Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106810
Revised: April 19, 2025
Accepted: June 13, 2025
Published online: July 27, 2025
Processing time: 140 Days and 3.4 Hours
Hepatocellular carcinoma (HCC), a primary malignancy of the liver and leading cause of cancer-related mortality worldwide, poses substantial therapeutic challenges, particularly in advanced and unresectable stages. Immune checkpoint inhibitors (ICIs) have emerged as critical therapeutic agents, targeting immune checkpoint pathways to restore antitumor immune responses. Combinations such as atezolizumab (anti-programmed cell death ligand 1 with bevacizumab (anti-vascular endothelial growth factor), as well as antibodies directed against cy
Core Tip: Combination of immune checkpoint inhibitor (ICI) therapy has improved efficacy and survival in patients with unresectable hepatocellular carcinoma (HCC). However, a substantial proportion still fail to respond due to resistance driven by the tumor microenvironment. Emerging strategies targeting gut microbiota offer promising avenues to overcome this barrier. Certain microbial taxa have been associated with enhanced T-cell infiltration and improved ICI responses. Additionally, microbial metabolites like butyrate and desaminotyrosine exert immunomodulatory effects that may restore sensitivity to ICIs. Dual-target approaches combining ICIs and microbiota modulation hold potential to improve progression-free and overall survival in HCC.
- Citation: Pamungkas KMN, Lesmana Dewi PIS, Alamsyah AZ, Dewi NLPY, Dewi NNGK, Mariadi IK, Sindhughosa DA. Microbiome dysbiosis and immune checkpoint inhibitors: Dual targets in Hepatocellular carcinoma management. World J Hepatol 2025; 17(7): 106810
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106810.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106810
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, accounting for 80%–90% of all liver cancer cases, and ranks as the third leading cause of cancer-related mortality worldwide[1,2]. Its global burden is particularly pronounced in Asia, where countries such as Thailand, Vietnam, and Cambodia report incidence rates of 22-24 cases per 100000, while Mongolia has the highest incidence globally, exceeding 80 per 100000[3]. Additionally, recent epidemiological shifts have shown rising incidence in previously low-burden regions, including Iran, Iraq, and Nepal[4].
Immune checkpoint inhibitors (ICIs) have emerged as a promising therapeutic strategy for HCC by enhancing an
Recent studies have proposed the gut microbiome as a key modulator of ICIs efficacy, given its role in shaping systemic immunity and its association with cancer progression and therapeutic outcomes[11,12]. Alterations in microbial composition, particularly within the microbiome, represents a potential target for enhancing immunotherapeutic efficacy in HCC. This review examines the evolving relationship between gut microbiome and immunotherapy in HCC, with an emphasis on microbiome-informed strategies to improve clinical outcomes.
Patients with Barcelona-clinic liver cancer stage B or C HCC with sufficient liver function and performance status but can no longer undergo liver replacement therapy due to disease progression or extrahepatic spread, systemic therapy should be considered[13]. HCC demonstrates resistance to conventional cytotoxic chemotherapy through multiple intricate molecular mechanisms. These include autophagy activation, apoptosis evasion, upregulation of drug efflux pumps, heightened intracellular drug metabolism, and enhanced deoxyribonucleic acid (DNA) repair processes, among others[14]. As of 2023, first-line treatment for HCC consists of targeted therapies, including multikinase inhibitors, anti-vascular endothelial growth factor (VEGF) agents, ICIs, or their combinations[15].
Immune checkpoints are membrane-bound proteins found on various cell types, including natural killer cells, dendritic cells (DCs), monocytes, tumor-associated macrophages, myeloid-derived suppressor cells, as well as B and T-lymphocytes[16]. ICIs have exhibited prolonged effectiveness in multiple solid tumors, including HCC, where the tumor's immunological profile makes it a suitable target for immune-based therapies. Evidence suggests that the infiltration of lymphocytes within HCC tumors is positively correlated with clinical outcomes, highlighting the pivotal role of immune mechanisms in the therapeutic management of HCC. The strength of the immune response and the activation of cytotoxic immunity are governed by the balance between costimulatory signals and immune checkpoints[17,18]. These checkpoints play a critical role in regulating the immune response, making them key targets in therapies designed to enhance antitumor immunity. T-cells are the main focus of immune checkpoint therapy for three fundamental reasons: Their selective recognition of peptide antigens from proteins in different cellular compartments, their ability to destroy antigen-expressing cells through cytotoxic CD8+ T-cells, and their crucial role in regulating immune responses via CD4+ helper T-cells[19]. In individuals with chronic liver inflammation, intrahepatic lymphocytes show increased programmed cell death 1 (PD-1) expression, whereas its ligands, programmed death-ligand (PD-L) 1 and PD-L2, are highly expressed in Kupffer cells, liver sinusoidal endothelial cells, and leukocytes[20,21]. Currently, two main classes of ICIs are utilized in clinical practice for advanced HCC: Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors and PD-1/PD-L1 inhibitors. These agents are typically recommended as second-line treatments for HCC in patients who do not respond to first-line therapy with sorafenib[22] (Table 1).
| Study name | Regimen | Line of therapy | No. of patients | ORR (%) | mPFS (months) | mOS (months) | Findings |
| ICI single therapy | |||||||
| CheckMate 459[7,36] | Nivolumab vs Sorafenib | 1st line | 743 | 15 | 3.7 vs 3.8 | 16.4 vs 14.7 | HR death 0.85 (95%CI: 0.72-1.00; P = 0.0522) |
| Keynote-224[9] | Pembrolizumab | 2nd line | 104 | 17 | 4.9 | 13.2 | Durable anti-tumour activity and improvement in BOR. CR increased vs the primary analysis (3.8% vs 1.0%) |
| Keynote-240[8] | Pembrolizumab vs Placebo | 2nd line | 413 | 18 vs 4 | 3.0 vs 2.8 | 13.9 vs 10.6 | Did not met the threshold HR of 0.781 (95%CI: 0.611 to 0.998; P = 0.0238) and 0.775 (95%CI: 0.609-0.987; P = 0.0186) for OS and PFS |
| Keynote-394[37] | Pembrolizumab vs Placebo | 2nd line (Asian) | 453 | 13.9 vs 1.3 | 2.6 vs 2.3 | 14.6 vs 13.0 | Significance OS/PFS benefit (HR = 0.79; 95%CI: 0.63-0.99; P = 0.018) |
| HIMALAYA[38] | STRIDE (Durva + Tremeli) vs Sorafenib | 1st line | 1171 | 20.1 | 3.78 vs 4.07 | 16.4 vs 13.8 | Significance OS (HR = 0.78; 96%CI: 0.65–0.92; P = 0.0035) |
| RATIONALE-301[39] | Tislelizumab vs Sorafenib | 1st line | 674 | 14.3 vs 5.4 | 2.3 vs 3.3 | 15.9 vs 14.1 | OS non-inferior (HR = 0.85; 95%CI: 0.712-1.019) |
| Sangro et al[40] 2013 | Tremelimumab | 2nd line | 21 | 17.6 | NA | 8.2 | Median TTP 6.48 months (95%CI: 3.95–9.14) |
| ICI combination therapies | |||||||
| CheckMate 040[47] | Nivolumab + Ipilimumab | 2nd line | 148 | 32-31 | 2.96-4.0 | 22.8-12.5 | Arm A, the 12-mOS rate was 61% (95%CI: 0.46-0.73) |
| CheckMate 9DW[46] | Nivolumab + Ipilimumab vs Sorafenib/Lenvatinib | 1st line | 1084 | 36 vs 13 | 9.1 vs 9.2 | 23.7 vs 20.6 | Significantly improve OS (HR = 0.79, 95%CI: 0.65-0.96; P = 0.018) |
| IMbrave150[43] | Atezolizumab + Bevacizumab vs Sorafenib | 1st line | 336 vs 165 | 27.3 vs 11.9 | 6.8 vs 4.3 | 19.1 vs 13.4 | HR death 0.58 (95%CI 0.42–0.79; P < 0.001) |
| AMETHISTA[44] | Atezolizumab + Bevacizumab (Single arm) | 1st line | 152 | 26.9 | 8.51 | 18.23 | TEAEs in 28.9% |
| COSMIC-312[45] | Atezolizumab Cabozantinib vs sorafenib | 1st line | 837 | NA | 6.8 vs 4.2 | 15.4 vs 15.5 | HR death 0.63 (95%CI: 0.44-0.91, P = 0.0012) |
CTLA-4 is an intracellular protein found in resting T-cells and is constitutively expressed on regulatory T-cells (Tregs). Its activation occurs exclusively within lymph nodes, where it plays a crucial role in modulating immune responses[23]. On Tregs, CTLA-4 is essential for inhibiting effector T-cell activity through multiple mechanisms. Furthermore, CTLA-4 plays an important role in regulating T-cell activation under normal physiological conditions, helping to prevent excessive immune responses[24]. CTLA-4 contributes to immunosuppression within the tumor microenvironment by improving the activity and differentiation of Tregs and disrupting the DC function[25]. Upon activation of the T-cell receptor by CD28, CTLA-4 is transported to the cell surface. Once expressed on T-cells, it binds to CD80 and CD86, thereby inhibiting their interaction with CD28, which is essential for the transmission of costimulatory signals. This interaction mediates inhibitory signals to T-cells, leading to the suppression of their proliferation and activation[22,26,27]. CTLA-4 signaling promotes tumor progression by inhibiting antigen presentation by antigen-presenting cells and stimulating the pro
PD-1 is an immune inhibitory receptor expressed on T-cells, B-cells, and others. It regulates immune responses through a bidirectional signaling mechanism. PD-L1, also known as CD274 or B7-H1, is broadly expressed on various somatic cells in response to proinflammatory cytokines and primarily functions to inhibit T-cell activity[33]. PD-L2 (CD273 or B7-DC), on the other hand, is less frequently expressed on APC. The binding of PD-1 to its ligands triggers apoptosis in antigen-specific T-cells within lymph nodes while concurrently inhibiting apoptosis in Tregs, thereby modulating immune tolerance and suppression[22,34]. Prolonged engagement between PD-1 and PD-L1 can amplify inhibitory signaling, fostering an immunosuppressive microenvironment. Cancer cells have adapted to exploit this pathway by continuously expressing PD-L1 or PD-L2, which activates PD-1 in tumor-infiltrating lymphocytes (TILs), enabling immune evasion[24,35]. Therapeutic antibodies targeting PD-1, such as pembrolizumab and nivolumab, or PD-L1, including durvalumab and atezolizumab, block this interaction, thereby mitigating immunosuppression and restoring an immunostimulatory tumor microenvironment[32].
ICIs have transformed the therapeutic landscape of advanced HCC. Various clinical trials have evaluated PD-1, PD-L1, and CTLA-4 inhibitors either as monotherapy or in combination, with results summarized in Table 1. Monotherapy trials with nivolumab (CheckMate 459)[36], pembrolizumab (KEYNOTE-224[9], KEYNOTE-240[8], KEYNOTE-394[37]), durvalumab (HIMALAYA)[38], tislelizumab (RATIONALE-301)[39], and tremelimumab consistently demonstrated ORR ranging from 15% to 20%, with manageable toxicity profiles. However, only KEYNOTE-394[37] achieved its prespecified overall survival (OS) and progression-free survival (PFS) endpoints, particularly in Asian populations. Despite early promise, the confirmatory CheckMate 459[36] and KEYNOTE-240[8] trials failed to meet statistical significance for OS improvement, leading to withdrawal of nivolumab’s Food and Drug Administration approval for second-line therapy in 2021. Tremelimumab also showed high disease control rate, highlighting its potential as a promising therapeutic option for HCC[40-42].
Dual-agent regimens have shown greater efficacy. The STRIDE regimen durvalumab combined with a single priming dose of tremelimumab improved OS over sorafenib with sustained long-term benefit, as shown in the HIMALAYA trial[38]. Likewise, the combination of atezolizumab and bevacizumab (IMbrave150[43], AMETHISTA[44] trials) led to a paradigm shift, becoming a first-line standard due to superior survival outcomes compared to sorafenib. The COSMIC-312 trial showed the combination of atezolizumab and cabozantinib significantly improved PFS. However, interim results showed no substantial OS benefit compared to sorafenib, pending the final survival analysis[45]. The combination of ipilimumab and nivolumab, studied in CheckMate9DW and CheckMate 040, also showed promising results in first-line and second-line settings, with exploratory analyses suggesting that “inflammatory signatures” may predict better outcomes[46,47]. Overall, these findings support the use of ICIs either alone or in combination, particularly in immunologically enriched tumors.
Although some studies have shown superior efficacy of ICIs, clinical responses are still variable. The resistance is still a major challenge, affecting both monotherapy or combination regimen. Anti-PD-1 monotherapy reaches RR of 42%-45%, whereas combination with anti-CTLA-4 boosted the RR to 58%. Nonetheless, resistance to PD-1 inhibitor monotherapy developed in approximately 55% of patients, and in 40% of those receiving combination regimens. Furthermore, 25% of initial responders demonstrated acquired resistance within a two-year period[48]. Likewise, combination therapy is more at risk of developing higher rates of grade 3–4 treatment-related adverse events[49]. Mechanisms underlying this cha
Dysbiosis refers to an imbalanced state of the gut microbiome, often caused by antibiotics, diet, and infection[52]. This disruption may alter the immune signaling through the production of less-immunogenic lipopolysaccharide (LPS), thus disrupting inflammation pathways[53]. Studies have indicated the impact of dysbiosis on immunotherapy. A study using mice with antibiotic-associated dysbiosis failed to control tumor growth under anti-CTLA-4 immunotherapy[54]. In humans, a cohort study of advanced cancer patients treated with ICIs also revealed that administration of broad-spectrum antibiotics impacted both the RR and longer response time of ICIs[55]. In the context of HCC, dysbiosis may aggravate immune resistance by facilitating immune tolerance mechanisms. A key pathway involves Tregs differentiation which is recruited into the tumor microenvironment[56]. These Tregs can suppress cytotoxic T-cells activity, and impair anti-tumor immunity, contributing to immunotherapy resistance[57]. Moreover, Tregs enhance the expression of CTLA-4, a target for checkpoint blockage, which makes it susceptible to ICIs[58].
Many clinical trials about HCC revealed limited benefit and response to ICIs, highlighting HCC-specific resistance in many patients (Table 1). To date, ICIs-based therapy has indeed shown better outcomes compared to previous first-line standard of therapy, like sorafenib. However, long-term responses remain limited due to resistance mechanisms[59]. For instance, the combination of atezolizumab and bevacizumab showed an ORR of 34% in sorafenib-naïve patients, with only one patient gaining a complete response[60]. These suggest special resistance mechanisms in HCC which limit ICIs treatment. This includes the presence of cold tumor conditions characterized by low T-cell infiltration[61] and PD-L1 expression[62]. The cause of this condition is unclear, but it is thought to be linked to immune deficiency or innate immune response dysfunction that is related to T-lymphocyte rejection[63]. This immune dysfunction may create an unfavorable microenvironment that resists ICIs therapy. Given its role in immune regulation, gut microbiome mo
The gastrointestinal tract constitutes the body's largest immune organ and is integral to maintaining physiological and immunological homeostasis. It harbors a diverse and complex community of microorganisms-comprising bacteria, archaea, eukaryotes, viruses, and parasites—collectively referred to as the gut microbiota[65,66]. This microbial eco
This axis is anatomically and functionally mediated by the portal vein, biliary system, and systemic circulation, allowing the liver to influence gut homeostasis via bile acids (BAs) and antimicrobial molecules, while gut-derived microbial metabolites and components, such as LPS, influence hepatic function[68,69]. Meta-analyses of 16s ribosomal-ribonucleic acid gene sequences have identified seven dominant phyla within the gut microbiota: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verucomicrobia, Fusobacteria, and Cyanobacteria[70,71].
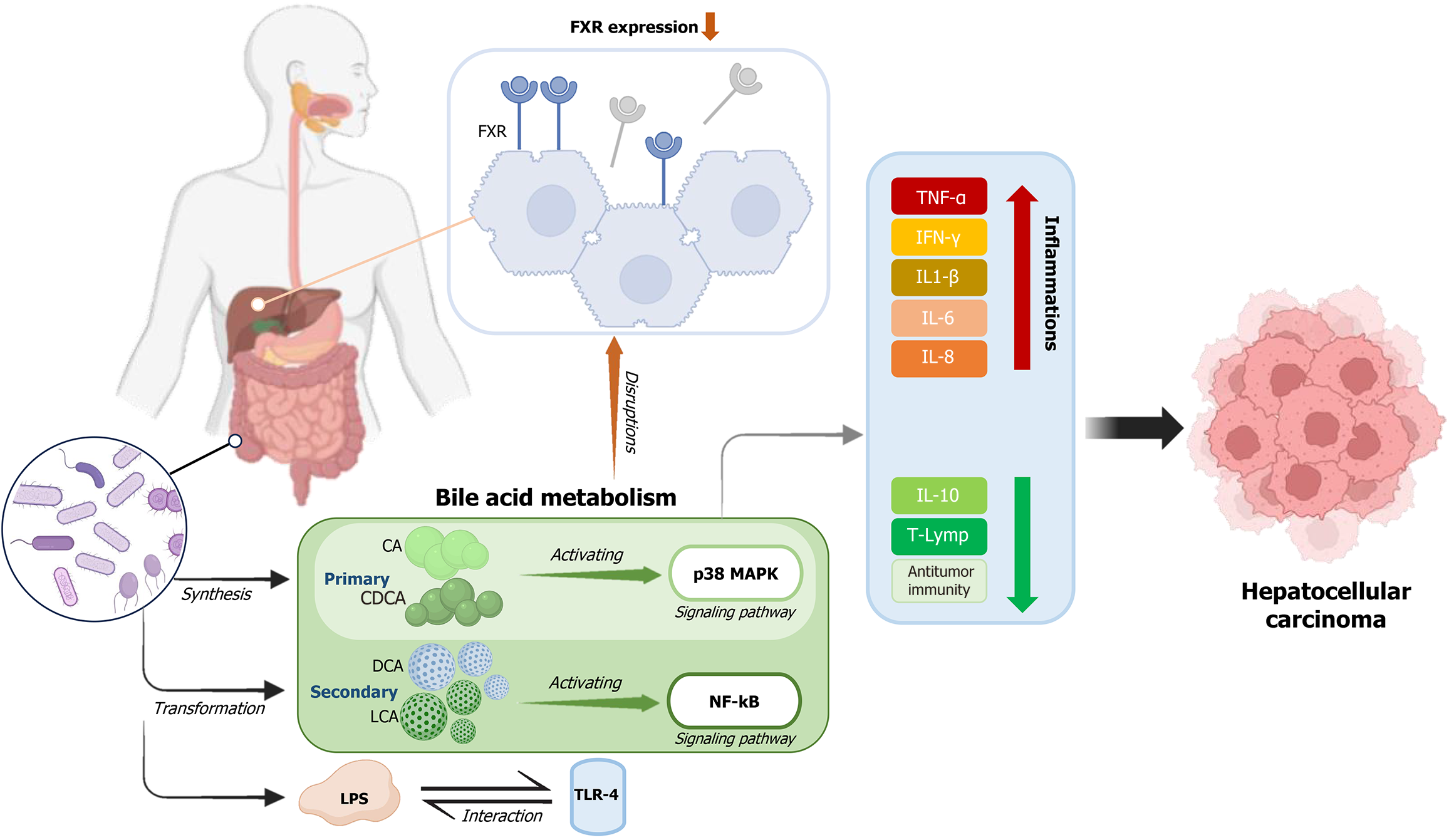
Synthesized from cholesterol in the liver, BAs undergo extensive enterohepatic circulation and are modified by the gut microbiota through processes such as deconjugation, dehydroxylation, and epimerization[72]. This interplay regulates the composition of both BAs and microbial populations. However, dysregulated BA-metabolism-particularly the accumulation of hydrophobic BAs like cholic acid (CA) and chenodeoxycholic acid can trigger hepatocyte apoptosis through p38 mitogen-activated protein kinase signaling, promote chronic inflammation, and drive hepatic carcinogenesis[66,73] (Figure 1).
Microbial dysbiosis further contributes to hepatocarcinogenesis through immune activation and the release of pro-inflammatory metabolites. Elevated LPS levels activate toll-like receptor 4 (TLR4) on hepatic stellate cells, stimulating fibrogenesis and accelerating tumor progression[74]. Additionally, secondary BAs such as deoxycholic acid (DCA) can induce DNA damage and activate pro-inflammatory pathways, including nuclear factor kappa B, thereby enhancing the secretion of cytokines such as interleukin-1β, interleukin-6, tumor necrosis factor-alpha, IFN-γ, interleukin-8, and reactive oxygen species (ROS)[69]. These processes contribute to a tumor-promoting hepatic microenvironment, particularly in obesity-associated HCC.
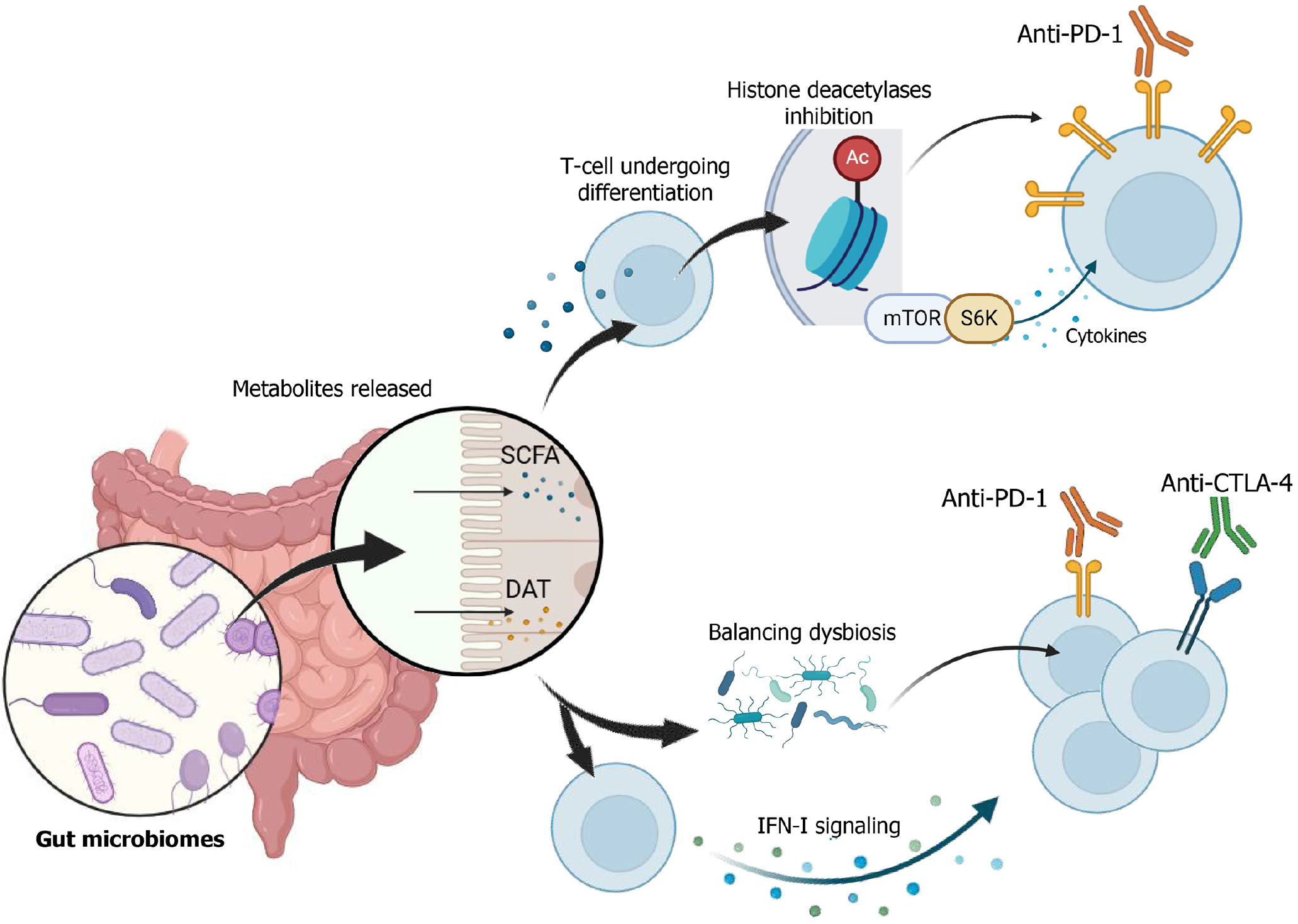
Beyond its role in liver carcinogenesis, the gut microbiome also plays a pivotal role in modulating the efficacy of ICIs. Specific microbial metabolites, including short-chain fatty acids (SCFAs) such as butyrate and desamino tyrosine (DAT), have demonstrated immunomodulatory effects that enhance ICIs responses. SCFAs can inhibit histone deacetylases, regulate mTOR-S6K signaling, and influence PD-1 ligand expression, thereby altering T-cell function and antitumor immunity[75-77]. DAT has been shown to potentiate type I interferon signaling, promote cytotoxic T-cell differentiation, and enhance the efficacy of anti-PD-1 and anti-CTLA-4 therapies[78,79].
Importantly, DAT also shifts microbial composition in favor of taxa such as Burkholderiales and Bacteroidales-bacterial orders associated with favorable ICIs responses[54,79]. In vivo studies confirm that DAT supplementation delays tumor growth and enhances immunotherapy outcomes, even in the presence of dysbiosis. Additionally, the concept of antigenic mimicry, wherein microbial peptides share structural homology with tumor antigens, has been proposed as a mechanism through which the microbiome enhances T-cell mediated antitumor responses. This has been demonstrated in both murine models and clinical studies[80].
Collectively, these findings position the gut microbiome as a critical determinant of immunotherapy responses. Its capacity to modulate host immunity, influence hepatic oncogenesis, and enhance ICIs efficacy underscores its potential as both a predictive biomarker and therapeutic target in HCC (Figure 2).
Microbiota dysbiosis plays a pivotal role in modulating the efficacy of ICIs. The presence of specific microbial taxa has been correlated with enhanced therapeutic responses to ICIs. Modulation of the gut microbiota can be achieved through various strategies, including fecal microbiota transplantation (FMT), administration of probiotics and prebiotics, dietary and lifestyle modifications, as well as the use of antibiotics (Table 2). Several studies have demonstrated the capacity of gut microbiota to enhance ICIs efficacy. For instance, Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium have been shown to exert immunomodulatory effects by downregulating Tregs, promoting the activation of Batf3 DC, and enhancing Th1 immune responses during PD-L1 inhibitor therapy[81]. Similarly, Bifidobacterium spp. has been reported to potentiate the local effects of anti-CD47 immunotherapy and to promote tumor-specific CD8+ T cell responses in the context of PD-L1 inhibition[82]. In addition, Bacteroides fragilis, Bacteroides thetaiotaomicron, and members of the Burkholderiales order have been implicated in driving interleukin-12–dependent Th1 responses during CTLA-4 inhibitor therapy[54]. Moreover, a combination of Lactobacillus rhamnosus and Escherichia coli Nissle 1917 has been shown to reshape the gut microbiome by increasing the abundance of beneficial bacteria such as Prevotella and Oscilibacter, which produce anti-inflammatory metabolites. These changes contribute to reduced Th17 polarization and promote the differentiation of anti-inflammatory Tregs/Tr1 cells within the gut[83].
| Ref. | Country | Samples (n) | Microbiota enrichment or microbiota modulation | ICIs | Outcomes |
| Zheng et al[138] (2020) | China | HCC with BCLC C (n = 8) | Firmicutes, Bacteroidetes, Proteobacteria dominated both in responder and non-responder | Camrelizumab (Anti-PD-1) | Several beneficial lactic acid bacteria, including four Lactobacillus species (L. oris, L. vaginalis, L. mucosae, and L. gasseri), Streptococcus thermophilus, and Bifidobacterium dentium, were significantly enriched, contributing to the support of host metabolism and immune function. Additionally, commensal bacteria enriched in responders—particularly members of the Ruminococcaceae family and Akkermansia muciniphila—promoted host health by preserving intestinal barrier integrity and mitigating systemic immunosuppression |
| Mao et al[113] (2021) | China | Unresectable HCC (n = 35) or advanced biliary tract cancer (n = 30) | Bacteroidetes, Proteobacteria, and Firmicutes dominated in both the clinical benefit response (CBR) group and the non-clinical benefit response (NCB) group | Anti-PD-1 (patients that progressed from gemcitabine plus cisplatin and the first-line chemotherapy) | The CBR group had more Bacteroidetes (P = 0.028), while Proteobacteria trended higher in the NCB group (P = 0.067). In HCC patients, Veillonellaceae was enriched in the NCB group and linked to poorer outcomes. In contrast, Erysipelotrichaceae bacterium-GAM147 and Ruminococcus callidus were associated with longer progression-free survival. These findings suggest that gut microbiota composition influences ICI response and survival |
| Shen et al[114] (2021) | Taiwan | Advanced HCC (n = 36) | Bifidobacterium, coprococcus, acidaminococcus | Anti-PD-1/anti-PD-L1 monotherapy or in combination with an immunomodulatory agent | Responders showed higher levels of Succinivibrio and Tyzzerella subgroup 4, while nonresponders had more Akkermansia. However, in patients with disease control, significant enrichment was observed in Bifidobacterium, Alloprevotella, Blautia, Megasphaera, Succinatimonas, Lachnospira, Acidaminococcus, Tyzzerella subgroup 4, and Coprococcus subgroup 3. Notably, by week 8 of ICI therapy, the increased abundance of Bifidobacterium, Acidaminococcus, and Coprococcus was no longer present |
| Lee et al[140] (2022) | Taipei | Unresectable HCC | Firmicutes and bacteroides | Nivolumab and pembrolizumab | Lachnospiraceae and Veillonellaceae were enriched in responders, while Prevotellaceae and Enterobacteriaceae increased in progressive disease with reduced Lachnospiraceae and Veillonellaceae. Secondary bile acids (e.g., UDCA, MDCA, tauro-UDCA, UCA) were higher in responders and correlated positively with Lachnoclostridium and Ruminococcus gnavus, but negatively with Prevotella 9. High Lachnoclostridium and low Prevotella 9 were linked to improved overall survival (median OS: 22.8 months) |
| Ahmed et al[55] (2018) | New York | Advance cancer (n = 60), HCC | Systemic antibiotic. Broad spectrum antibiotic (including cephalosporin). Narrow spectrum antibiotic (including vancomycin) | Anti-PD-1 or anti-PDL-1 | Broad-spectrum antibiotics were linked to poorer treatment responses compared to narrow-spectrum antibiotics. Using antibiotics shortly before or after starting ICI therapy was associated with shorter progression-free survival. Overall, patients who received antibiotics had worse overall survival than those who did not |
Epigenetic modulation by probiotics has also been implicated in the prevention of HCC pathogenesis. A synergistic formulation comprising Saccharomyces cerevisiae and Lactobacillus acidophilus, in combination with selenium and glutathione, has been shown to prevent carbon tetrachloride (CCl₄)-induced liver fibrosis through activation of silent information regulator 1 (SIRT1) in hepatocytes. Activation of SIRT1 attenuates ROS, endoplasmic reticulum stress, and inflammation associated with CCl₄ exposure[84]. Furthermore, probiotics such as Streptococcus thermophilus, Lactobacillus rhamnosus, and Weissella cibaria have demonstrated the ability to regulate aflatoxin metabolite contamination. Aflatoxins, which are secondary metabolites produced by Aspergillus flavus and Aspergillus parasiticus, are well-established car
Inulin and fructo-oligosaccharides (FOS), classified as prebiotics, have demonstrated antitumor effects primarily through the modulation of gut microbiota. These prebiotics stimulate the growth of Bifidobacterium species, which has been associated with enhanced efficacy of ICIs therapy in murine cancer models[90]. In particular, inulin contributes to the modulation of gut microbiota composition, promotes systemic memory T-cell responses, and enhances anti-PD-1 efficacy, thereby improving ICIs treatment outcomes[91]. FOS has also been shown to suppress colonization resistance by Clostridium difficile. Members of Clostridium clusters XIVa and XI are known to facilitate the transformation of primary to secondary BAs, a process that has been implicated in hepatic carcinogenesis[92].
Dietary interventions have also been linked to improved ICIs responsiveness. The ketogenic diet, characterized by high fat content as the primary source of calories, has been shown to stimulate the production of pro-inflammatory cytokines, enhance CD8+ T cell-mediated cytolysis, increase infiltration of CD4+ T cells, and improve T cell cytotoxic activity. Moreover, it's found to down regulate ICs expression (CTLA-4 and PD-1/PDL-1) on TILs, thereby mitigating immune escape mechanisms. The ketogenic diet also activates AMPK, which promotes PD-L1 degradation[93,94]. Additionally, adherence to a ketogenic dietary pattern has been associated with increased abundance of beneficial gut microbes such as Akkermansia muciniphila, which has been shown to restore responsiveness to ICIs[95]. Furthermore, diets rich in dietary fiber can be fermented by gut microbiota to produce SCFAs, which exhibit anti-inflammatory and antitumorigenic properties. High-fiber diets also promote greater microbial diversity and richness in the gut ecosystem[96]. This dietary pattern contributes to a lower fecal pH, which reduces the proliferation of carcinogenic bacteria involved in BAs meta
On the other hand, antibiotics also play a significant role in modulating the gut-liver axis and influencing HCC development. Vancomycin, a first-generation glycopeptide antibiotic, disrupts gram-positive bacterial cell wall synthesis[98]. It selectively depletes intestinal gram-positive bacteria, particularly members of the Lachnospiraceae, Ruminococcaceae, Bifidobacteria, and Clostridia families[99], thereby offering protection against HCC driven by secondary BAs. Secondary BAs have been implicated in establishing a pro-carcinogenic hepatic microenvironment[100]. Vancomycin also reduced liver cancer development by reducing bacteria expressing butyryl-CoA: Acetate transferase which are predominantly involved in SCFAs production in inulin-fed TRLR5- deficient mice. Butyrate, one of SCFA, is reported to have a double-sword effect, known as ‘butyrate paradox’. Under certain pathological conditions such as cholestasis, butyrate may exert pro-oncogenic effects and further exacerbate gut dysbiosis[101]. Moreover, vancomycin has been found to promote hematopoietic stem cells senescence by depleting Clostridium clusters XI and XIVa, which leads to a reduction in the concentration of DCA, a metabolite known to cause DNA damage and contribute to hepatocarcinogenesis[102]. In murine models overexpressing the MYC oncogene, the combined administration of vancomycin, primaxin, and neomycin significantly reduced both the number and size of HCC tumors[103]. Rifaximin is an antibiotic with no significant side effect on gut microbiome, acts improving intestinal permeability by inhibiting the LPS-TLR4 signalling pathway and suppressing portal endotoxin. Importantly, rifaximin has a low rate of antimicrobial resistance, making it a promising candidate for long-term therapeutic strategies in HCC management[104,105].
FMT is a therapeutic approach that involves transferring fecal material from a healthy donor to a recipient’s gastrointestinal tract, directly modifying the gut microbiota and potentially offering clinical benefits[106]. FMT can be administered orally through lyophilized or frozen capsules or directly via colonoscopy or gastroscopy[107,108]. Currently, no clinical trials have specifically examined the use of FMT in patients with HCC. However, preclinical studies using animal models have shown encouraging results. One study found that FMT from wild-type mice enhanced anticancer responses in HCC-bearing mice[109]. FMT also showed favorable effect to overcome ICIs-associated colitis and toxicity by modulation gut microbiota[110].
The gut microbiota plays a crucial role in tumor development. The administration of specific probiotics derived from gut microbiota has emerged as a strategy for microbiota manipulation[111]. Common probiotics, including Lactobacillus and Bifidobacterium species, are widely used to enhance the efficacy of immune ICIs, particularly in cases where antibiotics have disrupted gut microbiota. For example, Lactobacillus rhamnosus synergizes with ICIs, restoring microbial diversity and composition while increasing beneficial bacteria such as Bifidobacterium pseudolongum and Bacteroides[112]. Clinical studies on unresectable HCC patients undergoing anti-PD-1 therapy reveal that Ruminococcus calidus and Erysipelotrichaceae bacterium-GAM147 are more abundant in patients with prolonged PFS and OS. Conversely, higher Veillonellaceae abundance is associated with poorer outcomes[113]. Additionally, enrichment of Bifidobacterium, Coprococcus, and Acidaminococcus correlates with ICIs efficacy in HCC patients. Those gut microbiota were associated with disease control rather than objective response. The abundance of Bifidobacterium, Acidaminococcus, and Coprococcus significantly di
Prebiotics are a food source for microbiota. Fermentation of prebiotic can produce a favorable substance for preventing and reducing HCC. Specific dietary sources can provide prebiotics properties such as soluble fiber diets, including fructans, gums, pectins. Some plants like banana, garlic, chicory root, onions, asparagus, leeks, Jerusalem artichokes, rye, barley and wheat are non-digestible polysaccharide, highly contain both of FOS and inulin, which may have antitumor effect[102,115]. Non-digestible oligosaccharides found in nuts, tea, wine, vegetables, and fruit, contain prebiotic sub
Dietary intervention showed a favorable effect on HCC by preventing its risk factor. The most recently studied Mediterranean diet reported on reducing obesity, non-alcoholic fatty liver disease and type 2 diabetes mellitus. Furthermore, a population based study showed reduction in HCC incidence in liver cirrhosis patients who adhere to a mediterranean diet[119]. The Mediterranean diet contains low saturated fat and cholesterol, high monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA), complex carbohydrates, fibers, and polyphenols, increasing the number of Lactobacillus, Bifidobacterium, and Faecalibacterium[120-123]. However, high fiber contained in Mediterranean diet, particularly fermented fiber, might be unfavourable due to inducing dysbiosis, resulting in high BA production, and hyperbilirubinemia. This insight underscores the need for modification of the Mediterranean diet[123]. Diets containing MUFA and omega-3 PUFA have been found to reduce risk of HCC development[124-126] In animal study, high MUFA and PUFA diets gain a higher microbial diversity, including Bifidobacteria, which reportedly have protective effects against HCC[124,127]. In animal models with prostate and renal cell carcinoma, a low-protein diet in addition to anti-PD-1 amplifies the capacity of tumor-associated macrophages to eradicate tumor cells[95]. Compared with the low fiber diet, high fiber diet showed a higher effectiveness of anti-PD-1 on melanoma progression (OR = 5.3, 95%CI: 1.02-26.3)[128].
Antibiotic administration in HCC patients undergoing ICIs therapy showing inconsistent results. A study of 414 HCC patients treated with anti-PD-1 monotherapy suggest that administration of beta-lactam or quinolones 30 days before or after ICIs initiation are associated with extended PFS[129]. In contrast, some studies reported that exposure to antibiotics in HCC with ICIs therapy demonstrates a worsen HCC outcome. A study consisting of 4100 patients with HCC found an unfavorable effect of antibiotic therapy[130]. Moreover, a study in Hong Kong involving 395 patients with HCC also reported higher mortality rate after antibiotic use during immunotherapy[131]. In 59 patients with advanced HCC received nivolumab suggest antibiotic administration shortening OS (P = 0.04) when compared to patients who did not receive antibiotics. Furthermore, the higher risk of death during ICIs therapy was linked with administration of antibiotic against anaerobes compared to antibiotic against aerobes[132,133].
The rapid evolution of therapeutic options for HCC has created significant knowledge gaps, paving the way for future research[134]. One major challenge is determining the optimal management strategy following progression on ICIs-based therapies, now established as front-line treatments. Questions remain regarding the potential benefit of switching to alternative ICIs combinations for patients who fail to respond to initial regimens. Additionally, the availability of various front-line combinations highlights the urgent need for biomarker development to identify patients most likely to benefit from specific therapies[135]. Another critical area is the treatment of patients with advanced cirrhosis (Child-Pugh B or C), who constitute a large portion of the HCC population yet are often excluded from clinical trials[32]. While the phase II CheckMate 040 trial demonstrated that nivolumab was tolerable in patients with Child-Pugh B cirrhosis[7], future studies must clarify treatment approaches for HCC in this subgroup, where survival is more influenced by liver function than tumor burden[136].
Given the rising global incidence of HCC and the scarcity of actionable mutations, it is crucial to unravel host-intrinsic and host-extrinsic factors contributing to HCC and shaping therapeutic outcomes[1]. Predictive biomarkers that can guide treatment decisions remain elusive, making it unclear which patients will derive the most benefit from specific therapies. As the ICIs combination landscape expands, such as ICIs plus anti-VEGF or dual ICIs therapies, a deeper understanding of predictive biomarkers and precise patient stratification could significantly enhance treatment outcomes[32]. Recent biomarker analyses from trials of atezolizumab-bevacizumab have provided insights, showing that pre-existing immunity, VEGF receptor 2 expression, Tregs levels, and myeloid inflammation signatures correlate with better outcomes. Conversely, high Tregs/Teff ratios, glypican-3 expression, and elevated alpha-fetoprotein levels were associated with reduced benefit. These findings emphasize the complexity of biomarker-driven therapy in HCC[137].
Emerging areas of research offer promising avenues to improve HCC management. For instance, preliminary evidence suggests that the gut microbiota plays a critical role in modulating responses to ICIs, making it a promising target for enhancing the efficacy of immunotherapy in HCC[138]. The gut microbiota influences systemic immunity, with specific bacterial taxa associated with favorable responses to ICIs in preclinical and clinical studies. For instance, a higher abundance of Akkermansia muciniphila and Bifidobacterium species has been linked to enhanced anti-tumor immunity and better outcomes with ICIs. Combining ICIs with interventions targeting the gut microbiota, such as FMT, probiotics, prebiotics, antibiotics, or dietary intervention, may help optimize treatment responses. Recent studies have also explored the use of dietary modifications and microbiota-derived metabolites, such as SCFAs, to modulate the tumor immune microenvironment. However, further prospective studies are needed to better define the predictive role of the microbiota and validate its therapeutic potential in combination with ICIs for HCC[11,139,140].
The gut microbiome plays a pivotal role in regulating liver function, metabolic processes, and immune responses through its metabolites and bidirectional communication via the gut-liver axis. Certain microbial taxa, including Ruminococcaceae, Akkermansia muciniphila, Lactobacillus species, Streptococcus thermophilus, Bifidobacterium dentium, Erysipelotrichaceae bacterium, and Lachnoclostridium, contribute to maintaining intestinal barrier integrity, mitigating systemic immunosuppression, modulating responses to ICIs, and enhancing patient survival. Modulating the gut microbiome in conjunction with ICIs offers promising potential for improving HCC treatment. Integrating microbiome-targeted strategies with existing ICIs, such as anti-CTLA-4 and anti-PD-1 antibodies, may improve clinical outcomes.
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3883] [Article Influence: 970.8] [Reference Citation Analysis (3)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11434] [Article Influence: 3811.3] [Reference Citation Analysis (4)] |
| 3. | Tran NH. Shifting Epidemiology of Hepatocellular Carcinoma in Far Eastern and Southeast Asian Patients: Explanations and Implications. Curr Oncol Rep. 2022;24:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 5. | Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1656] [Cited by in RCA: 1743] [Article Influence: 290.5] [Reference Citation Analysis (0)] |
| 6. | Schwabe RF, Greten TF. Gut microbiome in HCC - Mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 7. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 744] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 8. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1341] [Article Influence: 268.2] [Reference Citation Analysis (0)] |
| 9. | Kudo M, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer DH, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Yau T, Gurary EB, Siegel AB, Wang A, Cheng AL, Zhu AX; KEYNOTE-224 Investigators. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer. 2022;167:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 10. | Merle P, Blanc JF, Edeline J, Le Malicot K, Allaire M, Assenat E, Guarssifi M, Bouattour M, Péron JM, Laurent-Puig P, Levrero M, Costentin C, Guiu B, Sokol H, Tougeron D, Aparicio T, Nault JC, Phelip JM. Ipilimumab with atezolizumab-bevacizumab in patients with advanced hepatocellular carcinoma: The PRODIGE 81-FFCD 2101-TRIPLET-HCC trial. Dig Liver Dis. 2023;55:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (0)] |
| 12. | Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, Lv ZD, Wang DS, Li YH, Zhang DS, Xu RH. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2:100383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | Kinsey E, Lee HM. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers (Basel). 2024;16:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 14. | Lohitesh K, Chowdhury R, Mukherjee S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight. Cancer Cell Int. 2018;18:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 16. | Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017;68:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Hato T, Goyal L, Greten TF, Duda DG, Zhu AX. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2278] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 19. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10352] [Article Influence: 796.3] [Reference Citation Analysis (34)] |
| 20. | Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50:1625-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ, Yang DL. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020;20:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2013] [Cited by in RCA: 2253] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 24. | Kowalczyk A, D'Souza CA, Zhang L. Cell-extrinsic CTLA4-mediated regulation of dendritic cell maturation depends on STAT3. Eur J Immunol. 2014;44:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Hargadon KM. Tumor microenvironmental influences on dendritic cell and T cell function: A focus on clinically relevant immunologic and metabolic checkpoints. Clin Transl Med. 2020;10:374-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4619] [Article Influence: 659.9] [Reference Citation Analysis (0)] |
| 27. | Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 481] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 28. | Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998;188:1849-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 288] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 750] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 30. | Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015;33:1889-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1625] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 31. | Eroglu Z, Kim DW, Wang X, Camacho LH, Chmielowski B, Seja E, Villanueva A, Ruchalski K, Glaspy JA, Kim KB, Hwu WJ, Ribas A. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer. 2015;51:2689-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | van Bömmel F, Berg T, Lordick F. Immune checkpoint inhibition (ICI) in current systemic therapies for hepatocellular carcinoma (HCC). ESMO Gastrointest Oncol. 2023;1:27-39. [DOI] [Full Text] |
| 33. | Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 706] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 34. | Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1404] [Cited by in RCA: 1385] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 35. | Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 1219] [Article Influence: 152.4] [Reference Citation Analysis (0)] |
| 36. | Sangro B, Park J, Finn R, Cheng A, Mathurin P, Edeline J, Kudo M, Han K, Harding J, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo S, Kelley R, Begic D, Chen G, Neely J, Tschaika M, Yau T. LBA-3 CheckMate 459: Long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol. 2020;31:S241-S242. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Qin S, Fang W, Ren Z, Ou S, Lim HY, Zhang F, Lee KC, Choi HJ, Tong J, Tao M, Xu A, Cheng A, Lu CH, Chiu CF, Abdul Wahid MI, Kamble S, Norquist JM, Zhong W, Li C, Chen Z. A Phase 3 Study of Pembrolizumab versus Placebo for Previously Treated Patients from Asia with Hepatocellular Carcinoma: Health-Related Quality of Life Analysis from KEYNOTE-394. Liver Cancer. 2024;13:389-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1:EVIDoa2100070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 658] [Article Influence: 219.3] [Reference Citation Analysis (0)] |
| 39. | Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, Macarulla TM, Tomasello G, Boisserie F, Hou J, Li X, Song J, Zhu AX. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 40. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 750] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 41. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 42. | Zarlashat Y, Mushtaq H, Pham L, Abbas W, Sato K. Advancements in Immunotherapeutic Treatments for Hepatocellular Carcinoma: Potential of Combination Therapies. Int J Mol Sci. 2024;25:6830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 43. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 44. | Martinelli E, Masi G, Piscaglia F, Cabibbo G, Di Maio M, Gasbarrini A, Iavarone M, Pellegrini E, Mazzaferro V, Ballestrero A, Garufi C, Bergamo F, Celsa C, Marino D, Tovoli F, Ponziani F, Pressiani T, Astolfi C, Ciardiello F, Daniele B, Rimassa L. P-57 Atezolizumab in combination with bevacizumab in patients with unresectable HCC previously untreated with systemic therapy: Interim analysis results from phase IIIb Italian AMETHISTA trial. Ann Oncol. 2023;34:S34. [DOI] [Full Text] |
| 45. | Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:995-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 349] [Article Influence: 116.3] [Reference Citation Analysis (1)] |
| 46. | Decaens T, Yau T, Kudo M, Sangro B, Qin S, Da Fonseca L, Karachiwala H, Park J, Gane E, Pinter M, Tai D, Santoro A, Pizarro G, Chiu C, Schenker M, He A, Wang Q, Ogata T, Hreiki J, Galle P. 965MO Nivolumab (NIVO) plus ipilimumab (IPI) vs lenvatinib (LEN) or sorafenib (SOR) as first-line (1L) treatment for unresectable hepatocellular carcinoma (uHCC): Expanded analyses from CheckMate 9DW. Ann Oncol. 2024;35:S657. [DOI] [Full Text] |
| 47. | Matloubieh JE, Licona-freudensten AP, Baran AM, Lada MJ, Jones CE, Peyre CG, Hezel AF, Noel MS, Dunne RF, Tejani MA. Surveillance for locally advanced esophageal and gastroesophageal junction (GEJ) cancers: Patterns of recurrence and methods of detection. JCO. 2019;37:32-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 48. | Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 626] [Cited by in RCA: 1011] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 49. | Wu K, Yi M, Qin S, Chu Q, Zheng X, Wu K. The efficacy and safety of combination of PD-1 and CTLA-4 inhibitors: a meta-analysis. Exp Hematol Oncol. 2019;8:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 50. | Lim SY, Shklovskaya E, Lee JH, Pedersen B, Stewart A, Ming Z, Irvine M, Shivalingam B, Saw RPM, Menzies AM, Carlino MS, Scolyer RA, Long GV, Rizos H. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat Commun. 2023;14:1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 51. | Fujiwara Y, Mittra A, Naqash AR, Takebe N. A review of mechanisms of resistance to immune checkpoint inhibitors and potential strategies for therapy. Cancer Drug Resist. 2020;3:252-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 1082] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 53. | Vatanen T, Kostic AD, d'Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M; DIABIMMUNE Study Group, Xavier RJ. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:842-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 54. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 2549] [Article Influence: 254.9] [Reference Citation Analysis (0)] |
| 55. | Ahmed J, Kumar A, Parikh K, Anwar A, Knoll BM, Puccio C, Chun H, Fanucchi M, Lim SH. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7:e1507670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 56. | Fidelle M, Rauber C, Alves Costa Silva C, Tian AL, Lahmar I, de La Varende AM, Zhao L, Thelemaque C, Lebhar I, Messaoudene M, Pizzato E, Birebent R, Mbogning Fonkou MD, Zoppi S, Reni A, Dalban C, Leduc M, Ferrere G, Durand S, Ly P, Silvin A, Mulder K, Dutertre CA, Ginhoux F, Yonekura S, Roberti MP, Tidjani-Alou M, Terrisse S, Chen J, Kepp O, Schippers A, Wagner N, Suárez-Gosálvez J, Kobold S, Fahrner JE, Richard C, Bosq J, Lordello L, Vitali G, Galleron N, Quinquis B, Le Chatelier E, Blanchard L, Girard JP, Jarry A, Gervois N, Godefroy E, Labarrière N, Koschny R, Daillère R, Besse B, Truntzer C, Ghiringhelli F, Coatnoan N, Mhanna V, Klatzmann D, Drubay D, Albiges L, Thomas AM, Segata N, Danlos FX, Marabelle A, Routy B, Derosa L, Kroemer G, Zitvogel L. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science. 2023;380:eabo2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 106] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 57. | Yin Y, Sakakibara R, Honda T, Kirimura S, Daroonpan P, Kobayashi M, Ando K, Ujiie H, Kato T, Kaga K, Mitsumura T, Nakano R, Sakashita H, Matsuge S, Ishibashi H, Akashi T, Hida Y, Morohoshi T, Azuma M, Okubo K, Miyazaki Y. High density and proximity of CD8(+) T cells to tumor cells are correlated with better response to nivolumab treatment in metastatic pleural mesothelioma. Thorac Cancer. 2023;14:1991-2000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Michielin O, Weide B, Romero P, Speiser DE. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140-6145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 59. | Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, Impera V, Dubois M, Persano M, Migliari M, Pretta A, Liscia N, Astara G, Scartozzi M. Immune Checkpoint Inhibitors in the Treatment of HCC. Front Oncol. 2020;10:601240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 60. | Pishvaian M, Lee M, Ryoo B, Stein S, Lee K, Verret W, Spahn J, Shao H, Liu B, Iizuka K, Hsu C. Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018;29:viii718-viii719. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res. 2016;22:1865-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 685] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 62. | Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 2222] [Article Influence: 370.3] [Reference Citation Analysis (0)] |
| 63. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4184] [Article Influence: 380.4] [Reference Citation Analysis (0)] |
| 64. | Manfredi GF, Celsa C, John C, Jones C, Acuti N, Scheiner B, Fulgenzi CAM, Korolewicz J, Pinter M, Gennari A, Mauri FA, Pirisi M, Minisini R, Vincenzi F, Burlone M, Rigamonti C, Donadon M, Cabibbo G, D'Alessio A, Pinato DJ. Mechanisms of Resistance to Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023;10:1955-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1710] [Cited by in RCA: 2057] [Article Influence: 257.1] [Reference Citation Analysis (7)] |
| 66. | Luo W, Guo S, Zhou Y, Zhao J, Wang M, Sang L, Chang B, Wang B. Hepatocellular Carcinoma: How the Gut Microbiota Contributes to Pathogenesis, Diagnosis, and Therapy. Front Microbiol. 2022;13:873160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Ohtani N, Hara E. Gut-liver axis-mediated mechanism of liver cancer: A special focus on the role of gut microbiota. Cancer Sci. 2021;112:4433-4443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 68. | Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. 2018;7:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Wang J, Wang X, Zhuo E, Chen B, Chan S. Gutliver axis in liver disease: From basic science to clinical treatment (Review). Mol Med Rep. 2025;31:10. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 70. | Laterza L, Rizzatti G, Gaetani E, Chiusolo P, Gasbarrini A. The Gut Microbiota and Immune System Relationship in Human Graft-versus-Host Disease. Mediterr J Hematol Infect Dis. 2016;8:e2016025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 71. | Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 1352] [Article Influence: 450.7] [Reference Citation Analysis (0)] |
| 72. | Xie C, Pocha C. Crosstalk between Gut Microbiota and Hepatocellular Carcinoma. Gastrointest Disord. 2023;5:127-143. [DOI] [Full Text] |
| 73. | Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1094] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 74. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1028] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 75. | Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 76. | Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396-406.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 770] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 77. | Qiu Q, Lin Y, Ma Y, Li X, Liang J, Chen Z, Liu K, Huang Y, Luo H, Huang R, Luo L. Exploring the Emerging Role of the Gut Microbiota and Tumor Microenvironment in Cancer Immunotherapy. Front Immunol. 2020;11:612202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 78. | Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman MJ, Boon ACM, Lenschow DJ, Stappenbeck TS. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 411] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 79. | Joachim L, Göttert S, Sax A, Steiger K, Neuhaus K, Heinrich P, Fan K, Orberg ET, Kleigrewe K, Ruland J, Bassermann F, Herr W, Posch C, Heidegger S, Poeck H. The microbial metabolite desaminotyrosine enhances T-cell priming and cancer immunotherapy with immune checkpoint inhibitors. EBioMedicine. 2023;97:104834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 80. | Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, Onder L, Lütge M, Novkovic M, Nindl V, Ramos G, Arnoldini M, Slack EMC, Boivin-Jahns V, Jahns R, Wyss M, Mooser C, Lambrecht BN, Maeder MT, Rickli H, Flatz L, Eriksson U, Geuking MB, McCoy KD, Ludewig B. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 81. | Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 2132] [Article Influence: 304.6] [Reference Citation Analysis (1)] |
| 82. | Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1979] [Cited by in RCA: 2843] [Article Influence: 284.3] [Reference Citation Analysis (2)] |
| 83. | Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306-E1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 84. | Liu Y, Liu Q, Hesketh J, Huang D, Gan F, Hao S, Tang S, Guo Y, Huang K. Protective effects of selenium-glutathione-enriched probiotics on CCl(4)-induced liver fibrosis. J Nutr Biochem. 2018;58:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 85. | Ehrlich KC, Mack BM. Comparison of expression of secondary metabolite biosynthesis cluster genes in Aspergillus flavus, A. parasiticus, and A. oryzae. Toxins (Basel). 2014;6:1916-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 86. | Sun Z, Lu P, Gail MH, Pee D, Zhang Q, Ming L, Wang J, Wu Y, Liu G, Wu Y, Zhu Y. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology. 1999;30:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | O'Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 552] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 88. | Kim J, Lee HK. The Role of Gut Microbiota in Modulating Tumor Growth and Anticancer Agent Efficacy. Mol Cells. 2021;44:356-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Son MY, Cho HS. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J Microbiol Biotechnol. 2023;33:849-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 90. | Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, Lewis IA, Geuking MB, McCoy KD. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 846] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 91. | Albouery M, Bretin A, Buteau B, Grégoire S, Martine L, Gambert S, Bron AM, Acar N, Chassaing B, Bringer MA. Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients. 2021;13:1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 935] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 93. | Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C, Zhang Z, Xu K, Duan J, He S, Zhang J, Wan L, Liu T, Zhou X, Hung MC, Freeman GJ, Wei W. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell. 2021;81:2317-2331.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 94. | Ferrere G, Tidjani Alou M, Liu P, Goubet AG, Fidelle M, Kepp O, Durand S, Iebba V, Fluckiger A, Daillère R, Thelemaque C, Grajeda-Iglesias C, Alves Costa Silva C, Aprahamian F, Lefevre D, Zhao L, Ryffel B, Colomba E, Arnedos M, Drubay D, Rauber C, Raoult D, Asnicar F, Spector T, Segata N, Derosa L, Kroemer G, Zitvogel L. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 95. | Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, Wei H, Yang X, Sung JJY, Yu J. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 543] [Article Influence: 135.8] [Reference Citation Analysis (0)] |
| 96. | Abreu Y Abreu AT, Milke-García MP, Argüello-Arévalo GA, Calderón-de la Barca AM, Carmona-Sánchez RI, Consuelo-Sánchez A, Coss-Adame E, García-Cedillo MF, Hernández-Rosiles V, Icaza-Chávez ME, Martínez-Medina JN, Morán-Ramos S, Ochoa-Ortiz E, Reyes-Apodaca M, Rivera-Flores RL, Zamarripa-Dorsey F, Zárate-Mondragón F, Vázquez-Frias R. Dietary fiber and the microbiota: A narrative review by a group of experts from the Asociación Mexicana de Gastroenterología. Rev Gastroenterol Mex (Engl Ed). 2021;86:287-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Tanaka Y, Shimizu S, Shirotani M, Yorozu K, Kitamura K, Oehorumu M, Kawai Y, Fukuzawa Y. Nutrition and Cancer Risk from the Viewpoint of the Intestinal Microbiome. Nutrients. 2021;13:3326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE(2)-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 99. | Nazzal L, Soiefer L, Chang M, Tamizuddin F, Schatoff D, Cofer L, Aguero-Rosenfeld ME, Matalon A, Meijers B, Holzman R, Lowenstein J. Effect of Vancomycin on the Gut Microbiome and Plasma Concentrations of Gut-Derived Uremic Solutes. Kidney Int Rep. 2021;6:2122-2133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek JD Jr, Zhang L, Wang WB, Hao S, Flythe MD, Gonzalez DJ, Cani PD, Conejo-Garcia JR, Xiong N, Kennett MJ, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell. 2018;175:679-694.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 101. | Singh V, Yeoh BS, Abokor AA, Golonka RM, Tian Y, Patterson AD, Joe B, Heikenwalder M, Vijay-Kumar M. Vancomycin prevents fermentable fiber-induced liver cancer in mice with dysbiotic gut microbiota. Gut Microbes. 2020;11:1077-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 102. | Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1651] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 103. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1037] [Article Influence: 148.1] [Reference Citation Analysis (0)] |
| 104. | Fujinaga Y, Kawaratani H, Kaya D, Tsuji Y, Ozutsumi T, Furukawa M, Kitagawa K, Sato S, Nishimura N, Sawada Y, Takaya H, Kaji K, Shimozato N, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Effective Combination Therapy of Angiotensin-II Receptor Blocker and Rifaximin for Hepatic Fibrosis in Rat Model of Nonalcoholic Steatohepatitis. Int J Mol Sci. 2020;21:5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 414] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 106. | Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, Hu HM, Hsu PI, Wang JY, Wu DC. Fecal microbiota transplantation: Review and update. J Formos Med Assoc. 2019;118 Suppl 1:S23-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 107. | Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, Zidi B, Zhang S, Badger JH, Vetizou M, Cole AM, Fernandes MR, Prescott S, Costa RGF, Balaji AK, Morgun A, Vujkovic-Cvijin I, Wang H, Borhani AA, Schwartz MB, Dubner HM, Ernst SJ, Rose A, Najjar YG, Belkaid Y, Kirkwood JM, Trinchieri G, Zarour HM. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 1032] [Article Influence: 258.0] [Reference Citation Analysis (0)] |
| 108. | Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 992] [Article Influence: 198.4] [Reference Citation Analysis (0)] |
| 109. | Hu C, Xu B, Wang X, Wan WH, Lu J, Kong D, Jin Y, You W, Sun H, Mu X, Feng D, Chen Y. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology. 2023;77:48-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 110. | Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, Parra ER, Francisco-Cruz A, Raju GS, Stroehlein JR, Campbell MT, Gao J, Subudhi SK, Maru DM, Blando JM, Lazar AJ, Allison JP, Sharma P, Tetzlaff MT, Wargo JA, Jenq RR. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 550] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 111. | Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, Okamoto T, Hamatake M, Tsuchiya-Kawano Y, Otsubo K, Inoue K, Yoneshima Y, Tanaka K, Okamoto I, Nakanishi Y, Mori M. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: A multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int J Cancer. 2021;149:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 112. | Gao G, Ma T, Zhang T, Jin H, Li Y, Kwok LY, Zhang H, Sun Z. Adjunctive Probiotic Lactobacillus rhamnosus Probio-M9 Administration Enhances the Effect of Anti-PD-1 Antitumor Therapy via Restoring Antibiotic-Disrupted Gut Microbiota. Front Immunol. 2021;12:772532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 113. | Mao J, Wang D, Long J, Yang X, Lin J, Song Y, Xie F, Xun Z, Wang Y, Wang Y, Li Y, Sun H, Xue J, Song Y, Zuo B, Zhang J, Bian J, Zhang T, Yang X, Zhang L, Sang X, Zhao H. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. 2021;9:e003334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 114. | Shen YC, Lee PC, Kuo YL, Wu WK, Chen CC, Lei CH, Yeh CP, Hsu C, Hsu CH, Lin ZZ, Shao YY, Lu LC, Liu TH, Chen CH, Wu MS, Huang YH, Cheng AL. An Exploratory Study for the Association of Gut Microbiome with Efficacy of Immune Checkpoint Inhibitor in Patients with Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:809-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 115. | Sousa VMCD, Santos EFD, Sgarbieri VC. The Importance of Prebiotics in Functional Foods and Clinical Practice. FNS. 2011;02:133-144. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, Nicoll AJ. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients. 2021;13:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 117. | Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 118. | Fotschki B, Juśkiewicz J, Jurgoński A, Sójka M. Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver. Nutrients. 2021;13:833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 119. | Bajaj JS, Idilman R, Mabudian L, Hood M, Fagan A, Turan D, White MB, Karakaya F, Wang J, Atalay R, Hylemon PB, Gavis EA, Brown R, Thacker LR, Acharya C, Heuman DM, Sikaroodi M, Gillevet PM. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology. 2018;68:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 120. | Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 121. | Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, De Lorenzo A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020;13:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 122. | Gutiérrez-Díaz I, Fernández-Navarro T, Sánchez B, Margolles A, González S. Mediterranean diet and faecal microbiota: a transversal study. Food Funct. 2016;7:2347-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 123. | Rajapakse J, Khatiwada S, Akon AC, Yu KL, Shen S, Zekry A. Unveiling the complex relationship between gut microbiota and liver cancer: opportunities for novel therapeutic interventions. Gut Microbes. 2023;15:2240031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 124. | López-Salazar V, Tapia MS, Tobón-Cornejo S, Díaz D, Alemán-Escondrillas G, Granados-Portillo O, Noriega L, Tovar AR, Torres N. Consumption of soybean or olive oil at recommended concentrations increased the intestinal microbiota diversity and insulin sensitivity and prevented fatty liver compared to the effects of coconut oil. J Nutr Biochem. 2021;94:108751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 125. | Lyons CL, Finucane OF, Murphy AM, Cooke AA, Viollet B, Vieira PM, Oldham W, Kahn BB, Roche HM. Monounsaturated Fatty Acids Impede Inflammation Partially Through Activation of AMPK. FASEB J. 2016;30. [DOI] [Full Text] |
| 126. | Rocha DM, Bressan J, Hermsdorff HH. The role of dietary fatty acid intake in inflammatory gene expression: a critical review. Sao Paulo Med J. 2017;135:157-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 127. | Fu Y, Wang Y, Gao H, Li D, Jiang R, Ge L, Tong C, Xu K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediators Inflamm. 2021;2021:8879227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 128. | Spencer CN, Gopalakrishnan V, Mcquade J, Andrews MC, Helmink B, Khan MW, Sirmans E, Haydu L, Cogdill A, Burton E, Amaria R, Patel S, Glitza I, Davies M, Posada E, Hwu W, Diab A, Nelson K, Tawbi H, Wong M, Jenq RR, Cohen L, Daniel-macdougall C, Wargo JA. Abstract 2838: The gut microbiome (GM) and immunotherapy response are influenced by host lifestyle factors. Cancer Res. 2019;79:2838-2838. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, Saeed A, Jun T, Dharmapuri S, Gampa A, Wang Y, Khan U, Muzaffar M, Navaid M, Lee PC, Bulumulle A, Yu B, Paul S, Nimkar N, Bettinger D, Hildebrand H, Abugabal YI, Pressiani T, Personeni N, Nishida N, Kudo M, Kaseb A, Huang YH, Ang C, Pillai A, Rimassa L, Naqash AR, Sharon E, Cortellini A, Pinato DJ. Early Antibiotic Exposure Is Not Detrimental to Therapeutic Effect from Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2021;10:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 130. | Pinato DJ, Li X, Mishra-Kalyani P, D'Alessio A, Fulgenzi CAM, Scheiner B, Pinter M, Wei G, Schneider J, Rivera DR, Pazdur R, Theoret MR, Casak S, Lemery S, Fashoyin-Aje L, Cortellini A, Pelosof L. Association between antibiotics and adverse oncological outcomes in patients receiving targeted or immune-based therapy for hepatocellular carcinoma. JHEP Rep. 2023;5:100747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 131. | Cheung KS, Ka LL, Leung WK. Antibiotics Associated with Lower Survival in Hepatocellular Cancer Patients Receiving Immune Checkpoint Inhibitors Independent of Tumor Status. Liver Cancer. 2023;12:91-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Cheung KS, Lam LK, Seto WK, Leung WK. Use of Antibiotics during Immune Checkpoint Inhibitor Treatment Is Associated with Lower Survival in Hepatocellular Carcinoma. Liver Cancer. 2021;10:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 133. | Alshammari K, Alsugheir F, Aldawoud M, Alolayan A, Algarni MA, Sabatin F, Mohammad MF, Alosaimi A, Sanai F, Odah H, Alshehry A, Aldibasi O, Al Saleh AS, Jazieh A. Association between antibiotic exposure and survival in patients with hepatocellular carcinoma treated with nivolumab. JCO. 2021;39:e16186-e16186. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Bejjani AC, Finn RS. Hepatocellular Carcinoma: Pick the Winner-Tyrosine Kinase Inhibitor Versus Immuno-oncology Agent-Based Combinations. J Clin Oncol. 2022;40:2763-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 135. | Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 177] [Reference Citation Analysis (0)] |
| 136. | Kudo M. Combination immunotherapy with anti-VEGF/TKI for hepatocellular carcinoma: present and future perspective. Hepatobiliary Surg Nutr. 2021;10:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, Yau T, Kaseb AO, Burgoyne AM, Dayyani F, Spahn J, Verret W, Finn RS, Toh HC, Lujambio A, Wang Y. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28:1599-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 344] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 138. | Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, Li P, Qin N, Fang W. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 139. | Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3310] [Article Influence: 472.9] [Reference Citation Analysis (0)] |
| 140. | Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, Yu-Lun K, Chou SH, Luo JC, Hou MC, Huang YH. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |