Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106993
Revised: April 27, 2025
Accepted: July 2, 2025
Published online: July 27, 2025
Processing time: 128 Days and 3.4 Hours
Pancreatic cancer has limited treatment options and poor prognosis owing to late diagnosis and aggressive biology. Current therapies include surgery, chemo
A 45-year-old male presented with jaundice in December 2022. Initial investigations revealed a pancreatic head mass and liver metastases; a liver biopsy confirmed moderately differentiated adenocarcinoma. The patient received multimodal therapies, including gemcitabine, albumin-bound paclitaxel, nimotuzumab, and proton radiotherapy, which initially resulted in significant shrinkage of the pancreatic lesion and a reduction in liver metastases. However, the disease eventually progressed, prompting further evaluation at our MTB clinic. Genetic testing revealed a homologous recombination deficiency (HRD) score of 58 (HRD-positive) and a pathogenic BRCA2 mutation (p.T3033fs), sugge
The patient’s clinical course highlights the potential of MTB in providing significant benefits for advanced pancreatic cancer with liver metastases.
Core Tip: We report the case of a 45-year-old male with pancreatic head adenocarcinoma and liver metastases who underwent treatment with gemcitabine, albumin-bound paclitaxel, nimotuzumab, and proton radiotherapy. The tumor subsequently progressed, and the patient visited our molecular tumor boards (MTB) clinic. Repeat genetic testing revealed homologous recombination deficiency positivity (score 58) and a pathogenic BRCA2 mutation (p.T3033fs), prompting a shift to olaparib combined with immunotherapy (tislelizumab and atezolizumab) and hepatic arterial infusion chemotherapy (5-fluorouracil + leucovorin + oxaliplatin). This approach stabilized the disease and reduced liver metastases. MTB was crucial in interpreting genetic profiles and guiding personalized treatment, demonstrating the potential of MTB-driven strategies for treating advanced pancreatic cancer with liver metastases.
- Citation: Yan Y, Ren ZZ, Wang WY, Tang J, Zhang YW. Molecular tumor boards in pancreatic cancer with liver metastasis: A case report. World J Hepatol 2025; 17(7): 106993
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106993.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106993
With the advancements in molecular oncology, cancer treatment has entered an era of precision medicine. In clinical practice, biomarkers or "driver genes" are often identified before or during the treatment of various cancers such as colorectal cancer[1], non-small cell lung cancer, breast cancer, and acute myeloid leukemia[2]. Testing indicators, such as tumor mutational burden (TMB) and microsatellite instability (MSI) status, in patients can help assess whether they might benefit from immunotherapy[3]. Genetic testing has been incorporated into routine clinical practice to assist in the decision-making process for many cancers. However, this progress brings new challenges such as how to appropriately interpret patients' genetic test results and design the most suitable anticancer treatment plan. To address this challenge, a novel collaborative model was initiated in 2011 at the University of Michigan, involving clinical experts, pathologists, molecular biologists, and bioinformatics experts to form a molecular tumor boards (MTB)[4].
The board discusses and proposes treatment plans that MTB patients might benefit from. This collaborative model has since been adopted and further developed, with many hospitals in China beginning to use the MTB format to guide cancer treatment[5].
Among all cancers in China, pancreatic cancer ranks 8th in incidence and 6th in mortality[6]. Owing to its insidious onset, approximately 80% of pancreatic cancer patients are diagnosed at an advanced stage[7]. Current treatment methods primarily include surgery and chemotherapy, with targeted immunotherapy options tailored based on genetic testing results. For instance, patients with wild-type KRAS may benefit from chemotherapy combined with nimotuzumab[8], whereas those with MSI-H or mismatch repair deficient may consider immune checkpoint inhibitors (ICIs) in later lines of treatment[9]. BRAC mutations can lead to homologous recombination deficiency (HRD), and the use of PARP inhibitors (such as olaparib, niraparib, and fluozoparib) can induce synthetic lethality, leading to tumor cell death[10]. Therefore, the Chinese Society of Clinical Oncology guidelines recommend that advanced pancreatic cancer patients with BRAC1/2 germline mutations who show no disease progression after 16 weeks of platinum-based therapy should consider maintenance therapy with olaparib[11]. Although this approach extends progression-free survival (PFS) in these patients, the overall survival (OS) is not beneficial[12]. Hence, formulating treatment plans based on genetic monitoring results is crucial and highlights the significance of MTB. This article reports the role of a multidisciplinary collaborative model of MTB in the treatment of patients with pancreatic cancer and liver metastasis.
A 45-year-old male presented with progressive pancreatic cancer and liver metastases in October, 2023.
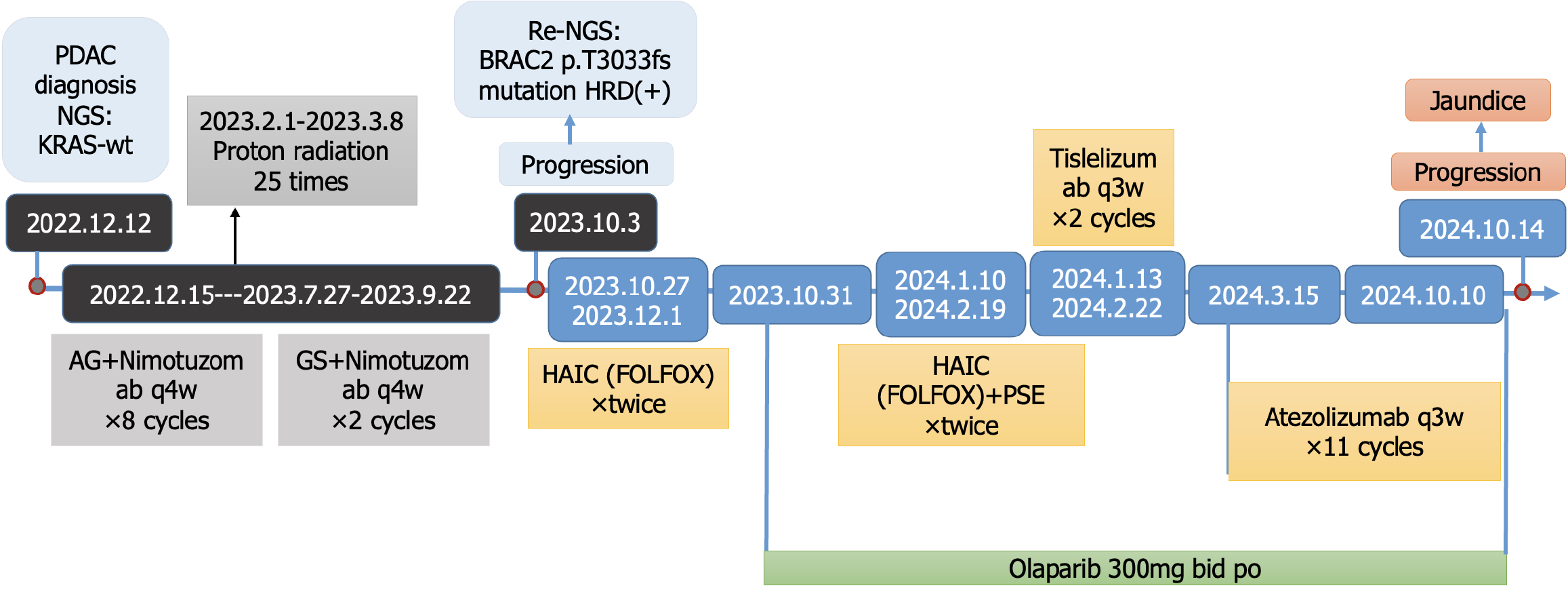
The patient presented with jaundice in December 2022 at an external hospital where a pancreatic head mass and liver metastasis were diagnosed. Liver biopsy indicated moderately differentiated adenocarcinoma. Next-generation sequencing (NGS) results showed wild-type KRAS; mutations in ATRX, CUL3, and PTPRT; TMB = 2.1; and microsatellite stable. From December 15, 2022, to July 27, 2023, the patient was treated with gemcitabine (1 g/m2) on days 1, 8, and 15, combined with albumin-bound paclitaxel (125 mg/m2) on the same days, and nimotuzumab (400 mg) on day 1, weekly for 4 weeks per cycle (a total of eight cycles, with albumin-bound paclitaxel discontinued in the last two cycles due to bone pain side effects). Proton radiotherapy was administered 25 times from February 1, 2023 (targeting the pancreatic head and part of the liver with metastases). A review on May 19, 2023, showed stability: Significant shrinkage of the pancreatic head lesion and a reduction in liver metastases. From July 28, 2023, to September 22, 2023, the treatment was adjusted to tegafur-gimeracil-oteracil potassium (S-1), gemcitabine and nimotuzumab for two cycles. A review on August 25, 2023, showed further shrinkage of the pancreatic head lesion and a partial reduction in liver metastases. In September 2023, the patient received a Chinese medicine called Tongguan Teng injection for 7 days but stopped due to fatigue. In October 2023, the progression of liver lesions led to a consultation at our hospital's MTB clinic.
The patient had no significant prior medical history.
The patient denied any food or drug allergies and had no family history of cancer.
Physical examination revealed that the patient was 170 cm tall and weighed 75 kg. No significant abnormalities were observed.
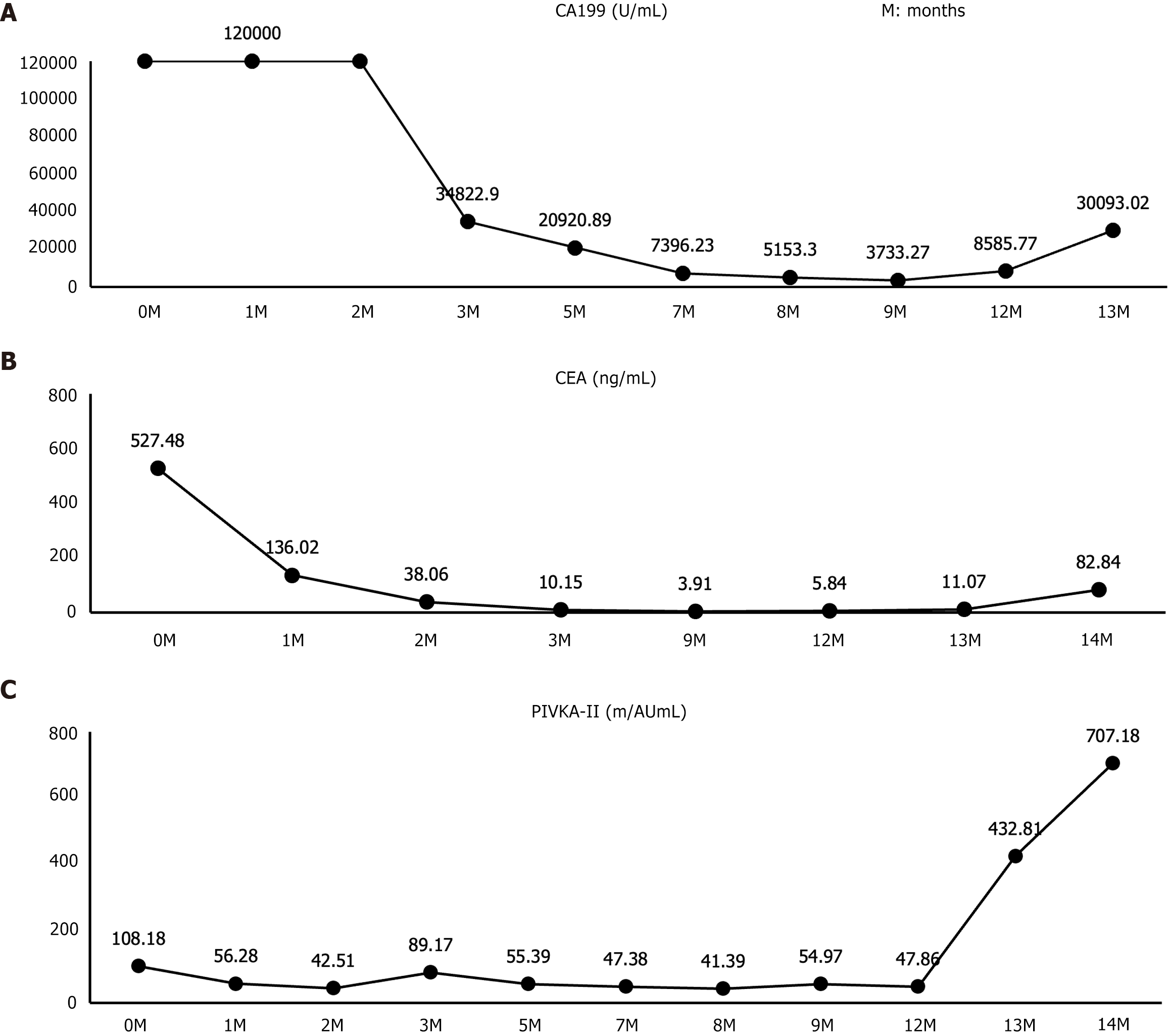
Liver function tests on October 26, 2023: Alanine aminotransferase 18.8 U/L, aspartate aminotransferase 36.7 U/L, total bilirubin 28.4 μmol/L, direct bilirubin 14.4 μmol/L. Serum levels of tumor markers on October 26, 2023: Carbohydrate antigen 19-9 (CA19-9) > 120000 U/mL; carcinoembryonic antigen (CEA) 527.48 ng/mL; protein induced by vitamin K absence II (PIVKA-II) 108.18 mAU/mL.
Upper abdominal magnetic resonance imaging (MRI) on October 26, 2023 revealed multiple liver metastases, slightly enlarged retroperitoneal lymph nodes suggestive of metastasis, and ascites.
Moderately differentiated adenocarcinoma of the pancreatic head with liver metastases.
Peripheral blood gene testing revealed HRD positivity and a pathogenic BRCA2 mutation, suggesting sensitivity to PARP inhibitors and platinum drugs. Tumor microenvironment testing revealed PD-L1 expression and CD8+ T cell infiltration. Based on the above test results, the treatments administered were as follows: Hepatic arterial infusion chemotherapy (HAIC) (FOLFOX regimen: Oxaliplatin 85 mg/m2 in total, 50 mg in 1 day for 3 days, and 400 mg/m2 5-fluorouracil plus 400 mg/m2 leucovorin per day for 3 days) on October 27, 2023, and December 1, 2023. Partial splenic artery embolization on January 10, 2024, and February 19, 2024. Olaparib 300 mg twice daily starting October 31, 2023. Immunotherapy: Tislelizumab 200 mg (January 13, 2024, and February 22, 2024) and atezolizumab 1200 mg IV every 21 days (March 15, 2024, to October 10, 2024).
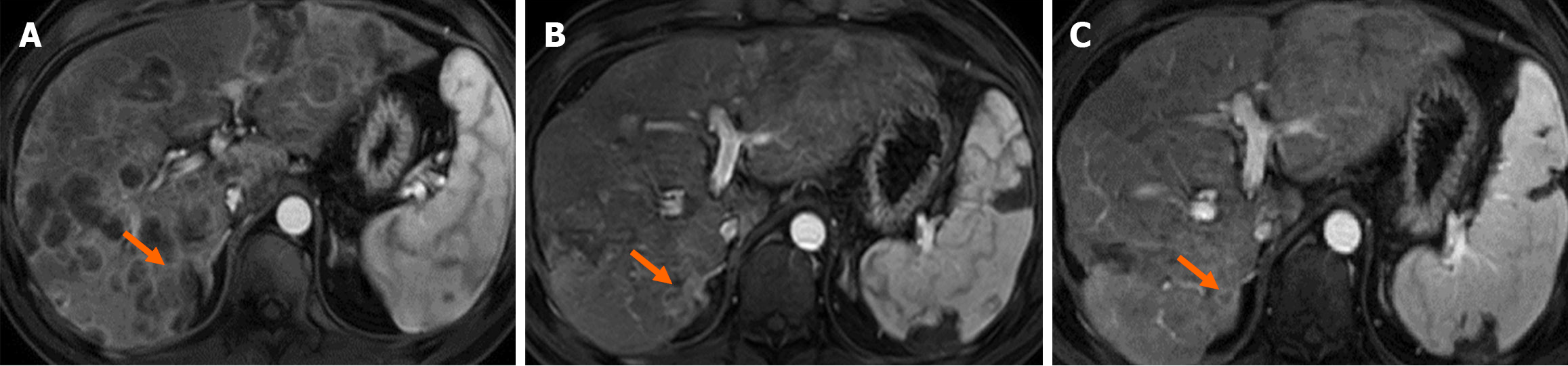
Genetic testing-guided therapies (olaparib, FOLFOX, and immunotherapy) led to further stabilization and a partial reduction in liver metastases. Contrast-enhanced upper abdominal MRI on May 19, 2024, showed marked regression of the liver lesions. On July 25, 2024, the lesions displayed further reduction in size. The alterations in the MRI images are shown in Figure 1.
On October 14, 2024, MRI revealed an increase in some liver lesions accompanied by elevated liver enzymes and jaundice.
The serum tumor markers (CA19-9, CEA, and PVIKA-II) were monitored throughout the treatment period (Figure 2). The overall treatment process is illustrated in Figure 3.
This case highlights the importance of genetic profiling for identifying actionable targets and guiding personalized treatment strategies, particularly after the progression of initial multimodal therapies.
Based on the NOTABLE study[8], nimotuzumab combined with gemcitabine has been approved for the treatment of KRAS wild-type locally advanced or metastatic pancreatic cancer. This study showed that the combination of nimotuzumab and gemcitabine resulted in a PFS of 4.2 months and a median OS of 10.9 months. To further improve efficacy, this patient was treated with nimotuzumab and gemcitabine combined with albumin-bound paclitaxel, along with local radiotherapy, achieving a PFS of 10.5 months at first progression, which was significantly longer than that of the two-drug combination of nimotuzumab and gemcitabine.
BRAC1/2 genes are related to homologous recombination repair (HRR), and their mutations can lead to HRD. Besides BRAC1/2, mutations in HRR-related genes, such as PALB2, ATM, and RAD51, can also cause HRD[13]. The abnormal expression and methylation of HRR-related proteins can lead to HRD[14]. Therefore, patients without BRAC gene mutations may still be HRD-positive. HRD-positive patients with advanced epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer can benefit from the combination of olaparib and bevacizumab[15]. This patient harbored a somatic mutation in BRAC2 and was HRD-positive, suggesting a potential benefit from olaparib. Some studies suggest that BRAC2 mutations enhance tumor immunogenicity and increase responsiveness to ICIs[16]. In vitro experiments have shown that PARP inhibitors can upregulate PD-L1 expression in tumor cells, enhancing the antitumor effects when combined with PD-L1 inhibitors[17]. In addition, a retrospective study of patients with HRD-positive pancreatic and biliary tract cancers showed that the combination of nivolumab and ipilimumab achieved an objective response rate of 42% and a disease control rate of 58%. However, owing to the limited sample size, further validation of efficacy is needed[18]. Based on these findings and the presence of PD-L1 expression in the tumor microenvironment, the patient was advised by the MTB to regularly use ICIs. Previous studies demonstrated the beneficial effects of HAIC in pancreatic cancer patients with liver metastases[19-21]. Therefore, in addition to PARP inhibitors and ICI therapy, this patient may have derived additional clinical benefits from the combination of HAIC for the local management of intrahepatic metastatic lesions.
In the POLO study[12], patients with advanced pancreatic cancer with BRAC1/2 germline mutations who showed no disease progression after 16 weeks of platinum-based therapy had an objective response rate of 23.1% and a median PFS of 7.4 months with olaparib. In this case, after the initial treatment progression, the patient was treated with HAIC using the FOLFOX regimen combined with systemic targeted immunotherapy, stabilizing the liver lesions for approximately 11.5 months, demonstrating the beneficial role of HAIC combined with systemic targeted immunotherapy in such patients.
This study demonstrated innovation by questioning the conventional notion that “pancreatic cancer lacks actionable targets”. After disease progression following previous therapies in October 2023, the MTB recommended a second round of genomic sequencing to identify molecular developments. This sequencing uncovered new therapeutic opportunities. To overcome clinical sampling issues owing to a patient’s refusal to undergo repeat liver biopsy, peripheral blood NGS was used alongside analyzing tumor microenvironment in the original biopsy tissue, demonstrating a practical diagnostic alternative. Addressing the discrepancy in the POLO trial (no OS benefit despite PFS prolongation), this study proposed a novel approach of combining olaparib with HAIC and immunotherapy after a thorough evaluation to investigate the survival benefits in this patient subgroup.
However, the sequential use of multiple therapies complicates the attribution of clinical benefits to a single intervention. However, the synergistic or antagonistic interactions between treatments remain unclear. The duration of follow-up after initiating olaparib and combination therapy may have been insufficient to assess long-term outcomes, including OS, durability of response, and delayed adverse effects.
This case highlights the potential of MTB in the management of advanced pancreatic cancer with liver metastases, for which conventional therapies often yield limited benefits. Despite the initial response to multimodal therapies, disease progression in this 45-year-old patient prompted MTB-guided genomic analysis, which revealed HRD positivity and a pathogenic BRCA2 mutation (p.T3033fs). These findings enabled a precision-based shift to olaparib combined with immunotherapy and HAIC (FOLFOX), resulting in disease stabilization and partial regression of liver metastases. This case reinforces the use of MTB as pivotal tools for integrating genomic insights into clinical decision-making, particularly in pancreatic cancer, where actionable mutations are rare but critical for extending survival. Future efforts should focus on expanding access to MTB and validating their efficacy in larger cohorts to establish standardized frameworks for precision oncology.
We would like to express our sincere gratitude to Yu-Nuo Zhang for her active role and dedication in coordinating the MTB clinic, which has been essential to the smooth progress of our work.
| 1. | Isermann T, Sers C, Der CJ, Papke B. KRAS inhibitors: resistance drivers and combinatorial strategies. Trends Cancer. 2025;11:91-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi H. Advances in pathogenesis research and challenges in treatment development for acute myeloid leukemia. Int J Hematol. 2024;120:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Lemery S, Keegan P, Pazdur R. First FDA Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication. N Engl J Med. 2017;377:1409-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 493] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 4. | Roychowdhury S, Iyer MK, Robinson DR, Lonigro RJ, Wu YM, Cao X, Kalyana-Sundaram S, Sam L, Balbin OA, Quist MJ, Barrette T, Everett J, Siddiqui J, Kunju LP, Navone N, Araujo JC, Troncoso P, Logothetis CJ, Innis JW, Smith DC, Lao CD, Kim SY, Roberts JS, Gruber SB, Pienta KJ, Talpaz M, Chinnaiyan AM. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111ra121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 459] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 5. | Lam TC, Cho WC, Au JS, Ma ES, Lam ST, Loong HH, Wong JWH, Wong SNM, Lee VH, Leung RC, Lau JK, Kam MT, Mok FS, Lim FM, Nyaw JS, Tin WW, Cheung KM, Chan OS, Kwong PW, Cheung FY, Poon DMC, Chik JY, Lam MH, Chan LW, Wong SC, Cao YB, Hui CV, Chen JZ, Chang JH, Kong SF, El Helali A; Precision Oncology Working Group (POWG). Consensus Statements on Precision Oncology in the China Greater Bay Area. JCO Precis Oncol. 2023;7:e2200649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1520] [Article Influence: 253.3] [Reference Citation Analysis (1)] |
| 7. | De Dosso S, Siebenhüner AR, Winder T, Meisel A, Fritsch R, Astaras C, Szturz P, Borner M. Treatment landscape of metastatic pancreatic cancer. Cancer Treat Rev. 2021;96:102180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 8. | Qin S, Li J, Bai Y, Wang Z, Chen Z, Xu R, Xu J, Zhang H, Chen J, Yuan Y, Liu T, Yang L, Zhong H, Chen D, Shen L, Hao C, Fu D, Cheng Y, Yang J, Wang Q, Qin B, Pan H, Zhang J, Bai X, Zheng Q. Nimotuzumab Plus Gemcitabine for K-Ras Wild-Type Locally Advanced or Metastatic Pancreatic Cancer. J Clin Oncol. 2023;41:5163-5173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 695] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 10. | Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34:360-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 440] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 11. | Cui J, Jiao F, Li Q, Wang Z, Fu D, Liang J, Liang H, Xia T, Zhang T, Zhang Y, Dai G, Zhang Z, Wang J, Bai Y, Bai Y, Bi F, Chen D, Cao D, Chen J, Fang W, Gao Y, Guo J, Hao J, Hua H, Huang X, Liu W, Liu X, Li D, Li J, Li E, Li Z, Pan H, Shen L, Sun Y, Tao M, Wang C, Wang F, Xiong J, Zhang T, Zhang X, Zhan X, Zheng L, Ren G, Zhang T, Zhou J, Ma Q, Qin S, Hao C, Wang L. Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of pancreatic cancer. J Natl Cancer Cent. 2022;2:205-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Kindler HL, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, Bordia S, McGuinness D, Cui K, Locker GY, Golan T. Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J Clin Oncol. 2022;40:3929-3939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 13. | Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol. 2013;5:a012740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 636] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 14. | LaRose M, Manji GA, Bates SE. Beyond BRCA: Diagnosis and management of homologous recombination repair deficient pancreatic cancer. Semin Oncol. 2024;51:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | LYNPARZA® (olaparib) tablets, for oral use. [cited 26 Jun, 2025]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/208558s028lbl.pdf. |
| 16. | Samstein RM, Krishna C, Ma X, Pei X, Lee KW, Makarov V, Kuo F, Chung J, Srivastava RM, Purohit TA, Hoen DR, Mandal R, Setton J, Wu W, Shah R, Qeriqi B, Chang Q, Kendall S, Braunstein L, Weigelt B, Blecua Carrillo Albornoz P, Morris LGT, Mandelker DL, Reis-Filho JS, de Stanchina E, Powell SN, Chan TA, Riaz N. Mutations in BRCA1 and BRCA2 differentially affect the tumor microenvironment and response to checkpoint blockade immunotherapy. Nat Cancer. 2021;1:1188-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 17. | Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, Li CW, Chou CK, Lim SO, Chang SS, Litton J, Arun B, Hortobagyi GN, Hung MC. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin Cancer Res. 2017;23:3711-3720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 755] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 18. | Terrero G, Datta J, Dennison J, Sussman DA, Lohse I, Merchant NB, Hosein PJ. Ipilimumab/Nivolumab Therapy in Patients With Metastatic Pancreatic or Biliary Cancer With Homologous Recombination Deficiency Pathogenic Germline Variants. JAMA Oncol. 2022;8:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Ouyang H, Ma W, Si T, Liu D, Chen P, Gerdtsson AS, Song J, Ni Y, Luo J, Yan Z. Systemic Chemotherapy With or Without Hepatic Arterial Infusion Chemotherapy for Liver Metastases From Pancreatic Cancer: A Propensity Score Matching Analysis. Clin Colorectal Cancer. 2023;22:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Peng C, Xu B, Xiao J, Zhou C, Li X, Shi H, Qiang W, Wang T, Zhao J, Liu F, Li G, Li H, Chen C, Shi L. Hepatic Artery Infusion of Floxuridine in Combination With Systemic Chemotherapy for Pancreatic Cancer Liver Metastasis: A Propensity Score-Matched Analysis in Two Centers. Front Oncol. 2021;11:652426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Homma H, Doi T, Mezawa S, Takada K, Kukitsu T, Oku T, Akiyama T, Kusakabe T, Miyanishi K, Niitsu Y. A novel arterial infusion chemotherapy for the treatment of patients with advanced pancreatic carcinoma after vascular supply distribution via superselective embolization. Cancer. 2000;89:303-313. [PubMed] [DOI] [Full Text] |