy and/or direct measurement of the hepatic venous pressure gradient to help assess progression and guide further management. Liver stiffness greater than 9.6 kPa on elastography was found to be associated with a 5-fold increased risk of progression to cirrhosis and liver-related death[9].
For patients exhibiting an inadequate response to UDCA, defined as persistently elevated ALP levels above the upper limit of normal after one year of UDCA therapy in the absence of cirrhosis, combination therapy is recommended[10]. This typically involves continuing UDCA while adding a second agent, such as OCA or a peroxisome proliferator-activated receptor (PPAR) agonist[11]. OCA was approved by the FDA in May 2016 for use in combination with UDCA in patients with PBC who have shown an inadequate response, or as monotherapy in patients who are unable to tolerate UDCA[7]. Approximately 3% to 5% of patients are intolerant of UDCA, often due to adverse effects such as persistent diarrhea, pruritus, or other gastrointestinal symptoms that make continued use difficult[12].
OCA is a synthetic derivative of chenodeoxycholic acid, a primary human bile acid, and functions as a potent ligand for the farnesoid X receptor (FXR), a nuclear receptor critical for bile acid homeostasis. FXR serves as a key transcriptional regulator of bile acid metabolism and is predominantly expressed in the liver and intestine[13]. The signaling cascade inducer by OCA inhibits cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid synthesis, thereby modulating bile acid homeostasis and reducing hepatic bile acid production[11].
The optimal therapeutic dose of OCA for PBC appears to be 10 mg daily. According to the FDA approved drug label, OCA treatment is initiated at a dose of 5 mg daily and may be titrated to 10 mg daily after three months if well tolerated. A biochemical response to OCA, typically observed within six months of therapy in combination with UDCA, is defined as an ALP level ≤ 1.67 times the upper limit of normal[10,14].
Randomized clinical trials have demonstrated that OCA at a 10 mg dose significantly reduces ALP levels in patients with PBC, with a median decrease of 53.9% (Q1: -62.5, -Q3: 29.3) compared to placebo, which exhibited minimal change
Long-term cohort studies have also provided evidence supporting OCA’s biochemical efficacy. In patients with PBC, ALP concentrations were significantly reduced from baseline at multiple time points: 12 months [-105.2 U/L (SD 87.6)], 24 months [-101.0 U/L (98.5)], 36 months [-108.6 U/L (95.7)], and 48 months [-95.6 U/L (121.1); P < 0.0001]. Additionally, total bilirubin with significant reductions were also observed at 12 months [-0.9 μmol/L (SD 4.1); P = 0.0042] and 48 months [-0.8 μmol/L (3.8); P = 0.016][16].
Furthermore, a propensity score-matched analysis compared 209 OCA-treated patients with untreated individuals from two disease registries (Global PBC, n = 1381; UK-PBC, n = 2135) over a six-year follow-up period[17]. OCA use was associated with a significantly lower risk of mortality or liver transplantation compared to no OCA treatment (HR: 0.29, 95%CI: 0.10–0.83 and HR: 0.30, 95%CI: 0.12-0.75, respectively). These findings support the potential benefits of long-term OCA therapy in improving clinical outcomes in PBC patients.
The FDA has updated safety guidance on OCA for treating PBC[17]. OCA is contraindicated in patients with de
The latest safety update emphasizes the need for close monitoring of PBC patients without cirrhosis who are receiving OCA. Liver biochemistries should be assessed every three months, and clinical signs of liver decompensation, such as jaundice, should be closely monitored, given the reported cases of liver injury in patients without cirrhosis[18].
Additionally, pruritus is a well-documented, dose-dependent adverse effect of OCA therapy. A double-blind, placebo-controlled, 12-month follow-up randomized controlled trial demonstrated a higher incidence of pruritus among patients receiving OCA compared to placebo (56% in the 5-10 mg group, 68% in the 10 mg group, and 38% in the placebo group)[10]. However, despite its prevalence, treatment discontinuation due to pruritus remains below 10% at recommended therapeutic doses[19]. The pruritus associated with OCA typically resolves over time and can be effectively managed with bile acid sequestrants such as cholestyramine or with rifampicin. For patients with a history of pruritus, optimizing pruritus management before initiating OCA therapy is recommended, and dose titration at the start of treatment may enhance tolerability[20].
For managing severe pruritus in patients with PBC, cholestyramine is the first-line treatment recommended by the American Association for the Study of Liver Diseases (AASLD). The initial dose is 4 g per day, titratable up to 16 g daily based on response and tolerance. It should be taken at least 1 hour after or 4 hours before other medications to prevent interference with their absorption. Some patients may prefer colestipol, an alternative available in tablet form[21]. For those who do not respond to or cannot tolerate cholestyramine, rifampicin is a recommended second-line option. The AASLD advises starting at 150 mg twice daily, increasing to 300 mg twice daily if needed. Due to potential risks, such as hepatotoxicity, hemolytic anemia, renal failure, and thrombotic thrombocytopenic purpura, liver biochemistries should be closely monitored. Rifampicin is contraindicated in patients with bilirubin levels above 2.5 mg/dL[21]. Additionally, sedating antihistamines such as diphenhydramine and hydroxyzine may be helpful for managing sleep disturbances associated with pruritus.
PPAR agonists, including fibrates, seladelpar, and elafibranor, have shown potential as adjunctive therapies for PBC by modulating bile acid synthesis, metabolism, and transport. Their anti-cholestatic effects are enhanced through crosstalk with FXR and pregnane X receptor, while downregulation of nuclear factor-kappa B (NF-κB) contributes to their anti-inflammatory properties[22,23].
Fenofibrate (PPAR-α agonist) and bezafibrate (pan-PPAR agonist) have demonstrated efficacy in improving liver biochemistries, particularly in patients with incomplete responses to UDCA[24,25]. Although primarily approved for lipid-lowering, fibrates have long been recognized for reducing biochemical markers of cholestasis, such as ALP and GGT[26]. Adding fibrates to UDCA therapy may offer other clinical benefits as well, particularly in pruritus and fatigue relief, as itching recurrence or worsening has been reported after bezafibrate discontinuation[25].
However, in the United States, bezafibrate is unavailable, and fenofibrate use in PBC remains off-label. Fibrates are contraindicated in decompensated cirrhosis due to safety concerns. Adverse effects of fibrates include myalgia, reversible elevated creatinine, hepatotoxicity, and an increased risk of rhabdomyolysis[27,28]. Drug interactions primarily involve CYP3A4 metabolism. Fibrates may potentiate warfarin effects, necessitating dose adjustments, and increase statin toxicity, particularly with lipophilic statins (atorvastatin, lovastatin, simvastatin)[29]. Additionally, fenofibrate may enhance calcineurin inhibitor nephrotoxicity, requiring renal function monitoring, and concurrent use with colchicine elevates rhabdomyolysis risk.
Bezafibrate has demonstrated efficacy in improving biochemical responses in patients with PBC. The BEZURSO trial, a randomized, placebo-controlled study, evaluated bezafibrate (400 mg/day) as an add-on to UDCA in 100 patients with an incomplete response to UDCA based on Paris-II criteria. After two years of treatment, the primary endpoint—complete normalization of ALP, aspartate transaminase (AST), ALT, total bilirubin, albumin, and prothrombin time, was achieved in 30% of patients on bezafibrate, while none in the placebo group met this criterion. Additionally, 67% of bezafibrate-treated patients achieved ALP normalization, with improvements in liver stiffness and pruritus reduction[28].
Long-term cohort studies further suggest that adding bezafibrate to UDCA therapy not only enhances GLOBE and UK-PBC scores but also improves long-term prognosis, particularly in early-stage PBC[30,31]. A Japanese nationwide retrospective cohort study further supports these findings, demonstrating that UDCA-bezafibrate combination therapy was associated with a significantly reduced risk of all-cause mortality (adjusted HR: 0.325, 95%CI: 0.19-0.55) and liver-related mortality or need for liver transplantation (adjusted HR: 0.275, 95%CI: 0.13) compared to UDCA monotherapy[31].
Clinical studies have identified bezafibrate as an effective agent for managing pruritus in PBC. In the double-blind, randomized, placebo-controlled FITCH trial, 45% of patients treated with bezafibrate achieved a ≥ 50% reduction in moderate to severe pruritus, compared to only 11% in the placebo group (P = 0.003). Bezafibrate also significantly reduced pruritus intensity measured by the visual analog scale, both in the morning (P = 0.01 vs placebo) and evening
A randomized controlled trial assessed the efficacy of fenofibrate (200 mg/day) in combination with UDCA in PBC patients with an incomplete UDCA response. After 12 months, 20.8% of patients receiving combination therapy achieved normalization of ALP, GGT, and total bilirubin, whereas none in the UDCA monotherapy group met this primary outcome (absolute difference: 20.8 percentage points; 95%CI: 4.6-37.0)[34]. Real-world evidence also supported triple therapy with UDCA, OCA, and fibrates (bezafibrate or fenofibrate), demonstrating significant reductions in GGT, ALT, AST, and total bilirubin, alongside pruritus improvement in difficult-to-treat PBC[26].
A meta-analysis of six non-randomized studies involving 102 PBC patients with incomplete UDCA response further demonstrated significant biochemical improvements with fenofibrate (100–200 mg/day) as adjunct therapy, with 69% of patients achieving a biochemical response (≥ 40% ALP reduction or normalization) with UDCA plus fenofibrate (OR: 82.8, 95%CI: 21.6-317.2; P = 0.024)[24]. Additionally, a propensity-score-matching multicenter retrospective study of 120 PBC patients found that fenofibrate addition was associated with a reduced risk of mortality, hepatic decompensation, or liver transplantation (adjusted HR: 0.40, 95%CI: 0.17-0.93)[35]. These findings suggest that fenofibrate may offer both biochemical and survival benefits when used as an adjunct to UDCA in PBC management.
Tropifexor (TXR, also known as LJN452) is a potent non–bile acid FXR agonist under investigation for chronic liver diseases, including PBC and MASH[36]. A phase 2, double-blind, placebo-controlled trial (NCT02516605) evaluated its safety, tolerability, and efficacy as a second-line therapy in PBC patients with an inadequate response to UDCA[37].
In this study, among 61 patients enrolled, 11, 9, 12, and 8 patients received 30-, 60-, 90-, and 150-μg doses of tropifexor, respectively, while 21 patients received placebo. Three patients in the 150-μg group discontinued due to adverse events. Pruritus was the most common adverse event, occurring in 52.5% of tropifexor-treated patients vs 28.6% in the placebo group. By day 28, tropifexor achieved a 26%-72% reduction in GGT at doses of 30-150 μg (P < 0.001 at 60-150 μg vs placebo), with no significant difference in quality-of-life scores. Tropifexor was generally well tolerated with im
Seladelpar (MBX-8025) is a PPAR-δ agonist that has emerged as a promising therapy for patients with PBC, particularly those who do not respond adequately to other treatments. Through selective activation of PPAR-δ—a nuclear receptor expressed in hepatocytes, cholangiocytes, Kupffer cells, and stellate cells—it modulates bile acid metabolism and inflammation[38,39].
One notable benefit of seladelpar is its capacity to alleviate pruritus, a common and distressing symptom for many patients with PBC. In contrast to OCA, which may exacerbate pruritus, seladelpar has demonstrated substantial reductions in pruritus severity—an effect potentially linked to its ability to lower interleukin-31 (IL-31) levels[40].
Multiple clinical trials have highlighted seladelpar’s therapeutic promise. These studies document sustained reductions in ALP, ALT, AST, total bilirubin, GGT, and immunoglobulin M (IgM), accompanied by improvements in pruritus, sleep quality, and fatigue. Although an early Phase II trial (NCT02609048) exploring higher doses (50 mg and 200 mg daily) was discontinued due to transient aminotransferase elevations[41], subsequent research using lower doses (2-10 mg daily) has confirmed seladelpar’s efficacy and safety[42,43]. Seladelpar does not have many drug-drug interactions, unlike other PPAR agonists which often interact with CYP metabolized drugs, which makes it an advantageous option in patients at higher risk for polypharmacy.
Phase III trials have further demonstrated seladelpar’s clinical utility. In the ENHANCE study (NCT03602560), 78% of patients receiving 10 mg daily met the primary endpoint (ALP < 1.67 × ULN, ≥ 15% ALP reduction, and normal total bilirubin), compared with 12.5% in the placebo group. Although discontinued early because of an unrelated histologic finding in a concurrent nonalcoholic steatohepatitis (NASH) trial, the results strongly support seladelpar’s potential advantages[43]. Likewise, the RESPONSE trial (NCT04620733) revealed that 61.7% of participants attained the primary endpoint at 12 months, with 25% achieving complete ALP normalization[44].
Additional studies are assessing seladelpar’s long-term safety and effectiveness. The ASSURE trial (NCT03301506) is an open-label investigation compiling comprehensive long-term data. A Phase I study (NCT04950764) is examining the tolerability of a 10 mg dose in patients with compensated cirrhosis and hepatic impairment, while the AFFIRM trial (NCT06051617), a randomized, double-blind, placebo-controlled Phase III study, continues to explore seladelpar’s effects on clinical outcomes in patients with PBC and compensated cirrhosis[44,45].
Importantly, seladelpar is not recommended for patients with decompensated liver cirrhosis. This recommendation is based on safety considerations and the lack of established efficacy in this patient population. Most of the existing studies, including the AFFIRM trial, specifically include only individuals with compensated cirrhosis.
With its capacity to enhance biochemical markers, reduce pruritus, and maintain a positive safety profile, seladelpar appears to be a promising option for PBC. As ongoing research further clarifies its role, seladelpar may become an integral treatment for a broader population, including those with more advanced liver disease.
Elafibranor is a dual PPAR-α/δ agonist recently approved by the FDA as a second-line therapy for PBC in patients who cannot tolerate or have an insufficient response to UDCA. It may be administered alongside UDCA or, in specific circumstances, as monotherapy, provided the patient does not have decompensated cirrhosis. Most clinical studies have examined elafibranor at a dose of 80 mg per day, based on its demonstrated efficacy and safety profile[46].
Evidence of elafibranor’s utility in PBC initially came from a phase II international, randomized, double-blind, placebo-controlled trial (NCT03124108) in non-cirrhotic patients with an inadequate response to UDCA. After 12 weeks, elafibranor significantly reduced ALP levels and improved additional markers of hepatic injury and inflammation [GGT, high-sensitivity C-reactive protein (hsCRP), and IgM]. Notably, it did not worsen pruritus, and no serious safety issues arose[47].
Building on these findings, the ongoing phase III ELATIVE trial (NCT04526665) is assessing elafibranor in patients with suboptimal UDCA response or unacceptable side effects. Interim data indicate that 51% of participants receiving elafibranor achieved a predefined biochemical response at 52 weeks—versus 4% in the placebo arm—along with higher rates of ALP normalization. Moreover, elafibranor produced beneficial changes in triglycerides and VLDL, while maintaining stable LDL and HDL. Improvements in moderate-to-severe pruritus have also been noted. The most frequently reported adverse events were mild gastrointestinal symptoms, transient elevations in creatine phosphokinase, and rare statin-related interactions, with no marked renal impairment detected[48].
Future research includes an open-label extension of ELATIVE and a confirmatory phase III trial, ELFIDENCE (NCT06016842), both designed to examine elafibranor’s long-term impact on liver biochemistry and clinical outcomes, including fibrosis progression and the likelihood of cirrhosis or liver transplantation[48]. Overall, current evidence suggests that elafibranor may represent a promising and well-tolerated treatment for patients with PBC, particularly those who remain above treatment targets with UDCA alone.
Another area that has garnered attention is apical sodium-dependent bile acid transporters (ASBT). ASBT inhibitors disrupt the enterohepatic circulation of bile acids and therefore, provide anti-cholestatic effects[11,49,50].
The GLIMMER trial, a Phase 2b, multicenter, randomized, parallel-group study, evaluated linerixibat (GSK2330672), an ASBT inhibitor, as a therapy for patients with PBC and moderate-to-severe pruritus (> 4 on a 0-10 numerical rating scale[49]. In this study, 147 subjects after 4 weeks of placebo, were randomized to linerixibat vs placebo for 12 weeks, followed by a single blind placebo, with primary end point to evaluate dose related change in mean worst daily itch (MWDI) score. Linerixibat doses evaluated were 20 mg daily, 90 mg daily, 180 mg daily, 40 mg twice daily and 90 mg twice daily. Linerixibat’s effect on pruritus was not significantly different compared to placebo in the primary intention to treat analysis but was noted to be associated with a significant dose-dependent reduction in pruritus in the per protocol population[49].
The most common adverse effect was diarrhea (11% for placebo vs 38%-68% for linerixibat) and abdominal pain (8% for placebo vs 9%-30% for linerixibat), mostly observed in patients taking higher doses of linerixibat. Limitations of this study include only quarter of the study population had only mild pruritus symptoms. Therefore, a full assessment in pruritus reduction may have been limited[49].
This study demonstrates the benefit of ASBT inhibitors as a promising therapy for the treatment of pruritus in PBC patients; however, further studies are needed to determine optimal dosing for MWDI reduction without adverse effects that would limit use of the medication.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) are a family of enzymes that produce reactive oxygen species (ROS) and are currently under investigation as adjuvant therapy for PBC[11,51]. These enzymes are thought to play a role in the development of PBC through the generation of ROS that can damage liver cells, thereby contributing to inflammation and fibrosis. A selective NOX 1/4 inhibitor, setanaxib, has been investigated in a phase 2 intention to treat trial. In this study, patients were administered setanaxib (400 mg daily or 400 mg twice daily) in addition to UDCA vs placebo with primary endpoint of percentage change in serum GGT[51]. GGT was used as a surrogate marker for inflammation, theoretically reflect the anti-inflammatory and anti-fibrotic properties of setanaxib[51].
The primary endpoint was not met with a statistically significant percentage change in serum GGT, although a larger reduction in GGT was noted in the setanaxib group when compared to placebo[51]. Additionally, the change in ALP from baseline to Week 24 was close to significant for the 400 mg twice daily group compared to placebo, demonstrating potential anti-cholestatic efficacy. When these findings were stratified by liver stiffness, the largest reductions in ALP and GGT were in patients with LSM > 9.6 kPa who were treated with setanaxib 400 mg twice daily[11,51]. In a post-hoc analysis, patients in the setanaxib 400 mg twice daily dosing demonstrated improvement in PBC-40 scores, particularly fatigue[52].
In general, the role of immunosuppression in PBC has always been rather controversial and little effectiveness has been demonstrated to date. Corticosteroids have generally been avoided due to systemic side effects and are used primarily in cases of PBC-autoimmune hepatitis overlap[53]. A placebo-controlled Phase 3 trial of budesonide in non-cirrhotic patients with PBC demonstrated a lack of improvement of hepatic inflammatory activity[11].
While the etiology of PBC has never fully been elucidated, evidence suggests that autoimmune reactions that target intrahepatic biliary epithelial cells play a role. The target antigen of AMA is a 2-oxo-acid dehydrogenase complex, which includes pyruvate dehydrogenase complex-E2 (PDC-E2). This has been explored as a potential target for immunomodulatory therapy for patients with AMA-positive PBC through engineered regulatory T-cell therapy[54].
Additionally, other ongoing trials have demonstrated PDC-E2 as an optimal target for therapy, such as utilizing an encapsulated PDC-E2 antigen to induce tolerance and reprogram the immune system (NCT05104853). While additional studies are needed, this ongoing novel research explores a promising new area for PBC therapies[11].
As previously mentioned, the hallmark of PBC is typically the presence of AMA antibodies, although 5% to 10% of patients are AMA-negative[55]. Compared to individuals with AMA-positive PBC, those with AMA-negative disease have similar rates of treatment response to UDCA. Similar adjusted rates for development of cirrhosis, including decompensated disease, as well as development of HCC or death from liver disease are similar between both patient populations[55]. Further research is needed to elucidate response to treatment between the two groups for the newer therapeutic options.
Patients who undergo liver transplantation for PBC still have a risk of recurrent primary biliary cirrhosis with recurrent rates ranging between 11% to 35%[56]. Short-term and long-term survival rates are excellent with one-year survival rates around 90%-95% and 5-year survival rates greater than 85%[57], UDCA used prophylactically in post-liver transplant patients has been associated with reduced recurrent PBC, graft loss, liver-related death, and all-cause mortality. The combination of cyclosporine in UDCA was associated with an even lower risk of both recurrent PBC and mortality[57].
While pregnancy generally is well-tolerated in PBC, there is a risk of preterm birth[58]. UDCA has been shown to reduce the likelihood of spontaneous preterm birth; there is likely protection for the fetus as well as benefit for the mother with PBC. UDCA is safe in pregnancy; however, up to 40% of patients do not have a biochemical response, although a normal physiologic rise in the ALP with pregnancy must be taken into account when assessing this[58]. Bezafibrate can be added to the administration of UDCA after the first trimester if the benefits outweigh the risks of fibrate administration[58]. While UDCA is safe during pregnancy, further data needs to be obtained for newer therapies in pregnancy. Patients with PBC who are pregnant should have an individualized approach to management.
PBC is a well-recognized cause of chronic, progressive, cholestatic liver disease. Figure 1 proposes a current treatment paradigm for PBC. UDCA continues to remain the cornerstone of first-line treatment choice for PBC. In general, all patients should be initiated on UDCA therapy at a dose of 13-15 mg/kg/day with laboratory re-assessment at every 3-6 months. It is well known that liver fibrosis at the start of therapy is a risk factor for progression of disease independent of treatment response. Those patients who have advanced fibrosis or cirrhosis with a complete response to UDCA have a reduced transplant-free survival compared to those without advanced fibrosis or cirrhosis and an incomplete response to therapy[11]. Therefore, we additionally advocate for fibrosis staging at the start of treatment initiation with non-invasive assessment such as LSM if available at an institution.
Inadequate response to UDCA (based on a number of available response criteria) at 12 months should prompt initiation of second-line therapy, including OCA or PPAR agonists, including fibrates, seladelpar or elafibranor. As outlined previously, the choice of second-line therapy initiation should be guided by the presence of other clinical symptoms like pruritis, degree of liver fibrosis, including cirrhosis, as well as drug availability and cost. If there is an adequate response after 6-12, we would advocate for patient enrollment in a trial exploring a novel therapy.
While UDCA currently remains the first-line therapy for PBC, the treatment paradigm continues to expand. Incorporation of early elastography, along with the development of predictive risk models, will hopefully enable providers to identify incomplete responders who may potentially benefit from combination therapy. While the role of early combination therapy, particularly for these patients with high-risk disease features, is an unmet question, ongoing investigations will hopefully elucidate this in coming years. Additionally, there are a number of investigational drugs currently under development, including immunotherapies, which will hopefully enable an even more personalized approach to patient care in the future.
| 1. | Trivella J, John BV, Levy C. Primary biliary cholangitis: Epidemiology, prognosis, and treatment. Hepatol Commun. 2023;7:e0179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 2. | Fiorucci S, Urbani G, Di Giorgio C, Biagioli M, Distrutti E. Current Landscape and Evolving Therapies for Primary Biliary Cholangitis. Cells. 2024;13:1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 549] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | John BV, Khakoo NS, Schwartz KB, Aitchenson G, Levy C, Dahman B, Deng Y, Goldberg DS, Martin P, Kaplan DE, Taddei TH. Ursodeoxycholic Acid Response Is Associated With Reduced Mortality in Primary Biliary Cholangitis With Compensated Cirrhosis. Am J Gastroenterol. 2021;116:1913-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 322] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, Ponsioen CY, Floreani A, Corpechot C, Mayo MJ, Battezzati PM, Parés A, Nevens F, Burroughs AK, Kowdley KV, Trivedi PJ, Kumagi T, Cheung A, Lleo A, Imam MH, Boonstra K, Cazzagon N, Franceschet I, Poupon R, Caballeria L, Pieri G, Kanwar PS, Lindor KD, Hansen BE; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-49.e5; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 344] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 7. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 8. | Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR; Dutch PBC Study Group. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 909] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 10. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 801] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 11. | Levy C, Manns M, Hirschfield G. New Treatment Paradigms in Primary Biliary Cholangitis. Clin Gastroenterol Hepatol. 2023;21:2076-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 12. | Invernizzi P, Floreani A, Carbone M, Marzioni M, Craxi A, Muratori L, Vespasiani Gentilucci U, Gardini I, Gasbarrini A, Kruger P, Mennini FS, Ronco V, Lanati E, Canonico PL, Alvaro D. Primary Biliary Cholangitis: advances in management and treatment of the disease. Dig Liver Dis. 2017;49:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569-3572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 612] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 14. | Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, Vincent C, Rust C, Parés A, Mason A, Marschall HU, Shapiro D, Adorini L, Sciacca C, Beecher-Jones T, Böhm O, Pencek R, Jones D; Obeticholic Acid PBC Monotherapy Study Group. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 15. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-61.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 16. | Trauner M, Nevens F, Shiffman ML, Drenth JPH, Bowlus CL, Vargas V, Andreone P, Hirschfield GM, Pencek R, Malecha ES, MacConell L, Shapiro D. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 17. | Murillo Perez CF, Fisher H, Hiu S, Kareithi D, Adekunle F, Mayne T, Malecha E, Ness E, van der Meer AJ, Lammers WJ, Trivedi PJ, Battezzati PM, Nevens F, Kowdley KV, Bruns T, Cazzagon N, Floreani A, Mason AL, Parés A, Londoño MC, Invernizzi P, Carbone M, Lleo A, Mayo MJ, Dalekos GN, Gatselis NK, Thorburn D, Verhelst X, Gulamhusein A, Janssen HLA, Smith R, Flack S, Mulcahy V, Trauner M, Bowlus CL, Lindor KD, Corpechot C, Jones D, Mells G, Hirschfield GM, Wason J, Hansen BE; GLOBAL PBC Study Group and the members of the UK-PBC Consortium. Greater Transplant-Free Survival in Patients Receiving Obeticholic Acid for Primary Biliary Cholangitis in a Clinical Trial Setting Compared to Real-World External Controls. Gastroenterology. 2022;163:1630-1642.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 18. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology. 2022;75:1012-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Leung KK, Deeb M, Hirschfield GM. Review article: pathophysiology and management of primary biliary cholangitis. Aliment Pharmacol Ther. 2020;52:1150-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Tanaka A, Ma X, Takahashi A, Vierling JM. Primary biliary cholangitis. Lancet. 2024;404:1053-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 21. | Rogal SS, Hansen L, Patel A, Ufere NN, Verma M, Woodrell CD, Kanwal F. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022;76:819-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 22. | Gallucci GM, Alsuwayt B, Auclair AM, Boyer JL, Assis DN, Ghonem NS. Fenofibrate Downregulates NF-κB Signaling to Inhibit Pro-inflammatory Cytokine Secretion in Human THP-1 Macrophages and During Primary Biliary Cholangitis. Inflammation. 2022;45:2570-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Corpechot C. Primary biliary cirrhosis and bile acids. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S13-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Reig A, Sesé P, Parés A. Effects of Bezafibrate on Outcome and Pruritus in Primary Biliary Cholangitis With Suboptimal Ursodeoxycholic Acid Response. Am J Gastroenterol. 2018;113:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Soret PA, Lam L, Carrat F, Smets L, Berg T, Carbone M, Invernizzi P, Leroy V, Trivedi P, Cazzagon N, Weiler-Normann C, Alric L, Rosa-Hezode I, Heurgué A, Cervoni JP, Dumortier J, Potier P, Roux O, Silvain C, Bureau C, Anty R, Larrey D, Levy C, Pares A, Schramm C, Nevens F, Chazouillères O, Corpechot C. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment Pharmacol Ther. 2021;53:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Carrion AF, Lindor KD, Levy C. Safety of fibrates in cholestatic liver diseases. Liver Int. 2021;41:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, Goria O, Potier P, Minello A, Silvain C, Abergel A, Debette-Gratien M, Larrey D, Roux O, Bronowicki JP, Boursier J, de Ledinghen V, Heurgue-Berlot A, Nguyen-Khac E, Zoulim F, Ollivier-Hourmand I, Zarski JP, Nkontchou G, Lemoinne S, Humbert L, Rainteau D, Lefèvre G, de Chaisemartin L, Chollet-Martin S, Gaouar F, Admane FH, Simon T, Poupon R. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018;378:2171-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 29. | Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99:3C-18C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Honda A, Tanaka A, Kaneko T, Komori A, Abe M, Inao M, Namisaki T, Hashimoto N, Kawata K, Takahashi A, Ninomiya M, Kang JH, Arakawa M, Yamagiwa S, Joshita S, Umemura T, Sato K, Kaneko A, Kikuchi K, Itakura J, Nomura T, Kakisaka K, Fujii H, Kawada N, Takikawa Y, Masaki T, Ohira H, Mochida S, Yoshiji H, Iimuro S, Matsuzaki Y, Takikawa H; Japan PBC Study Group. Bezafibrate Improves GLOBE and UK-PBC Scores and Long-Term Outcomes in Patients With Primary Biliary Cholangitis. Hepatology. 2019;70:2035-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | Tanaka A, Hirohara J, Nakano T, Matsumoto K, Chazouillères O, Takikawa H, Hansen BE, Carrat F, Corpechot C. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2021;75:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | de Vries E, Bolier R, Goet J, Parés A, Verbeek J, de Vree M, Drenth J, van Erpecum K, van Nieuwkerk K, van der Heide F, Mostafavi N, Helder J, Ponsioen C, Oude Elferink R, van Buuren H, Beuers U; Netherlands Association for the Study of the Liver-Cholestasis Working Group. Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial. Gastroenterology. 2021;160:734-743.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 33. | Medina-Morales E, Barba Bernal R, Gerger H, Goyes D, Trivedi HD, Ferrigno B, Patwardhan V, Bonder A. Pharmacological Therapy of Pruritus in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Clin Gastroenterol. 2023;57:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Li C, Zheng K, Chen Y, He C, Liu S, Yang Y, Li M, Zeng X, Wang L, Zhang F. A randomized, controlled trial on fenofibrate in primary biliary cholangitis patients with incomplete response to ursodeoxycholic acid. Ther Adv Chronic Dis. 2022;13:20406223221114198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Cheung AC, Lapointe-Shaw L, Kowgier M, Meza-Cardona J, Hirschfield GM, Janssen HL, Feld JJ. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther. 2016;43:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Sanyal AJ, Lopez P, Lawitz EJ, Lucas KJ, Loeffler J, Kim W, Goh GBB, Huang JF, Serra C, Andreone P, Chen YC, Hsia SH, Ratziu V, Aizenberg D, Tobita H, Sheikh AM, Vierling JM, Kim YJ, Hyogo H, Tai D, Goodman Z, Schaefer F, Carbarns IRI, Lamle S, Martic M, Naoumov NV, Brass CA. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med. 2023;29:392-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 37. | Schramm C, Wedemeyer H, Mason A, Hirschfield GM, Levy C, Kowdley KV, Milkiewicz P, Janczewska E, Malova ES, Sanni J, Koo P, Chen J, Choudhury S, Klickstein LB, Badman MK, Jones D. Farnesoid X receptor agonist tropifexor attenuates cholestasis in a randomised trial in patients with primary biliary cholangitis. JHEP Rep. 2022;4:100544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | Kouno T, Liu X, Zhao H, Kisseleva T, Cable EE, Schnabl B. Selective PPARδ agonist seladelpar suppresses bile acid synthesis by reducing hepatocyte CYP7A1 via the fibroblast growth factor 21 signaling pathway. J Biol Chem. 2022;298:102056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 39. | Haczeyni F, Wang H, Barn V, Mridha AR, Yeh MM, Haigh WG, Ioannou GN, Choi YJ, McWherter CA, Teoh NC, Farrell GC. The selective peroxisome proliferator-activated receptor-delta agonist seladelpar reverses nonalcoholic steatohepatitis pathology by abrogating lipotoxicity in diabetic obese mice. Hepatol Commun. 2017;1:663-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Kremer AE, Mayo MJ, Hirschfield GM, Levy C, Bowlus CL, Jones DE, Johnson JD, McWherter CA, Choi YJ. Seladelpar treatment reduces IL-31 and pruritus in patients with primary biliary cholangitis. Hepatology. 2024;80:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, Doerffel Y, Gitlin N, Gordon SC, Odin JA, Sheridan D, Wörns MA, Clark V, Corless L, Hartmann H, Jonas ME, Kremer AE, Mells GF, Buggisch P, Freilich BL, Levy C, Vierling JM, Bernstein DE, Hartleb M, Janczewska E, Rochling F, Shah H, Shiffman ML, Smith JH, Choi YJ, Steinberg A, Varga M, Chera H, Martin R, McWherter CA, Hirschfield GM. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Bowlus CL, Galambos MR, Aspinall RJ, Hirschfield GM, Jones DEJ, Dörffel Y, Gordon SC, Harrison SA, Kremer AE, Mayo MJ, Thuluvath PJ, Levy C, Swain MG, Neff GW, Sheridan DA, Stanca CM, Berg CP, Goel A, Shiffman ML, Vierling JM, Boudes P, Steinberg A, Choi YJ, McWherter CA. A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis. J Hepatol. 2022;77:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 43. | Hirschfield GM, Shiffman ML, Gulamhusein A, Kowdley KV, Vierling JM, Levy C, Kremer AE, Zigmond E, Andreone P, Gordon SC, Bowlus CL, Lawitz EJ, Aspinall RJ, Pratt DS, Raikhelson K, Gonzalez-Huezo MS, Heneghan MA, Jeong SH, Ladrón de Guevara AL, Mayo MJ, Dalekos GN, Drenth JPH, Janczewska E, Leggett BA, Nevens F, Vargas V, Zuckerman E, Corpechot C, Fassio E, Hinrichsen H, Invernizzi P, Trivedi PJ, Forman L, Jones DEJ, Ryder SD, Swain MG, Steinberg A, Boudes PF, Choi YJ, McWherter CA; ENHANCE Study Group*. Seladelpar efficacy and safety at 3 months in patients with primary biliary cholangitis: ENHANCE, a phase 3, randomized, placebo-controlled study. Hepatology. 2023;78:397-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 57] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 44. | Hirschfield GM, Bowlus CL, Mayo MJ, Kremer AE, Vierling JM, Kowdley KV, Levy C, Villamil A, Ladrón de Guevara Cetina AL, Janczewska E, Zigmond E, Jeong SH, Yilmaz Y, Kallis Y, Corpechot C, Buggisch P, Invernizzi P, Londoño Hurtado MC, Bergheanu S, Yang K, Choi YJ, Crittenden DB, McWherter CA; RESPONSE Study Group. A Phase 3 Trial of Seladelpar in Primary Biliary Cholangitis. N Engl J Med. 2024;390:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 45. | Long-Term Efficacy and Safety of Open-Label Seladelpar Treatment in Patients With Primary Biliary Cholangitis: Pooled Interim Results for Up to 3 Years From the ASSURE Study. Gastroenterol Hepatol (NY). 2024;20:8-9. [PubMed] |
| 46. | Ahmed IF, Rizwan F, Mansoor H, Fakhoury M, Shaik MH, Gandhi F, Belletieri C. Elafibranor (Iqirvo) unveiled: a groundbreaking FDA-approved therapy revolutionizing primary biliary cholangitis treatment. Ann Med Surg (Lond). 2024;86:6910-6912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Schattenberg JM, Pares A, Kowdley KV, Heneghan MA, Caldwell S, Pratt D, Bonder A, Hirschfield GM, Levy C, Vierling J, Jones D, Tailleux A, Staels B, Megnien S, Hanf R, Magrez D, Birman P, Luketic V. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021;74:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 48. | Kowdley KV, Bowlus CL, Levy C, Akarca US, Alvares-da-Silva MR, Andreone P, Arrese M, Corpechot C, Francque SM, Heneghan MA, Invernizzi P, Jones D, Kruger FC, Lawitz E, Mayo MJ, Shiffman ML, Swain MG, Valera JM, Vargas V, Vierling JM, Villamil A, Addy C, Dietrich J, Germain JM, Mazain S, Rafailovic D, Taddé B, Miller B, Shu J, Zein CO, Schattenberg JM; ELATIVE Study Investigators’ Group; ELATIVE Study Investigators' Group. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N Engl J Med. 2024;390:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 103] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 49. | Levy C, Kendrick S, Bowlus CL, Tanaka A, Jones D, Kremer AE, Mayo MJ, Haque N, von Maltzahn R, Allinder M, Swift B, McLaughlin MM, Hirschfield GM; GLIMMER Study Group. GLIMMER: A Randomized Phase 2b Dose-Ranging Trial of Linerixibat in Primary Biliary Cholangitis Patients With Pruritus. Clin Gastroenterol Hepatol. 2023;21:1902-1912.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 50. | Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 51. | Invernizzi P, Carbone M, Jones D, Levy C, Little N, Wiesel P, Nevens F; study investigators. Setanaxib, a first-in-class selective NADPH oxidase 1/4 inhibitor for primary biliary cholangitis: A randomized, placebo-controlled, phase 2 trial. Liver Int. 2023;43:1507-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 52. | Jones D, Carbone M, Invernizzi P, Little N, Nevens F, Swain MG, Wiesel P, Levy C. Impact of setanaxib on quality of life outcomes in primary biliary cholangitis in a phase 2 randomized controlled trial. Hepatol Commun. 2023;7:e0057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 53. | Younossi ZM, Bernstein D, Shiffman ML, Kwo P, Kim WR, Kowdley KV, Jacobson IM. Diagnosis and Management of Primary Biliary Cholangitis. Am J Gastroenterol. 2019;114:48-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 54. | Tewari R, Yang SJ, McClain ED, Hu A, Mortensen E, DeSchmidt A, Chen J, Kancharla A, Singh AK, James EA, Burman BE, Siddique A, Rawlings DJ, Patel C, Cerosaletti K, Buckner JH. Identification of a novel PDC-E2 epitope in primary biliary cholangitis: Application for engineered Treg therapy. J Autoimmun. 2024;149:103327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | John BV, Dahman B, Deng Y, Khakoo NS, Taddei TH, Kaplan DE, Levy C. Rates of decompensation, hepatocellular carcinoma and mortality in AMA-negative primary biliary cholangitis cirrhosis. Liver Int. 2022;42:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Silveira MG, Talwalkar JA, Lindor KD, Wiesner RH. Recurrent primary biliary cirrhosis after liver transplantation. Am J Transplant. 2010;10:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Manousou P, Arvaniti V, Tsochatzis E, Isgro G, Jones K, Shirling G, Dhillon AP, O'Beirne J, Patch D, Burroughs AK. Primary biliary cirrhosis after liver transplantation: influence of immunosuppression and human leukocyte antigen locus disparity. Liver Transpl. 2010;16:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Harrington L, Ovadia C, Heneghan M, Williamson C. A new diagnosis of primary biliary cholangitis in pregnancy treated with a combination of ursodeoxycholic acid and bezafibrate. Obstet Med. 2024;1753495X241293324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |